Abstract
In the red blood cell (RBC), adducin is present primarily as tetramers of α- and β-subunits at spectrin-actin junctions, or junctional complexes. Mouse RBCs also contain small amounts of γ-adducin. Platelets contain α- and γ-adducin only. Adducin functions as a barbed-end actin capping protein to regulate actin filament length and recruits spectrin to the ends of actin filaments. To further define adducin's role in vivo, we generated α-adducin knockout mice. α-Adducin is absent in all tissues examined in homozygous null mice. In RBCs, β- and γ-adducin are also absent, indicating that α-adducin is the limiting subunit in tetramer formation at the spectrin-actin junction. Similarly, γ-adducin is absent in α-null platelets. α-Adducin–null mice display compensated hemolytic anemia with features characteristic of RBCs in hereditary spherocytosis (HS), including spherocytes with significant loss of surface area, decreased mean corpuscular volume (MCV), cell dehydration, and increased osmotic fragility. Platelets maintain their normal discoid shape, and bleeding times are normal. α-Adducin–null mice show growth retardation at birth and throughout adulthood. Approximately 50% develop lethal communicating hydrocephalus with striking dilation of the lateral, third, and fourth ventricles. These data indicate that adducin plays a role in RBC membrane stability and in cerebrospinal fluid homeostasis.
Introduction
In mammals, 3 adducin proteins (α, β, γ) are encoded by distinct genes (Add1, Add2, and Add3, respectively).1 Adducins share extensive sequence and structural homology.2 α- and γ-adducin are ubiquitously expressed, whereas β-adducin expression is restricted to hematopoietic tissues and the brain.3 Adducins form heterodimers and heterotetramers via interactions at their N-terminal globular “head” domains and C-terminal MARCKS (myristoylated alanine rich C-kinase substrate) domains.4 In red blood cells (RBCs), α- and β-adducin interact in a 1:1 stoichiometry to form heterodimers and heterotetramers.2,5 In platelets and most other nonerythroid cells, α- and γ-subunits interact.6,7 Adducin promotes spectrin-actin assembly4,8 and caps actin filaments in a wide variety of cell types by phosphorylation- and calcium/calmodulin-dependent mechanisms. Adducin maintains the shape of resting platelets and participates in platelet activation as a substrate for protein kinase C and calpain signaling.7
Adducin localizes to the RBC junctional complex of the spectrin-based membrane skeleton, a 2-dimensional array located just beneath the lipid bilayer.2,9 The membrane skeleton confers strength and deformability to the RBC, allowing it to withstand high shear forces within the circulation and undergo extensive deformation to squeeze through the microvasculature intact. At the junctional complex, spectrin tetramers are cross-linked by short actin protofilaments.10 Actin protofilament length is strictly maintained in the RBC. Adducin caps the fast-growing barbed ends of the actin protofilaments,11 tropomodulin (Tmod) caps the slow-growing pointed ends,12 and tropomyosin (TM) stabilizes the protofilaments along their lengths.13 Both actin-capping and spectrin recruitment by adducin are inhibited by PKC phosphorylation of the MARCKS domain14 and by calmodulin binding.8 Rho-kinase phosphorylation, on the other hand, enhances adducin-actin interactions and spectrin recruitment.15,16 Recently, analysis of Rac1/Rac2 double-null mice revealed that Rac GTPases influence the phosphorylation status of RBC adducin.17 Together, these observations suggest a dynamic role for adducin in assembly of the junctional complex.
We and others demonstrated a role of adducin in RBC membrane stability using gene-targeted β-adducin (Add2)–null mice.3,18 β-Adducin–null mice display mild, compensated hemolytic anemia with characteristics of hereditary spherocytosis (HS), including reduced RBC deformability, increased osmotic fragility, sphero-elliptocytosis, dehydration, and increased mean cell hemoglobin content (MCHC). Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) revealed an intact membrane skeleton in β-null RBC ghost membranes with normal amounts of the major proteins spectrin, ankyrin, band 3, protein 4.1, and protein 4.2. α-Adducin, however, was decreased to approximately 30% of normal. Surprisingly, γ-adducin is present in wild-type mouse (but not human) RBC membranes, and is up-regulated approximately 5-fold in the β-null RBC ghosts, suggesting that αγ heterotetramers may compensate for the loss of αβ heterotetramers, accounting for the mild anemia observed in the absence of the β subunit.
To fully address the role of adducin in RBC stability in the mouse in vivo, all 3 adducins need to be deleted. To that end, we have generated α-adducin–null mice using a conventional gene knockout strategy. In this report, we focus on the overt phenotypic and hematopoietic consequences of α-adducin deficiency. α-Adducin–null mice display growth retardation throughout their life span with a compensated hemolytic anemia. RBCs are osmotically fragile and spherocytic with significant loss of membrane surface area. Surprisingly, β- and γ-adducin are absent in α-null RBC membranes despite normal mRNA expression. Hence, α-adducin is the limiting subunit in adducin heterodimer/tetramer assembly at the RBC junctional complex. A marked up-regulation of RBC membrane-bound CapZ (EcapZ) is seen and likely compensates for the lack of adducin in actin protofilament capping. Platelets maintain their normal discoid shape in the circulation, and bleeding times are normal. Finally, lethal communicating hydrocephalus occurs in approximately 50% of α-adducin–null mice, demonstrating for the first time a critical functional role in central nervous system cerebral spinal fluid (CSF) homeostasis.
Methods
Animals
All mice were maintained at The Jackson Laboratory in climate-controlled rooms (12-hour light cycle) and provided acidified water and chow (NIH 5K52; National Institutes of Health [NIH], Bethesda, MD) ad libitum. The Jackson Laboratory Animal Care and Use Committee approved all protocols.
Gene targeting
Mouse α-adducin genomic clones were isolated from a 129/Sv λFixII library (Stratagene, La Jolla, CA) using standard techniques.19 A 5.1-kilobase (kb) KpnI-XbaI fragment extending from intron 5 to intron 9 was subcloned into the KpnI/XbaI sites of the pPNTlox2 vector,20 and a 2.5-kb HpaI fragment within intron 12 was subcloned into the HpaI site (exon/intron organization according to Ensembl transcript ENSMUST0000011434021 ). Transfected E14 ES cells were cultured and selected in G418 and gancyclovir.20 ES cell genomic DNA was isolated, digested with EcoRI, and analyzed by Southern blotting using a flanking 1.7-kb fragment as hybridization probe to detect homologous recombination. Blastocyst injection and embryo transfer were performed using standard techniques.22 Male chimeras were mated to C57BL/6J females to generate heterozygotes. All mice for this study were maintained on a segregating C57BL/6J-129/Sv (B6;129) hybrid genetic background. Progeny were genotyped by the polymerase chain reaction (PCR) as described23 with forward primer (5′→3′) GCACTCCAGACACCAATGAAGTCTG and reverse primer (5′→3′) TCGACTTGGGACTGCTTCCGTTCTG yielding an 867–base pair (bp) wild-type fragment. Forward primer GTATCCACAGAGTCCACACGAGGAT and the neomycin-recognizing reverse primer GATAAATGCCTGCTCTTTACTGAAGGCTCT generated an 822-bp mutant fragment.
Northern blotting and RT-PCR analyses
Total RNA was isolated as previously described23 and transferred to Hybond-N+ membrane (Amersham Biosciences, Piscataway, NJ) for Northern blot analysis using standard protocols. Hybridization probes were generated by reverse-transcription PCR (RT-PCR) as described23 with wild-type spleen RNA as template. RT-PCR primer sequences used to generate all probes are provided in Table 1.
RT-PCR hybridization probes
| Gene . | Ensembl transcript ID . | Exon(s) . | Size, bp . | Forward primer . | Reverse primer . |
|---|---|---|---|---|---|
| α-adducin | ENSMUST00000114340 | 9 | 158 | CAAGTGCTGGAGGACCAGACAACTT | CTTATGCGGATGCTCGATAATCTGG |
| α-adducin | ENSMUST00000114340 | 10-12 | 444 | TACCCTTATCGATACCCTGCTCTGA | ATGATGGACAGAAGCCTTGTTCAGG |
| α-adducin | ENSMUST00000114340 | 15 | 498 | TGACGGCCTCCAAAGCCATCATTGA | AGGTCCTGTGTGTCCTCTCCTCATT |
| β-adducin | ENSMUST00000032069 | 12-14 | 360 | GAAGGCTGATGAAGTGGAAAAGTCC | AGAAGCTAGAACAGGAGCAGGAAGG |
| γ-adducin | ENSMUST00000050096 | 15 (3′UTR) | 704 | TAGCCAATCAGTCAGGGGTA | CTCAAAGTTTCCACTACGAG |
| actin | ENSMUST00000031564 | 3-6 | 929 | GAGGCCCAGAGCAAGAG | CCGGACTCATCGTACTC |
| neomycin | 278 | TGGAGAGGCTATTCGGCTATGACT | AGCAAGGTGAGATGACAGGAGATC |
| Gene . | Ensembl transcript ID . | Exon(s) . | Size, bp . | Forward primer . | Reverse primer . |
|---|---|---|---|---|---|
| α-adducin | ENSMUST00000114340 | 9 | 158 | CAAGTGCTGGAGGACCAGACAACTT | CTTATGCGGATGCTCGATAATCTGG |
| α-adducin | ENSMUST00000114340 | 10-12 | 444 | TACCCTTATCGATACCCTGCTCTGA | ATGATGGACAGAAGCCTTGTTCAGG |
| α-adducin | ENSMUST00000114340 | 15 | 498 | TGACGGCCTCCAAAGCCATCATTGA | AGGTCCTGTGTGTCCTCTCCTCATT |
| β-adducin | ENSMUST00000032069 | 12-14 | 360 | GAAGGCTGATGAAGTGGAAAAGTCC | AGAAGCTAGAACAGGAGCAGGAAGG |
| γ-adducin | ENSMUST00000050096 | 15 (3′UTR) | 704 | TAGCCAATCAGTCAGGGGTA | CTCAAAGTTTCCACTACGAG |
| actin | ENSMUST00000031564 | 3-6 | 929 | GAGGCCCAGAGCAAGAG | CCGGACTCATCGTACTC |
| neomycin | 278 | TGGAGAGGCTATTCGGCTATGACT | AGCAAGGTGAGATGACAGGAGATC |
SDS-PAGE and Western blotting
Tissues (whole brain, spleen, heart, kidney, lung) were homogenized in T-PER total protein extraction reagent (Pierce Biotechnology, Rockford, IL). Hemoglobin-depleted RBC ghosts were prepared by repeated lysis in 5 mM sodium phosphate (pH 7.6) containing 1 mM Na2 EDTA, 1 mM phenylmethyl sulfonyl fluoride (PMSF), 1 μM pepstatin A, 100 μM leupeptin, 10 μM E64, 1 mM Pefabloc, and 1 μM MDL-28170. All inhibitors were obtained from Sigma-Aldrich (St Louis, MO) except PMSF (Roche Applied Science, Indianapolis, IN). Because TM is selectively depleted if magnesium is not included in ghost preparations,24 2 mM MgCl2 was included in the lysis buffer for blotting with TM antibody. For platelet lysates, packed cells from whole blood were resuspended in mouse PBS (10 mM NaCl, 155 mM KCl, 10 mM glucose, 1 mM MgCl2, 2.5 mM KHPO4, pH 7.4) containing 1 mg/mL leupeptin, 1 mg/mL aprotinin, 1 mg/mL pepstatin A, 500 mM EGTA, 250 mM PMSF, and 100 mM Pefabloc. Platelet-rich plasma was obtained by low-speed centrifugation at 240g for 20 minutes followed by centrifugation at 260g 2 to 3 times or until all visible RBCs were removed. Following a final centrifugation at 500g, the platelet pellet was washed in mouse PBS and solubilized in equal volumes of 20% SDS and loading buffer.
SDS-PAGE was performed using 4% Steck25 gels or 10% Laemmli26 gels for RBC ghosts and 6.5% Laemmli gels for tissue proteins. For RBC ghosts, gels were loaded semiquantitatively (equal volume of packed ghosts/lane), as described.27 Ghost preparations from multiple animals of each genotype and multiple blots per antibody were performed to minimize loading variation. For platelet lysates and tissues, protein concentrations were determined by optical density (OD280nm) in 1% SDS. Gels were stained with Coomassie blue or transferred to Hybond–electrochemiluminescence (ECL) nitrocellulose membranes (Amersham Biosciences) for Western blotting as described.28 Primary polyclonal antibodies to spectrin, ankyrin, protein 4.1, protein 4.2, band 3, and β- and γ-adducin have been previously described.3,28,29 β-actin antibody was obtained from Abcam (Cambridge, MA); α-adducin, from Santa Cruz Biotechnology (Santa Cruz, CA); TM, from the Developmental Studies Hybridoma Bank (Iowa City, IA); and CapZα, CapZβ, and Tmod from Lifespan Biosciences (Seattle, WA). p55, GPC, and protein 4.9 antibodies were generated in our laboratories at New York Blood Center. Bound antibody was detected with horseradish peroxidase–conjugated IgG (Bio-Rad Laboratories, Hercules, CA) and visualized using ECL reagents from PerkinElmer (Waltham, MA). SDS-PAGE gels and Western blots were scanned and densitometry was performed using NIH ImageJ software (http://rsb.info.nih.gov/ij).
Blood analysis
Whole blood (250 μL) was collected via submandibular cheek puncture30 into Eppendorf tubes (Hamburg, Germany) containing 4 μL 3.5% EDTA (complete blood counts) or 7.5 U heparin (plasma chemistries). Complete blood counts were determined using an automated hematology analyzer (Advia 120 Multispecies Hematology Analyzer; Bayer Diagnostics, Tarrytown, NY). Peripheral blood smears were stained with Wright-Giemsa (Sigma-Aldrich). Blood urea nitrogen (BUN) and plasma electrolyte concentrations were obtained using a CX5 Delta (Beckman Coulter, Fullerton, CA) automated chemistry analyzer. Osmotic fragility curves were obtained as described previously.31 Ektacytometry was performed as described.32 Bleeding times were determined by the tail-tip method.33
Histology and electron microscopy
Liver, testis, and spleen were fixed in Bouin fixative. Whole brains and kidney were fixed in Bouin or Tellyesniczky fixative.34 Tissues were paraffin embedded, sectioned at 3 to 5 μM, and stained with hematoxylin and eosin for routine pathological analysis or with Prussian blue for detection of iron using standard techniques.35
For scanning electron microscopy (SEM), whole blood was collected from the retro-orbital sinus using heparinized microhematocrit tubes (Becton Dickinson, Franklin Lakes, NJ). Packed RBCs or platelet pellets (obtained by centrifugation) were washed in mouse PBS, fixed in 2% glutaraldehyde in cacodylate buffer (pH 7.2, 4°C), and processed for examination using a Hitachi 3000N VP (Hitachi High Technologies America, Pleasanton, CA) scanning electron microscope as previously described.35 For examination of platelet structure by transmission electron microscopy (TEM), blood was obtained by directly bleeding into 1.0 mL freshly prepared 1.5% glutaraldehyde in 0.01 M cacodylate buffer (pH 7.2) via submandibular cheek puncture and fixed overnight at 4°C. Platelet pellets were obtained by centrifugation, washed 3 times in freshly prepared cacodylate buffer, and resuspended in 1.0 mL buffer. Samples were postfixed in osmium and routinely processed for examination using a JEOL 1230 (JEOL, Tokyo, Japan) transmission electron microscope.
Image acquisition and assembly
SDS-PAGE gels and Western blot autoradiograms were scanned using a HP Scanjet 4070 Photosmart Scanner and HP Precisionscan Pro software (Hewlett-Packard, Palo Alto, CA) at 600 dpi. Images of peripheral blood and tissue sections were acquired using a Leica DMRXE microscope or Leica WILD M10 stereomicroscope equipped with a Leica DFC 300 FX cooled-CCD color camera and LeicaCam software (Leica Microsystems, Bannockburn, IL). Smears were examined with a 100×/1.4 oil-immersion objective; tissue sections, with a 20×/0.60 objective; and brain sections, with a 1.0× objective. SEM photomicrographs were acquired using PCI Quartz Imaging software built into the Hitachi S3000N VP scanning electron microscope. TEM images were captured on a Hamamatsu ORCA high-resolution camera (Hamamatsu, Okayama City, Japan) in the 2400 × 2400 pixel format using AMT software version 600.135 (Danvers, MA). Final images were assembled using Adobe Photoshop 7.0 (Adobe, San Jose, CA) and Macromedia Freehand MX (San Francisco, CA) software packages.
Statistical analysis
Differences between group data were tested for significance by one-way ANOVA. Tukey honestly significant differences (HSD) or the 2-tailed Student t test was used to identify significant differences between mean values using JMP v 7.0 software (SAS Institute, Cary, NC).
Results
Targeting of the α-adducin gene (Add1)
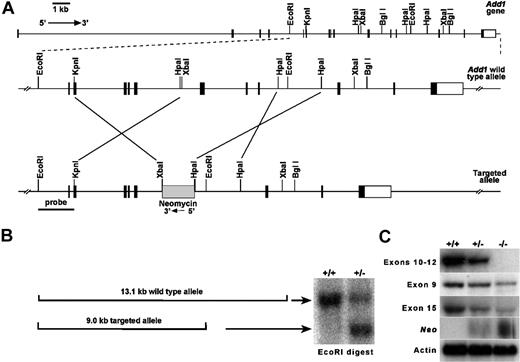
We used a conventional targeting strategy to delete exons 10 to 12 of the Add1 gene (Figure 1A). Correctly targeted clones were obtained (Figure 1B), injected into blastocysts, and transferred to pseudopregnant hosts using conventional methods. At birth, all expected genotypes were obtained in offspring of B6;129 heterozygous mating pairs with 36% wild-type (+/+), 48% heterozygotes (+/−), and 16% homozygous null (−/−). The deficit in homozygotes from expected (25%) suggests in utero loss. Northern blot analysis using a probe within the deleted region confirmed deletion of exons 10 to 12 (Figure 1C). However, probes upstream and downstream revealed diminished amounts of apparently normal-sized mRNA, and positive signal was obtained using a probe specific for the neomycin-resistance cassette (Figure 1C). These data raised the specter that we had created a hypomorphic allele rather than a null. However, Western blot analyses reveal a complete loss of α-adducin at the protein level in all tissues examined.
Targeting of the α-adducin gene, Add1. (A) Structure of the α-adducin locus according to Ensembl transcript identification ENSMUST00000114340 with the targeted allele shown below. Numbered boxes represent exons. Relevant restriction sites are indicated. (B) Homologous recombination generates a 13.1-kb wild-type and a 9.0-kb mutant allele upon digestion of ES cell DNA with EcoRI. (C) Northern blot analysis of E14.5 fetal liver with hybridization probes indicated. RT-PCR primers used to generate probes are given in Table 1. +/+ indicates wild- type; +/−, heterozygotes; and −/−, homozygous null.
Targeting of the α-adducin gene, Add1. (A) Structure of the α-adducin locus according to Ensembl transcript identification ENSMUST00000114340 with the targeted allele shown below. Numbered boxes represent exons. Relevant restriction sites are indicated. (B) Homologous recombination generates a 13.1-kb wild-type and a 9.0-kb mutant allele upon digestion of ES cell DNA with EcoRI. (C) Northern blot analysis of E14.5 fetal liver with hybridization probes indicated. RT-PCR primers used to generate probes are given in Table 1. +/+ indicates wild- type; +/−, heterozygotes; and −/−, homozygous null.
Loss of β- and γ-adducin in the absence of α-adducin
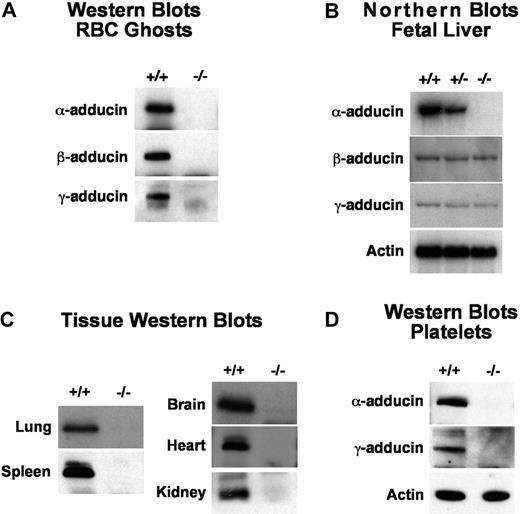
α-Adducin was not detectable by Western blotting of RBC ghost proteins in homozygous null mice (Figure 2A). Surprisingly, β- and γ-adducin were also absent despite normal or near-normal mRNA expression levels (Figure 2A,B). This is in contrast to previous observations in β-adducin–null RBCs where approximately 30% of α-adducin remains and γ-adducin is up-regulated, presumably forming αγ heterodimers and heterotetramers.3,18 Clearly, in the absence of the α-subunit, β- and γ-adducin stability is severely compromised in RBCs and no adducin complexes form at the spectrin-actin junctions. As α-adducin is ubiquitously expressed, we examined multiple additional tissues and confirmed its absence in all tissues and cells examined including platelets, lung, spleen, brain, heart, and kidney (Figure 2C,D). Notably, in platelets, where αγ heterodimers are normally present, γ-adducin is also completely lacking (Figure 2D). Thus, as in RBCs, the platelet heterologous binding partner is unstable in the absence of α-adducin. With the exception of the brain, there are no obvious abnormalities in any of these tissues; however, detailed functional analyses have yet to be completed.
Detection of gene products. (A) Western blots of RBC ghost proteins demonstrating loss of both β- and γ-adducin in α-adducin knockout mice. (B) Northern blots of E14.5 fetal livers showing loss of α-adducin mRNA but normal levels of β- and γ-adducin mRNA. The exon 10 to 12 α-adducin probe was used (Table 1). (C) Western blots demonstrating loss of α-adducin in all tissues examined. (D) Western blots demonstrating loss of platelet γ-adducin in α-adducin–null mice. +/+ indicates wild-type; +/−, heterozygotes; and −/−, homozygous null.
Detection of gene products. (A) Western blots of RBC ghost proteins demonstrating loss of both β- and γ-adducin in α-adducin knockout mice. (B) Northern blots of E14.5 fetal livers showing loss of α-adducin mRNA but normal levels of β- and γ-adducin mRNA. The exon 10 to 12 α-adducin probe was used (Table 1). (C) Western blots demonstrating loss of α-adducin in all tissues examined. (D) Western blots demonstrating loss of platelet γ-adducin in α-adducin–null mice. +/+ indicates wild-type; +/−, heterozygotes; and −/−, homozygous null.
Effect of adducin loss on other membrane skeleton and actin-binding proteins
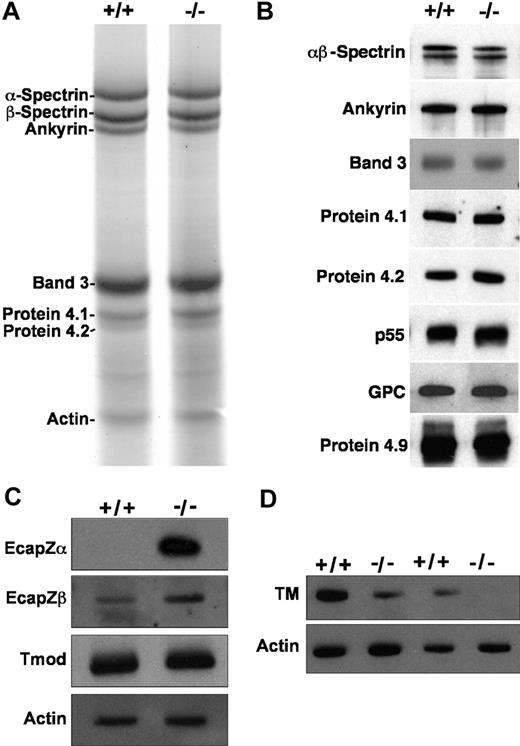
SDS-PAGE and/or Western blotting of RBC ghosts reveals an intact membrane skeleton with normal amounts of the major proteins α- and β-spectrin, ankyrin, band 3, protein 4.1, protein 4.2, protein 4.9, p55, and glycophorin C (Figure 3A,B). Actin appears normal on SDS-PAGE gels and Western blots of RBC ghost proteins; however, the spectrin-actin ratio is higher in homozygous mutants versus wild-type by scanning densitometry, although not significantly (4.7 ± 2.0 [X ± SD, n = 12] in homozygous null vs 3.3 ± 1.3 in wild-type [n = 7]; P = .13). A marked increase in membrane-associated EcapZ is seen in adducin-null RBC ghosts. EcapZα is undetectable in wild-type ghosts unless autoradiograms are dramatically overexposed, but present at very high levels in adducin-null ghosts, while EcapZβ is up-regulated approximately 3.5-fold by scanning densitometry of Western blot autoradiograms (Figure 3C). Thus, as has been described in β-adducin–null RBCs,36 EcapZ shifts from its normal, cytoplasmic location in the RBC to the membrane in the absence of adducin. Tmod appears unchanged in the absence of adducin (Figure 3C). TM is decreased in adducin-null RBCs to approximately 15% to 20% of normal (Figure 3D). The presence of normal Tmod levels is in contrast to previous observations in β-adducin–null RBCs, and the decrement in the major isoform of TM is more severe than in β-adducin–null RBCs.36
Membrane skeleton in α-adducin–null RBCs. The major membrane skeleton components α- and β-spectrin, ankyrin, band 3, proteins 4.1 and 4.2, and actin appear normal in α-adducin–null RBC ghost membranes by SDS-PAGE (A) and Western blotting (B). Other components of the junctional complex—p55, GPC, and protein 4.9 (dematin)—also appear normal. (C) The actin capping protein EcapZ is strikingly up-regulated in adducin-null RBC membranes. Tropomodulin (Tmod) appears normal. (D) TM is decreased to approximately 20% of normal in the absence of adducin. +/+ indicates wild-type; −/−, homozygous null.
Membrane skeleton in α-adducin–null RBCs. The major membrane skeleton components α- and β-spectrin, ankyrin, band 3, proteins 4.1 and 4.2, and actin appear normal in α-adducin–null RBC ghost membranes by SDS-PAGE (A) and Western blotting (B). Other components of the junctional complex—p55, GPC, and protein 4.9 (dematin)—also appear normal. (C) The actin capping protein EcapZ is strikingly up-regulated in adducin-null RBC membranes. Tropomodulin (Tmod) appears normal. (D) TM is decreased to approximately 20% of normal in the absence of adducin. +/+ indicates wild-type; −/−, homozygous null.
Hematologic studies in α-adducin–null mice
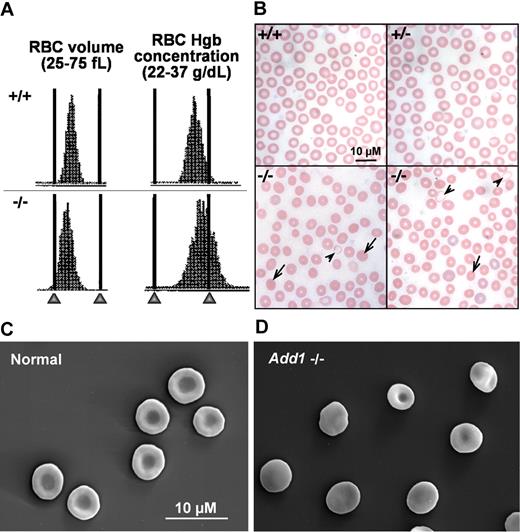
Peripheral blood analysis reveals mild compensated anemia in α-adducin–null mice (Table 2). In males, the hematocrit (Hct), mean corpuscular hemoglobin (MCH), and mean corpuscular volume (MCV) are significantly decreased versus wild-type, indicating a population of hypochromic, microcytic cells. Both the mean corpuscular hemoglobin concentration (MCHC) and hemoglobin distribution width (HDW) are significantly increased, indicating the presence of cells with increased hemoglobin concentration, or spherocytes. Histograms of RBC volume and hemoglobin concentration confirm populations of microcytic and dehydrated cells (Figure 4A), and Wright-Giemsa–stained peripheral blood smears and scanning electron microscopy are consistent with populations of hypochromic, microcytic cells and spherocytes (Figure 4B-D). The percentage of circulating reticulocytes is significantly elevated, indicating a compensatory increase in the rate of erythropoiesis. Spleens, however, did not significantly differ in weight (Table 2) or in iron content (data not shown).37 The red cell distribution width (RDW) is slightly, but significantly, increased. Similar counts were obtained in female α-adducin–null mice where the MCV and MCH are significantly decreased and the RDW, HDW, and reticulocytes are significantly increased (Table 2). However, the Hct and MCHC in females did not significantly differ from wild-type, and the RBC count was increased significantly (Table 2). Whether these data reflect true sex differences or simply the hybrid genetic background (B6;129) cannot be ascertained until backcrossing to generate a congenic strain on a pure inbred background is completed.
Hematologic values by genotype in adult mice
| Genotype . | RBC, × 1012/L . | Hgb, g/L . | Hct, % . | MCV, fL . | MCH, pg . | MCHC, g/L . | RDW, % . | HDW, g/L . | Retic, % . | Spleen weight, % BW . |
|---|---|---|---|---|---|---|---|---|---|---|
| Male | ||||||||||
| Add1+/+ (18) | 10.1 ± 0.5 | 161.9 ± 10.2 | 47.2 ± 2.4 | 47.0 ± 2.7 | 16.1 ± 0.8 | 343.5 ± 21.5 | 13.0 ± 0.7 | 19.2 ± 1.4 | 2.8 ± 0.6 | 0.32 ± 0.05 |
| Add1−1 (7) | 10.1 ± 0.7 | 160.6 ± 6.1 | 46.5 ± 2.3 | 46.3 ± 1.8 | 15.9 ± 0.8 | 344.8 ± 9.4 | 12.3 ± 0.4 | 17.8 ± 1.2 | 3.2 ± 0.9 | 0.36 ± 0.08 |
| Add1−1 (15) | 10.3 ± 1.0 | 155.7 ± 8.7 | 42.6 ± 2.7‡ | 41.7 ± 3.2‡ | 15.2 ± 1.0* | 365.5 ± 19.0† | 16.0 ± 1.6‡ | 27.6 ± 4.3‡ | 5.5 ± 2.3‡ | 0.36 ± 0.11 |
| Female | ||||||||||
| Add1+/+ (23) | 9.5 ± 0.9 | 156.3 ± 16.4 | 44.3 ± 4.0 | 45.7 ± 3.0 | 16.4 ± 0.5 | 360.3 ± 19.0 | 12.7 ± 0.9 | 19.9 ± 1.4 | 2.6 ± 0.9 | 0.43 ± 0.09 |
| Add1−1 (17) | 9.9 ± 0.0.7 | 160.1 ± 12.2 | 45.9 ± 3.2 | 46.2 ± 1.6 | 16.1 ± 0.8 | 348.9 ± 18.0 | 12.6 ± 0.4 | 18.7 ± 0.9 | 3.0 ± 1.0 | 0.44 ± 0.07 |
| Add1−1 (10) | 10.7 ± 0.9† | 161.5 ± 10.3 | 45.0 ± 2.6 | 42.2 ± 3.3† | 15.1 ± 1.4‡ | 365.7 ± 21.7 | 14.7 ± 1.9‡ | 26.8 ± 5.3‡ | 4.0 ± 1.2† | 0.46 ± 0.16 |
| Genotype . | RBC, × 1012/L . | Hgb, g/L . | Hct, % . | MCV, fL . | MCH, pg . | MCHC, g/L . | RDW, % . | HDW, g/L . | Retic, % . | Spleen weight, % BW . |
|---|---|---|---|---|---|---|---|---|---|---|
| Male | ||||||||||
| Add1+/+ (18) | 10.1 ± 0.5 | 161.9 ± 10.2 | 47.2 ± 2.4 | 47.0 ± 2.7 | 16.1 ± 0.8 | 343.5 ± 21.5 | 13.0 ± 0.7 | 19.2 ± 1.4 | 2.8 ± 0.6 | 0.32 ± 0.05 |
| Add1−1 (7) | 10.1 ± 0.7 | 160.6 ± 6.1 | 46.5 ± 2.3 | 46.3 ± 1.8 | 15.9 ± 0.8 | 344.8 ± 9.4 | 12.3 ± 0.4 | 17.8 ± 1.2 | 3.2 ± 0.9 | 0.36 ± 0.08 |
| Add1−1 (15) | 10.3 ± 1.0 | 155.7 ± 8.7 | 42.6 ± 2.7‡ | 41.7 ± 3.2‡ | 15.2 ± 1.0* | 365.5 ± 19.0† | 16.0 ± 1.6‡ | 27.6 ± 4.3‡ | 5.5 ± 2.3‡ | 0.36 ± 0.11 |
| Female | ||||||||||
| Add1+/+ (23) | 9.5 ± 0.9 | 156.3 ± 16.4 | 44.3 ± 4.0 | 45.7 ± 3.0 | 16.4 ± 0.5 | 360.3 ± 19.0 | 12.7 ± 0.9 | 19.9 ± 1.4 | 2.6 ± 0.9 | 0.43 ± 0.09 |
| Add1−1 (17) | 9.9 ± 0.0.7 | 160.1 ± 12.2 | 45.9 ± 3.2 | 46.2 ± 1.6 | 16.1 ± 0.8 | 348.9 ± 18.0 | 12.6 ± 0.4 | 18.7 ± 0.9 | 3.0 ± 1.0 | 0.44 ± 0.07 |
| Add1−1 (10) | 10.7 ± 0.9† | 161.5 ± 10.3 | 45.0 ± 2.6 | 42.2 ± 3.3† | 15.1 ± 1.4‡ | 365.7 ± 21.7 | 14.7 ± 1.9‡ | 26.8 ± 5.3‡ | 4.0 ± 1.2† | 0.46 ± 0.16 |
All mice were 4 or more weeks of age. All mice with overt signs of hydrocephalus or that developed hydrocephalus subsequent to obtaining complete blood counts were excluded. Number in parentheses is sample n. All values X plus or minus SD. Data shown exclude all mice showing any signs of hydrocephalus.
RBC indicates red blood cell count; Hgb, hemoglobin; Hct, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin content; RDW, red cell distribution width; HDW, hemoglobin distribution width; Retic, reticulocytes; and BW, body weight.
P < .05,
P < .01,
P < .001 versus sex-matched control (+/+) mice.
Adducin-null RBCs show features of HS. (A) RBC volume and hemoglobin concentration histograms indicate a population of small (left-shifted volume histogram) and dehydrated (right-shifted hemoglobin concentration histogram) in α-adducin–null (−/−, bottom) versus wild-type (+/+, top) RBCs. (B) Peripheral blood smears reveal a significant population of spherocytes ( ) with a small population of severely hypochromic cells (
) with a small population of severely hypochromic cells ( ). +/+ indicates wild-type; +/−, heterozygotes; and −/−, homozygous null. Bar represents 10 μM. (C) Biconcave RBCs from a normal littermate and (D) an α-adducin–null mouse showing significant spherocytosis. Bar represents 10 μM.
). +/+ indicates wild-type; +/−, heterozygotes; and −/−, homozygous null. Bar represents 10 μM. (C) Biconcave RBCs from a normal littermate and (D) an α-adducin–null mouse showing significant spherocytosis. Bar represents 10 μM.
Adducin-null RBCs show features of HS. (A) RBC volume and hemoglobin concentration histograms indicate a population of small (left-shifted volume histogram) and dehydrated (right-shifted hemoglobin concentration histogram) in α-adducin–null (−/−, bottom) versus wild-type (+/+, top) RBCs. (B) Peripheral blood smears reveal a significant population of spherocytes ( ) with a small population of severely hypochromic cells (
) with a small population of severely hypochromic cells ( ). +/+ indicates wild-type; +/−, heterozygotes; and −/−, homozygous null. Bar represents 10 μM. (C) Biconcave RBCs from a normal littermate and (D) an α-adducin–null mouse showing significant spherocytosis. Bar represents 10 μM.
). +/+ indicates wild-type; +/−, heterozygotes; and −/−, homozygous null. Bar represents 10 μM. (C) Biconcave RBCs from a normal littermate and (D) an α-adducin–null mouse showing significant spherocytosis. Bar represents 10 μM.
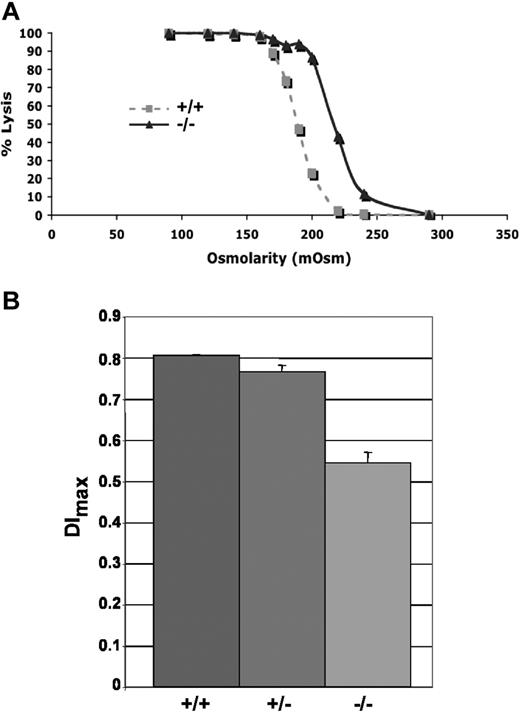
Adducin-null RBCs show increased osmotic fragility compared with wild-type RBCs (Figure 5A). Loss of membrane surface area in adducin-null RBCs was confirmed by ektacytometry. The maximum deformability index (DImax) measured at 250 dynes/cm2, a direct measure of membrane surface area, is significantly decreased in adducin-null RBCs (Figure 5B), confirming membrane loss. Together, the hematologic data, RBC morphology, volume and hemoglobin distribution histograms, right-shifted osmotic fragility profile, and decreased DImax indicate compensated hemolysis with dehydration and loss of membrane surface area, features characteristic of hereditary spherocytosis (HS).9
Increased osmotic fragility and ektacytometry. (A) Osmotic fragility curves indicate increased osmotic fragility in α-adducin–null RBCs. (B) The maximum value of the deformability index, DImax, at 250 dynes/cm2 is a direct measure of the mean RBC membrane surface area and is significantly reduced (P < .001) in α-adducin–null RBCs, confirming loss of membrane surface area (X ± SE). +/+ indicates wild-type; +/−, heterozygotes; and −/−, homozygous null.
Increased osmotic fragility and ektacytometry. (A) Osmotic fragility curves indicate increased osmotic fragility in α-adducin–null RBCs. (B) The maximum value of the deformability index, DImax, at 250 dynes/cm2 is a direct measure of the mean RBC membrane surface area and is significantly reduced (P < .001) in α-adducin–null RBCs, confirming loss of membrane surface area (X ± SE). +/+ indicates wild-type; +/−, heterozygotes; and −/−, homozygous null.
No overall differences in white blood cell counts were detected between adducin-null mice of either sex (Table 3). Platelet counts, however, were significantly elevated in adducin-null males compared with wild-type (Table 3). Platelet counts were also elevated in adducin-null females, although not significantly (P = .22). The mean platelet volume (MPV) was significantly lower in female adducin-null versus wild-type mice (Table 3). A decreased MPV was also seen in male adducin-null mice but it did not reach statistical significance (P = .07). In the circulation, α-adducin–null platelets maintain their discoid shape (Figure 6A,B). Moreover, adducin-null platelets appear to aggregate adequately: bleeding times do not significantly differ in α-adducin–null versus wild-type mice (Figure 6C). These data indicate that loss of adducin does not affect the shape of platelets in the circulation or their function in terms of the bleeding time assay.
White blood cell and platelet values by genotype in adult mice
| Genotype . | WBC, ×109/L . | PLT, ×1012/L . | MPV, fL . |
|---|---|---|---|
| Male | |||
| Add1+/+ | 6.6 ± 2.7 (18) | 0.98 ± 0.25 (14) | 5.8 ± 0.7 (18) |
| Add1−1 | 7.8 ± 2.4 (15) | 1.19 ± 0.18 (12)* | 5.4 ± 0.7 (15) |
| Female | |||
| Add1+/+ | 7.0 ± 2.6 (23) | 0.96 ± 0.17 (12) | 6.0 ± 0.6 (21) |
| Add1−1 | 7.8 ± 4.7 (10) | 1.05 ± 0.16 (10) | 5.3 ± 0.5 (10)† |
| Genotype . | WBC, ×109/L . | PLT, ×1012/L . | MPV, fL . |
|---|---|---|---|
| Male | |||
| Add1+/+ | 6.6 ± 2.7 (18) | 0.98 ± 0.25 (14) | 5.8 ± 0.7 (18) |
| Add1−1 | 7.8 ± 2.4 (15) | 1.19 ± 0.18 (12)* | 5.4 ± 0.7 (15) |
| Female | |||
| Add1+/+ | 7.0 ± 2.6 (23) | 0.96 ± 0.17 (12) | 6.0 ± 0.6 (21) |
| Add1−1 | 7.8 ± 4.7 (10) | 1.05 ± 0.16 (10) | 5.3 ± 0.5 (10)† |
All mice were 4 or more weeks of age. Number in parentheses is sample n. All values X plus or minus SD. Data shown exclude all mice showing any signs of hydrocephalus.
WBC indicates white blood cell count; PLT, platelet count; and MPV, mean platelet volume.
P < .05.
P < .01 verusus sex-matched control (+/+) mice.
Platelet structure and function. TEM (A) and SEM (B) reveal adducin-null platelets maintain their normal discoid state in the circulation. (C) Bleeding times. X plus or minus SE.
Platelet structure and function. TEM (A) and SEM (B) reveal adducin-null platelets maintain their normal discoid state in the circulation. (C) Bleeding times. X plus or minus SE.
Measurements of plasma BUN and electrolyte concentrations reveal no significant differences in adducin-null versus wild-type mice (Table 4). As BUN has been validated as a sensitive marker of kidney damage in mice,38 these data indicate that kidney function is normal in the age group tested (4-16 weeks).
Plasma blood urea nitrogen and electrolyte concentrations
| Genotype . | BUN, mg/dL . | Na, mM . | K, mM . | Cl, mM . | CO2, mM . |
|---|---|---|---|---|---|
| Add1+/+ | 24.9 ± 5.7 (21) | 150.7 ± 10.3 (36) | 8.6 ± 2.2 (25) | 123.8 ± 10.8 (36) | 14.9 ± 2.1 (36) |
| Add1−/− | 26.3 ± 8.9 (11) | 145.8 ± 9.1 (30) | 8.4 ± 2.5 (22) | 122.0 ± 9.7 (30) | 13.0 ± 2.4 (30) |
| Genotype . | BUN, mg/dL . | Na, mM . | K, mM . | Cl, mM . | CO2, mM . |
|---|---|---|---|---|---|
| Add1+/+ | 24.9 ± 5.7 (21) | 150.7 ± 10.3 (36) | 8.6 ± 2.2 (25) | 123.8 ± 10.8 (36) | 14.9 ± 2.1 (36) |
| Add1−/− | 26.3 ± 8.9 (11) | 145.8 ± 9.1 (30) | 8.4 ± 2.5 (22) | 122.0 ± 9.7 (30) | 13.0 ± 2.4 (30) |
All mice were 4 weeks of age or more. Number in parentheses is sample n. All values X plus or minus SD. Data shown exclude all mice showing any signs of hydrocephalus.
BUN indicates blood urea nitrogen; Na, plasma sodium; K, plasma potassium; Cl, plasma chloride; and CO2, plasma carbon dioxide.
Decreased growth and survival, and high incidence of lethal hydrocephaly in adducin-null mice
Homozygous α-adducin–null mice are smaller as neonates and throughout adulthood than their normal littermates (Figure 7A-C). Notably, 50 (51%) of 98 adducin-null mice developed hydrocephaly requiring euthanasia. Of these, 22 (44%) were female and 28 (56%), male. Hydrocephaly was never observed in heterozygous or wild-type littermates. On average, hydrocephaly became overtly visible (“domed” head) at 8 weeks of age. There is striking, progressive dilation of the lateral and third ventricles (Figure 7D). The cerebral aqueduct (aqueduct of Sylvius) is open, resulting in the accumulation of fluid within the fourth ventricle as well (not shown). Thus, α-adducin–null mice have a communicating hydrocephalus. As the condition becomes increasingly severe, there is a striking thinning of the cerebral cortex (Figure 7D), but no brain abnormalities that are not a direct consequence of hydrocephalus are seen, even when hydrocephaly is relatively mild. Recent studies have revealed that impaired motile cilia disrupting cerebrospinal fluid (CSF) flow is a major cause of hydrocephaly.39-41 In α-adducin–null mice, no histopathological alterations of the ependymal cells lining the ventricles are apparent by light microscopy, and they appear normally ciliated (not shown).
Growth retardation and lethal hydrocephalus in α-adducin–null mice. (A-C) α-Adducin–null mice are smaller at birth and throughout life compared with their normal littermates. Blue in panel C indicates X plus or minus SE. (D) Approximately 50% of α-adducin–null mice develop hydrocephalus with extreme dilation of the lateral and third ventricles. The fourth ventricle is also enlarged and the cerebral aqueduct is open (not shown), indicating communicating hydrocephalus. Significant thinning of the cortex is apparent as hydrocephalus worsens. Hydrocephalus was not observed in wild-type or heterozygous mice. C indicates cerebral cortex; CP, choroid plexus; HC, hippocampus; TA, thalamus; HTA, hypothalamus; LV, lateral ventricle; V3, third ventricle; +/+, wild-type; and −/−, homozygous null.
Growth retardation and lethal hydrocephalus in α-adducin–null mice. (A-C) α-Adducin–null mice are smaller at birth and throughout life compared with their normal littermates. Blue in panel C indicates X plus or minus SE. (D) Approximately 50% of α-adducin–null mice develop hydrocephalus with extreme dilation of the lateral and third ventricles. The fourth ventricle is also enlarged and the cerebral aqueduct is open (not shown), indicating communicating hydrocephalus. Significant thinning of the cortex is apparent as hydrocephalus worsens. Hydrocephalus was not observed in wild-type or heterozygous mice. C indicates cerebral cortex; CP, choroid plexus; HC, hippocampus; TA, thalamus; HTA, hypothalamus; LV, lateral ventricle; V3, third ventricle; +/+, wild-type; and −/−, homozygous null.
Discussion
Interactions at junctional complexes are critical in the maintenance of RBC membrane stability and mechanical properties. Here we focus on the actin-capping protein adducin, present at the RBC junctional complex where spectrin is cross-linked by actin into a 2-dimensional array. Adducin-null mice show features of compensated HS. RBCs are microcytic with a significantly decreased MCV. The MCHC is elevated, and numerous spherocytes are observed on peripheral blood smears and by SEM. RBC volume and hemoglobin concentration histograms indicate populations of small, dehydrated cells. RBCs are osmotically fragile, and ektacytometry confirms loss of membrane surface area.
Because α-adducin mRNA is expressed at much higher levels than β-adducin mRNA42 (Figure 2B), β-adducin was proposed to be the limiting subunit in heterotetramer formation at junctional complexes. Here, however, we show that α-adducin is limiting; in its absence, no stable adducin complexes form despite normal expression of β- and γ-adducin mRNA. Many examples exist in which the loss of one component of a complex destabilizes the remaining components.43 Loss of both β- and γ-adducin in the absence of the α-subunit in mice provides in vivo evidence that both αβ and αγ heterodimers and heterotetramers form in the mouse RBC membrane, but that stable βγ complexes do not. In addition, our observations suggest that neither β- nor γ-adducin by itself stably interacts with other RBC membrane proteins.
Adducin has been shown to regulate the length of actin filaments by capping the fast growing barbed ends and to recruit spectrin to the ends of actin filaments. Other components of the junctional complex include Tmod, TM, protein 4.1, protein 4.9, and p55. The levels of p55, protein 4.1, protein 4.9 (dematin), and Tmod appear normal in α-adducin–null mice by Western blot analyses and/or SDS-PAGE. However, as was previously reported for β-adducin–null RBCs,36 we find significantly up-regulated EcapZ, particularly EcapZα, which is normally confined to the RBC cytoplasm and is undetectable in wild-type RBC membranes.11 CapZ functions as a barbed-end capping protein in muscle.13,44 The advantage of adducin in normal RBCs, despite a lower affinity for F-actin compared with CapZ, is likely its ability to simultaneously bind spectrin and F-actin. Tmod was also reported to be increased by 65% in β-adducin–null RBCs.36 We see no evidence for this in α-adducin–null mice, suggesting that up-regulation and stable incorporation of Tmod at the junctional complex in β-adducin–null mice are dependent upon α- and/or γ-adducin, which are present in β-adducin–null RBCs but absent in α-adducin–null RBCs. We also demonstrate significantly diminished RBC membrane TM content in α-adducin–null mice. The decrement in TM is more severe in α-adducin–null RBCs, where it is present at approximately 15% to 20% normal levels, compared with β-adducin–null RBCs where it is present at 40% to 50% normal levels on Western blots.36 As was the case for Tmod, this observation suggests that α- and γ-adducin may ameliorate loss of TM in β-adducin–null RBCs.
Recently, it has been reported that selective depletion of TM weakens the spectrin–actin–protein 4.1 complex.24 Hence, one might speculate that the decreased level of TM in the absence of adducin is one basis for altered RBC stability and loss of membrane surface area. However, we see predominantly spherocytosis with no significant elliptocytosis in α-adducin–null RBCs, suggesting that “vertical” membrane linkages are disrupted. Classically, HS is associated with defects in the spectrin–ankyrin–band 3 vertical linkage within the RBC membrane,9 leading to lipid loss and decreased membrane surface area, compromising RBC deformability.45 Our demonstration of spherocytes in the absence of adducin suggests that adducin interacts vertically within the membrane. Recently, it has been shown that adducin and protein 4.9 independently bind GLUT1 in human RBCs.46 Our results are consistent with an independent membrane linkage for α-adducin, as we see no alteration in protein 4.9. As GLUT1 is not present in the membrane of mature mouse RBCs, another unknown protein must link adducin vertically. Further studies will be required to identify the physiologically relevant adducin ligand(s).
As in RBCs, loss of α-adducin in platelets leads to the absence of its normal binding partner, γ-adducin. However, despite the absence of adducin, platelets maintain their normal discoid shape in the circulation and are functionally normal at the level of the tail-tip bleeding time assay. That other proteins are compensating for the lack of adducin in platelets seems highly likely. Clearly, a more extensive and detailed analysis of platelets in adducin-null mice in the future will be required to clarify their dynamic structural characteristics and functional capacity, and to shed light on the mechanisms leading to the observed trend toward increased numbers of smaller platelets in the absence of adducin.
All 3 adducin genes are highly expressed in brain and are likely to perform several molecular roles in different cell types. Fifty percent of α-adducin knockout mice developed hydrocephalus. In contrast, β-adducin knockout mice had no morphologic or developmental abnormalities but were found to have impaired synaptic plasticity and learning.47 Studies of learning in α-adducin knockout mice will be addressed in future experiments. However, the observed differences in hydrocephalus suggest that, as in RBCs, α-adducin may be the limiting subunit in cells of the brain regulating CSF.
Recent studies in mouse models have indicated that absent or functionally defective ependymal cell motile cilia are a major cause of hydrocephalus.40,41,48-51 Cilia are not absent in α-adducin–null ependymal cells; however, more detailed analyses will be required to determine whether they are normal at the ultrastructural level and whether they are motile. Often hydrocephalus due to defects in cilia structure or function is accompanied by polycystic kidney disease (PCK) or features of primary ciliary dyskinesia (PCD). In addition to hydrocephaly, PCD is characterized by alterations in left-right symmetry (situs inversus), infertility, and chronic respiratory problems. We see no evidence of these sequelae in α-adducin–null mice; kidney histology appears normal, mice are fertile, organ symmetry is normal, and no respiratory distress has been noted. It should be noted that abnormalities in the subcommissural organ (SCO), specialized secretory ependymal cells located at the opening of the third ventricle into the cerebral aqueduct, are associated with hydrocephalus, although the mechanism is poorly understood.52,53 Detailed ultrastructural analysis of the SCO in α-adducin–null mice is ongoing.
Hydrocephalus is a significant problem in humans with an incidence of 1 to 3 per 1000 live births. The only causative human gene identified to date is the adhesion molecule L1-CAM, defects in which account for only approximately 6% of human cases.53 Defects in cell adhesion are also thought to underlie hydrocephalus in nonmuscle myosin II-B–null mice.54 It is noteworthy that a membrane skeleton has been documented in epithelial cells. In human bronchial epithelial cells, α- and γ-adducin are present but β-adducin is not.55 Depletion of α-adducin resulted in altered structure of the epithelia and loss of polarity.55 Loss of epithelial cell polarity was also seen in Caco-2 cells depleted of Tmod.56 Hence, it is clear that adducin in particular and a membrane skeleton in general are required to maintain cell polarity. It is tempting to speculate that the epithelia of the choroid plexus are likewise altered in the absence of adducin and ultimately disrupt CSF homeostasis. Further studies will be needed to address this hypothesis at a structural and mechanistic level.
That we see only 50% incidence of hydrocephaly in adducin-null mice points to the presence of segregating modifier genes in the hybrid B6;129 genetic background of the mice used in this study. It is well documented that the B6 genetic background is quite permissive in that there is a low rate of spontaneous hydrocephalus in this strain (1%–3%),57 and both the severity and penetrance of mutations known to cause hydrocephaly are exacerbated on the B6 background, whereas the 129 genetic background is resistant to hydrocephaly.51 It seems likely, therefore, that the hydrocephalic phenotype in adducin-null mice will be much more penetrant on a pure inbred B6 background and less so on 129 substrains. These observations have important implications for future studies of additional phenotypes relevant to adducin deficiency, such as systemic blood pressure, kidney function, and hemostasis, where one would clearly wish to avoid the physiological complications of severe hydrocephaly. We are currently producing congenic strains on both backgrounds to facilitate these and other studies in the future.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Leonard Shultz and David Serreze of The Jackson Laboratory for critical review of the paper.
This work was supported by National Institutes of Health grant HL075714 (L.L.P.), the American Heart Association (Dallas, TX) (L.L.P.), and the National Cancer Institute (Bethesda, MD) CA34196 (The Jackson Laboratory).
National Institutes of Health
Authorship
Contribution: R.F.R. created the knockout mice, maintained the mouse colony, designed and performed experiments, analyzed data, and assisted in paper preparation; S.L.C. performed gene targeting; B.G performed osmotic fragility studies; K.E.S. performed research; D.M.G. performed research and provided valuable reagents and insights; N.M. performed and analyzed ektacytometry studies; and L.L.P. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Luanne L. Peters, The Jackson Laboratory, 600 Main Street, Bar Harbor, ME 04609; e-mail: luanne.peters@jax.org.