Abstract
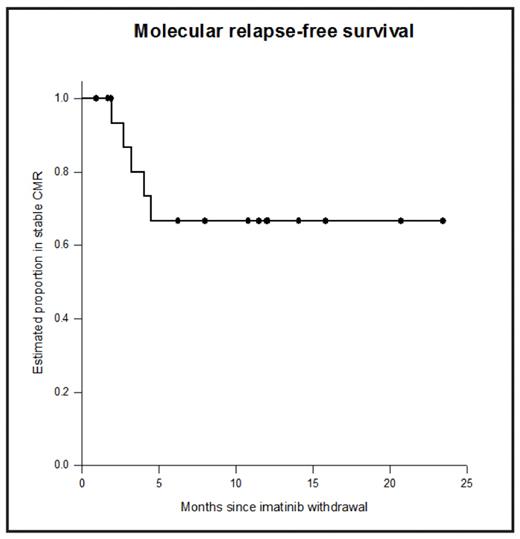
After 5 years of imatinib treatment 40–50% of chronic myeloid leukaemia (CML) patients will have stable undetectable BCR-ABL by real-time quantitative RT-PCR (RQ-PCR) using strict sensitivity criteria (‘complete molecular response’, CMR). Many patients who stop imatinib in CMR will relapse, but small numbers have been reported with sustained CMR after imatinib withdrawal. We designed a non-randomised prospective Phase 2 study of imatinib withdrawal in adult chronic phase CML patients in CMR for ≥2 years (ACTRN012606000118505). Patients were treated in multiple centres around Australia, and RQ-PCR for BCR-ABL was performed centrally: monthly for the first year after imatinib withdrawal, and 2-monthly in the second year. Molecular relapse was defined as a single PCR result above the level of major molecular response (MMR) or any two consecutive positive results. Molecular relapse was treated with imatinib and patients were monitored monthly for 12 months to assess response to retreatment. Patients were enrolled in two cohorts: imatinib de novo (IM only, n=5) and imatinib after prior interferon therapy (IFN-IM, n=13). The median duration of prior IFN was 39 months. Both cohorts continue to accrue. For all 18 patients the median age at study entry was 58 years; 44% were male. The median duration of imatinib treatment was 60 months (R40-89). The Kaplan-Meier estimate of the rate of sustained CMR after 12 months off treatment was 67% (95% confidence interval 40–85%, see Figure). Ten of 13 IFN-IM patients (77%) remain in CMR, and 7 of these have been in CMR for at least 12 months without treatment (maximum 23 months). The median follow-up in the IM only patients is currently only 7 months (R1-15), and 3/5 remain in CMR. All molecular relapses in both groups have occurred within 5 months of stopping imatinib. The median duration of prior imatinib treatment was not different in the 5 patients with loss of CMR (76 months) versus those in stable CMR (60 months; p=0.59). Among the 5 patients with loss of CMR the median time to molecular relapse was 3 months (range 2–5 months). Two relapsing patients lost MMR, and 3 had detectable BCR-ABL mRNA below this level. No patient has experienced haematological relapse or developed a kinase domain mutation. At last follow-up all 5 relapsing patients had regained CMR after a median of 5 months of re-treatment with imatinib. Patient-specific DNA Q-PCR assays were developed to test whether minimal residual disease (MRD) was detectable in genomic DNA in patients in CMR defined by RQ-PCR for BCR-ABL mRNA. Results are available for 6 patients, 3 of whom have relapsed. One relapsing patient had BCR-ABL DNA detected prior to imatinib withdrawal. In the remaining 2 relapsing patients BCR-ABL DNA was detected after imatinib withdrawal, but 2–3 months prior to the detection of BCR-ABL mRNA by RQ-PCR. BCR-ABL DNA increased by at least 1-log between the time of the first positive result and the detection of molecular relapse by RQ-PCR. The 3 patients in stable CMR had no detectable BCR-ABL DNA. In conclusion, with close molecular monitoring imatinib withdrawal in stable CMR appears to be safe: currently all patients are either in stable CMR off treatment or back in CMR after re-treatment. Withdrawal of effective treatment outside the setting of a clinical trial is not recommended. Monitoring of MRD by genomic DNA Q-PCR was able to detect molecular relapse prior to mRNA RQ-PCR, and shows promise for the prospective identification of patients at high risk of relapse. There is an apparent dichotomy of response between early molecular relapse and durable CMR, at least in patients treated with imatinib after IFN. It is too early to identify clinical or laboratory factors (such as prior IFN treatment) that may influence the probability of sustained CMR without treatment.
Disclosures: Ross:Novartis Pharmaceuticals: Honoraria, Research Funding. Grigg:Novartis Pharmaceuticals: Honoraria, Research Funding. Schwarer:Novartis Pharmaceuticals: Honoraria, Research Funding. Arthur:Novartis Pharmaceuticals: Honoraria, Research Funding. Loftus:Novartis Pharmaceuticals: Employment. Mills:Novartis Pharmaceuticals: Honoraria. Filshie:Novartis Pharmaceuticals: Honoraria, Research Funding. Seymour:Novartis Pharmaceuticals: Honoraria, Research Funding. Branford:Novartis Pharmaceuticals: Honoraria, Research Funding. Hughes:Novartis Pharmaceuticals: Honoraria, Research Funding, Speakers Bureau.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal