Abstract
Natural killer T (NKT) cells are innate-like T cells that recognize specific microbial antigens and also display autoreactivity to self-antigens. The nature of NKT-cell autoreactive activation remains poorly understood. We show here that the mitogen-activated protein kinase (MAPK) pathway is operative during human NKT-cell autoreactive activation, but calcium signaling is severely impaired. This results in a response that is biased toward granulocyte macrophage colony-stimulating factor (GM-CSF) secretion because this cytokine requires extracellular signal-regulated kinase (ERK) signaling but is not highly calcium dependent, whereas interferon-γ (IFN-γ), interleukin (IL)–4, and IL-2 production are minimal. Autoreactive activation was associated with reduced migration velocity but did not induce arrest; thus, NKT cells retained the ability to survey antigen presenting cells (APCs). IL-12 and IL-18 stimulated autoreactively activated NKT cells to secrete IFN-γ, and this was mediated by Janus kinase-signal transducers and activators of transcription (JAK-STAT)–dependent signaling without induction of calcium flux. This pathway did not require concurrent contact with CD1d+ APCs but was strictly dependent on preceding autoreactive stimulation that induced ERK activation. In contrast, NKT-cell responses to the glycolipid antigen α-galactosyl ceramide (α-GalCer) were dampened by prior autoreactive activation. These results show that NKT-cell autoreactivity induces restricted cytokine secretion and leads to altered basal activation that potentiates innate responsiveness to costimulatory cytokines while modulating sensitivity to foreign antigens.
Introduction
Natural killer T (NKT) cells are a subset of regulatory T cells that recognizes lipid antigens presented by CD1d molecules.1,2 Like innate lymphocytes, NKT cells are among the first responders during microbial infections, and their responses are not necessarily dependent on recognition of foreign antigens.3 NKT cells have attracted attention because they secrete large amounts of both Th1 and Th2 cytokines rapidly on stimulation and demonstrate a potent ability to modulate immune function.1,2,4 Remarkably, in some disease models, NKT cells promote proinflammatory immune responses, whereas in others they have a tolerogenic effect.5 It is not clear what confers the innate-like features of NKT cells or how they mediate contrasting immunologic outcomes in different contexts
Most NKT cells can be activated to secrete cytokines by exposure to CD1d+ antigen-presenting cells (APCs) in the absence of microbial antigens, and this appears to depend on presentation of cellular antigens by CD1d.6-9 NKT cells from germ-free mice and from human cord blood display a phenotype indicating prior activation,10-12 suggesting that NKT cells are autoreactive to self-antigens in vivo. NKT cells have been shown to recognize certain mammalian lipids as antigens presented by CD1d9,13 ; however, the specific self-antigens responsible for NKT cell autoreactivity to CD1d+ APCs remain unclear.14
NKT cells express semi-invariant T-cell receptors (TCRs) and also recognize certain classes of nonmammalian glycolipids.2 The prototypical nonself antigen is a synthetic glycolipid called α-galactosylceramide (α-GalCer), in which a galactose sugar is linked in an unusual α-anomeric configuration to a type of sphingolipid.15 Antigens of this kind can activate NKT cells beyond the level incurred by self-antigens, and α-GalCer appears to be a particularly strong agonist as evidenced by its ability to induce almost complete TCR down-regulation, sustained cytokine production, and a proliferative burst.16 The downstream effects of NKT-cell activation in vivo by administration of α-GalCer include widespread polyclonal activation of T cells, B cells and NK cells, and what has been called a “cytokine storm” because serum levels of both Th1 and Th2 cytokines become highly elevated.17,18 These findings have made it apparent that NKT cells mediate pleiotropic immunologic effects; however, the basis for this remains unclear.
Previous studies have shown that most CD4− NKT cells from human peripheral blood appear Th1-biased, whereas the CD4+ subset produced both Th1 and Th2 cytokines.19,20 Thus, the CD4+ and CD4− NKT-cell subsets may be responsible for different functional outcomes. NKT cells are also known to induce functionally distinct immune responses depending on the conditions of activation. Administration of α-GalCer results in elevated levels of both interferon-γ (IFN-γ) and interleukin-4 (IL-4); however, α-glycosyl lipid analogs that differ in the structure of the alkyl chains or the glycosidic linkage can produce Th1- or Th2-polarized responses.21-23 An important factor in this polarization effect is whether or not dendritic cell (DC) IL-12p70 production is stimulated, resulting in downstream IFN-γ secretion by NK cells.24,25 Hence, the structure of the lipid antigen affects the ability of NKT cells to activate DCs, which results in differential amplification of the cytokine response by other cell types.
It is not clear whether the cytokine output of individual NKT cells varies according to the antigenic stimulus. Features of lipid alkyl chains determine the binding affinity of an NKT cell TCR to the CD1d-glycolipid complex, and this is reflected in the intensity and duration of the calcium flux induced during activation.26 The generation of a sustained rise in cytoplasmic calcium ion concentration during T-cell activation is essential for a number of cellular processes that are regulated by calcium-dependent enzymes and for cytokine gene transcription by nuclear factor of activated T cells (NFAT).27,28 Strong calcium signaling in T cells has been associated with secretion of Th1 cytokines, whereas defective calcium flux is associated with anergy and altered cytokine production.29,30 Exposure to lipid bilayers containing CD1d molecules loaded with α-GalCer was sufficient to induce long-lasting NKT-cell calcium flux, and a lower affinity analog of α-GalCer resulted in a weaker response.26 However, activation of NKT cells by the low affinity analog resulted in reduced secretion of both IFN-γ and IL-4, and not a polarized response.26 Thus, antigen affinity affects the amount of cytokine produced by NKT cells, but it is not clear that it also qualitatively biases the response.
During microbial infection, 2 mechanistically distinct routes of NKT cell activation have been identified, and these do produce differentially biased cytokine responses. The “direct” route of NKT-cell activation involves recognition of certain bacterial antigens, such as α-glycosylceramide lipids from Sphingomonas or monogalactosyl-diacylglycerols from Borrelia.31-33 These bacterial antigens appear to be weaker agonists than α-GalCer but can stimulate enhanced NKT-cell secretion of both IFN-γ and IL-4.31,32 In addition, NKT cells can be “indirectly” activated during microbial infections by exposure to costimulatory cytokines, such as IL-12 and IL-18, that are produced by DCs in response to microbial TLR ligands.33-35 This pathway results in an NKT-cell response that is highly biased toward IFN-γ secretion, without enhanced IL-4 production. The requirement for TCR stimulation in this innate-like route of NKT-cell activation is not clear.
Here we have investigated the molecular signaling pathways involved in human NKT-cell activation. Our results indicate that NKT-cell autoreactivity elicits an activation pathway that is characterized by severely impaired calcium signaling and therefore results in the production of a restricted set of cytokines. This calcium-impaired activation process may constitute an intrinsic mechanism of control that limits secretion of potentially dangerous effector cytokines, such as IFN-γ and IL-4. In addition, our data indicate that, as a result of their autoreactivity, NKT cells can attain an altered basal activation level that allows them to respond to subsequent costimulatory cytokines without requiring concurrent TCR stimulation. This innate-like responsiveness to endogenous activation signals is counterbalanced by a reduced sensitivity to foreign antigen.
Methods
NKT cells and APCs
Human CD1d-restricted NKT-cell clones were generated and maintained as described.34 DCs were prepared by short-term culture of fresh human peripheral blood monocytes with granulocyte macrophage colony-stimulating factor (GM-CSF) and IL-4 as described.36 CD1d transfectants were generated from the 3023 human lymphoblastoid cell line as described.37 Studies were approved by the University of Wisconsin Minimal Risk Institutional Review Board, and written informed consent was obtained from all blood donors in accordance with the Declaration of Helsinki.
Glycolipid antigens
α-GalCer, OCH, and Borrelia burgdorferi glycolipid (BbGL) fraction IIf were synthesized as described.23,32,38 α-GalCer and OCH were dissolved in dimethyl sulfoxide, BbGL lipid was dissolved in 0.5% Tween 20, 150 mM of NaCl. Antigens were sonicated in a heated water bath at 37°C for 20 minutes before use.
NKT-cell stimulation assays
NKT cells were incubated in culture medium lacking IL-2 for 18 to 24 hours before all stimulation assays to ensure a fully rested state, and assays were carried out in medium lacking IL-2. Human CD1d-Fc fusion protein was prepared and used to stimulate NKT cells as described.34,39 For stimulation by APCs, CD1d transfectants or DCs were pulsed with lipids or vehicle overnight and then washed and coincubated with a 1:1 ratio of NKT cells. Supernatants were harvested after 18 to 24 hours and tested for cytokines using commercially available enzyme-linked immunosorbent assay (ELISA). For inhibitor experiments, titrated concentrations of cyclosporin A (CsA) or U0126 were added to the NKT cells 10 minutes before addition of the APCs. Ionomycin (100 nM), or IL-12 and IL-18 (2-5 units/mL and 50 ng/mL, respectively), were added to the culture medium concurrently with the NKT cells and APCs. For APC preexposure experiments, APCs were cell surface labeled with NHS-biotin (Pierce Chemical, Rockford, IL) before incubation with NKT cells. APCs were removed after 4 hours by magnetic sorting using antibiotin beads (Miltenyi Biotec, Auburn, CA). Flow cytometric analysis confirmed that the APC-depleted population was more than 99.5% pure.
Calcium flux
NKT cells were labeled with the calcium indicator dyes Fluo-4 and Fura-Red (Invitrogen, Carlsbad, CA) and analyzed by flow cytometry. NKT cells alone were acquired for 30 seconds to provide a baseline to set the threshold; then APCs were added and the sample was centrifuged for 15 seconds to initiate contact. The cells were then resuspended by vortexing briefly, and data were acquired for the following 6 to 8 minutes. Data were analyzed using FlowJo (TreeStar, Ashland, OR) software.
NFAT nuclear translocation
APCs and NKT cells were centrifuged to initiate contact and incubated at 37°C for the indicated times and then fixed and permeabilized (Cytofix/Cytoperm reagent; BD Biosciences, San Jose, CA). The samples were incubated with anti-NFAT1 antibody (clone 4G6; Santa Cruz Biotechnology, Santa Cruz, CA) and stained with a fluorescently labeled second antibody and 6-diamidino-2-phenylindole dihydrochloride. Slides were mounted in ProLong Gold antifade (Invitrogen) and were viewed on a Zeiss Axioplan 2 Imaging microscope (Carl Zeiss, Thornwood, NY) using a Plan-NEOFLUAR lens at 10×/.25 and 63×/1.40 oil DiC and analyzed using OpenLab software (Improvision, Waltham, MA).
NKT/APC interactions
For time-lapse microscopic analysis, poly-L-lysine–coated slides (Polysciences, Warrington, PA) were coated with human intercellular adhesion molecule-1 (ICAM-1)–Fc fusion protein (a generous gift of Dr Lloyd Klickstein, Brigham and Women's Hospital) and blocked with bovine serum albumin in phosphate-buffered saline. APCs and NKT cells were labeled with Vybrant DiD dye (National Institutes of Health [NIH], Bethesda, MD) and carboxyfluorescein diacetate succinimidyl ester (CFSE), respectively (Invitrogen). Microscopic analysis was performed on Bio-Rad Radiance 2100 MP Rainbow confocal system with Plan APO 20×/0.75 air lens (Bio-Rad, Hercules, CA). Videos were made by taking photographic images at 15-second intervals for a period of 40 to 60 minutes. Single-cell tracking analysis was performed using Image J software (NIH). Migration speed was determined by dividing the distance migrated by each T cell by the total time of analysis. For conjugation analysis, APCs were mixed with a 1:1 ratio of NKT cells and then pelleted and incubated for the indicated times at 37°C, resuspended by vigorous vortexing, and analyzed by flow cytometry. For the “zero” time point, contact was initiated by pelleting, but the cells were immediately vortexed without a 37°C incubation.
Extracellular signal-regulated kinase phosphorylation
APCs were pretreated with the MEK inhibitor U0126 for 30 minutes. NKT cells and APCS were pelleted and incubated at 37°C for the indicated times, then transferred to ice and immediately lysed by addition of a nondenaturing detergent buffer (Cell Lytic M; Sigma-Aldrich, St Louis, MO). Insoluble material was removed by centrifugation, and lysates were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was probed with rabbit polyclonal antiphospho-44/42-ERK antibody (Cell Signaling Technology, Danvers, MA), then stripped and reprobed with anti-ERK antibody (Millipore, Billerica, MA). Image J software was used for quantification. NKT-cell activation consistently resulted in 2 phospho-ERK bands, whereas activated APCs only showed the lower phospho-ERK band. Therefore, only the upper phospho-ERK band was quantified.
Results
Hierarchical pattern of cytokine production
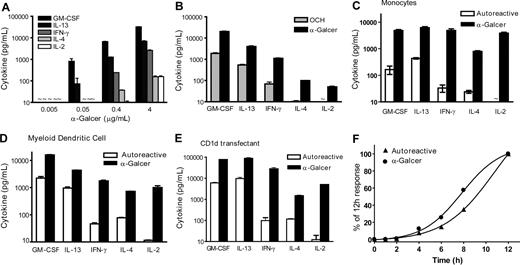
To investigate the relationship of TCR stimulation to cytokine production, we used plate-bound recombinant CD1d molecules to present titrated concentrations of α-GalCer to human CD4+ NKT- cell clones. A low dose of α-GalCer induced secretion of GM-CSF and IL-13 but failed to activate secretion of other cytokines (Figure 1A). Increasing the concentration of α-GalCer by 8- to 10-fold resulted in enhanced production of GM-CSF and IL-13 and induced detectable levels of IFN-γ and IL-4, whereas IL-2 required approximately 10-fold more α-GalCer (Figure 1A). Thus, the amount of antigen had both a qualitative and a quantitative effect on cytokine production. To assess whether antigen affinity also qualitatively affects the response, we tested plate-bound CD1d molecules that were loaded with a low affinity analog of α-GalCer. An α-glycosyl lipid called “OCH” that contains a severely truncated phytosphingosine chain has been shown to be a weak NKT cell agonist.26 Whereas α-GalCer induced the full spectrum of cytokines, the same concentration of OCH activated secretion of GM-CSF and IL-13 but stimulated only minimal IFN-γ and IL-4 production and completely failed to induce detectable IL-2 (Figure 1B). Thus, a low affinity antigen resulted in a pattern of cytokine secretion that was similar to a suboptimal dose of higher affinity antigen. These results show that NKT-cell cytokine production proceeds hierarchically according to the strength of the TCR signal, with GM-CSF and IL-13 requiring the least stimulation, followed by IFN-γ and IL-4, then IL-2.
Hierarchical NKT-cell cytokine production. (A) NKT cells were stimulated by exposure to plate-bound CD1d molecules pulsed with the indicated concentrations of α-GalCer, and cytokine secretion was determined by ELISA. (B) Plate-bound CD1d was pulsed with 1 μg/mL α-GalCer or OCH. (C-E) NKT cells were stimulated by the indicated APCs that were untreated (Autoreactive) or pulsed with 100 ng/mL of α-GalCer. (F) NKT cells were incubated with CD1d-transfected APCs for the indicated times, and GM-CSF secretion was quantitated by ELISA. Data are represented as mean plus or minus SD (not always visible on the scales shown) with amounts below the limit of detection indicated by “∼.” Panels A to E show a representative experiment performed using clone J24N.22, and in each case similar results were observed using 2 or 3 other NKT cell clones. Panel F shows 1 representative experiment of 2 performed using clone J3N.5.
Hierarchical NKT-cell cytokine production. (A) NKT cells were stimulated by exposure to plate-bound CD1d molecules pulsed with the indicated concentrations of α-GalCer, and cytokine secretion was determined by ELISA. (B) Plate-bound CD1d was pulsed with 1 μg/mL α-GalCer or OCH. (C-E) NKT cells were stimulated by the indicated APCs that were untreated (Autoreactive) or pulsed with 100 ng/mL of α-GalCer. (F) NKT cells were incubated with CD1d-transfected APCs for the indicated times, and GM-CSF secretion was quantitated by ELISA. Data are represented as mean plus or minus SD (not always visible on the scales shown) with amounts below the limit of detection indicated by “∼.” Panels A to E show a representative experiment performed using clone J24N.22, and in each case similar results were observed using 2 or 3 other NKT cell clones. Panel F shows 1 representative experiment of 2 performed using clone J3N.5.
We next investigated the cytokine secretion patterns resulting from autoreactive activation of NKT cells by CD1d+ APCs. NKT cells were incubated with freshly isolated human peripheral blood monocytes that were pulsed with α-GalCer or were untreated. The untreated monocytes stimulated NKT-cell secretion of GM-CSF and IL-13 and modest levels of IFN-γ and IL-4 but little or no IL-2, whereas those that were pulsed with α-GalCer activated production of all of the cytokines (Figure 1C). Similar results were obtained using human myeloid DCs generated by in vitro differentiation (Figure 1D). Because monocytes and myeloid DCs can produce costimulatory factors, such as IL-12 and IL-18, which might influence NKT-cell responses independently of TCR stimulation, we also tested CD1d-transfected human lymphoblastoid cell lines that do not produce these cytokines. Despite expressing approximately 40-fold higher levels of CD1d than DCs, the CD1d transfectants induced a similar pattern of NKT-cell cytokine secretion (Figure 1E; Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). NKT-cell cytokine secretion reached a plateau after 12 hours of stimulation and did not increase substantially after this point (data not shown). Thus, because cytokines were analyzed after 16 hours, the different patterns observed in these experiments were probably not the result of delayed cytokine secretion in response to autoreactive activation. Moreover, the kinetics of GM-CSF secretion during the first 12 hours appeared similar for autoreactive and α-GalCer-mediated activation (Figure 1F), suggesting that autoreactive activation was a relatively efficient stimulus for this cytokine.
The CD4− NKT-cell clones that we tested showed only weak responses to untreated CD1d+ APCs; therefore, they were not informative for analyzing autoreactivity. However, we found that CD4− clones displayed a similar preferential secretion of GM-CSF in response to low doses of α-GalCer (Figure S2). Together, these results show that the autoreactive responses of human CD4+ NKT cells result in a cytokine secretion pattern that is qualitatively similar to responses induced by weak to moderate TCR signaling.
Defective calcium signaling during autoreactive activation
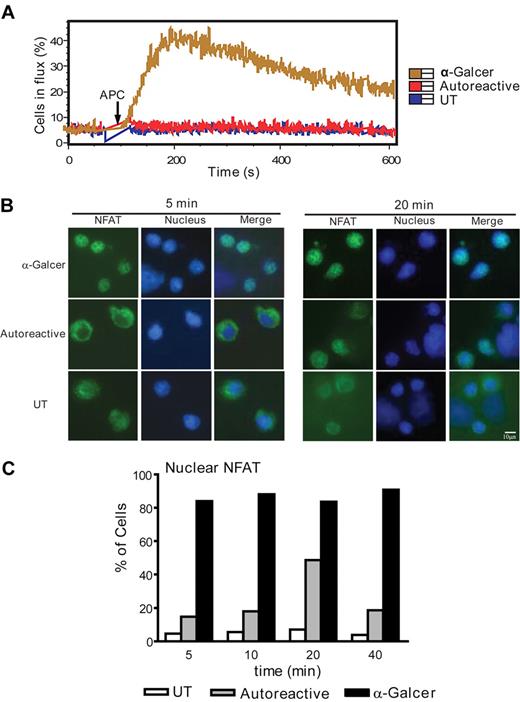
To investigate the signaling processes underlying their functional responses, we tested NKT cell calcium flux on contact with APCs. Whereas exposure to CD1d transfectants that were pulsed with α-GalCer elicited a marked calcium flux, NKT cells that were stimulated by untreated CD1d transfectants showed no detectable calcium flux and appeared indistinguishable from those that were exposed to untransfected APCs (Figure 2A). Similarly, NKT cells that were exposed to untreated myeloid DCs showed no detectable calcium flux within the first 10 minutes of exposure, whereas exposure to α-GalCer pulsed DCs led to a detectable response (data not shown).
Defective calcium signaling during NKT-cell autoreactive activation. (A) NKT cells were labeled with the intracellular calcium indicator dyes Fluo-4 and Fura-Red and stimulated by contact with APCs. UT indicates the untransfected parent cell (CD1d-negative); Autoreactive, unpulsed CD1d-transfected APCs; α-GalCer, antigen-pulsed CD1d transfectants. Intracellular calcium levels were assessed by flow cytometry. The cells in flux were defined as those cells with a ratio of Fluo-4 to Fura-Red that exceeded the 95% confidence interval of the baseline. The data shown are from 1 representative experiment of 3 using clone J24N.22, and similar results were obtained from 3 other clones. (B) NKT cells were coincubated for 5 minutes (left panels) or 20 minutes (right panels) with APCs, and NFAT1 activation was assessed by immunofluorescence analysis of nuclear translocation. NFAT1 is shown in green and nuclear staining (6-diamidino-2-phenylindole dihydrochloride) is shown in blue. (C) The percentage of NKT cells showing nuclear colocalization of NFAT1 after the indicated APC coincubation times. At least 80 events in 10 independent fields were quantitated. The data in panels B and C are from 1 representative experiment of 2 using clone J24N.22, and similar results were observed from analysis of 1 other NKT-cell clone.
Defective calcium signaling during NKT-cell autoreactive activation. (A) NKT cells were labeled with the intracellular calcium indicator dyes Fluo-4 and Fura-Red and stimulated by contact with APCs. UT indicates the untransfected parent cell (CD1d-negative); Autoreactive, unpulsed CD1d-transfected APCs; α-GalCer, antigen-pulsed CD1d transfectants. Intracellular calcium levels were assessed by flow cytometry. The cells in flux were defined as those cells with a ratio of Fluo-4 to Fura-Red that exceeded the 95% confidence interval of the baseline. The data shown are from 1 representative experiment of 3 using clone J24N.22, and similar results were obtained from 3 other clones. (B) NKT cells were coincubated for 5 minutes (left panels) or 20 minutes (right panels) with APCs, and NFAT1 activation was assessed by immunofluorescence analysis of nuclear translocation. NFAT1 is shown in green and nuclear staining (6-diamidino-2-phenylindole dihydrochloride) is shown in blue. (C) The percentage of NKT cells showing nuclear colocalization of NFAT1 after the indicated APC coincubation times. At least 80 events in 10 independent fields were quantitated. The data in panels B and C are from 1 representative experiment of 2 using clone J24N.22, and similar results were observed from analysis of 1 other NKT-cell clone.
To confirm the defect in calcium signaling, we analyzed activation of NFAT1, a transcription factor that is mobilized to migrate from the cytoplasm to the nucleus as a result of calcium signaling. Exposure of NKT cells to α-GalCer pulsed CD1d transfectants for 5 minutes resulted in complete colocalization of NFAT1 and nuclear staining (Figure 2B). In contrast, NKT cells that were autoreactively activated by CD1d transfectants or that were exposed to untransfected APCs for 5 minutes showed little or no nuclear localization of NFAT1 (Figure 2B). Although untransfected APCs never induced significant NKT cell NFAT1 translocation, some of the autoreactively activated NKT cells did show detectable nuclear NFAT1 localization, and this increased over the first 20 minutes and then dropped off (Figure 2C). Together, these results indicate that calcium signaling is not completely absent in autoreactively activated NKT cells, but the magnitude of the flux is severely impaired and calcium-dependent transcription factor activation is substantially delayed.
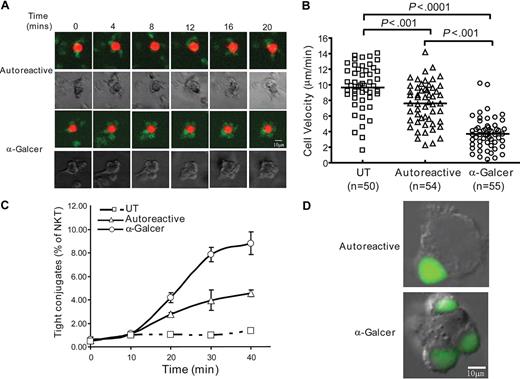
An important parameter resulting from calcium signaling is T-cell migration arrest. T cells move from APC to APC until they receive a calcium signal that causes them to stop migrating and form long-lasting contacts with APCs that are thought to be critical for productive activation.40,41 We therefore investigated the dynamics of NKT-cell interactions with APCs. Time-lapse photography revealed that NKT cells migrating across an ICAM-1–coated surface nearly immediately underwent arrest when α-GalCer pulsed DCs were introduced but did not stop moving when untreated DCs were added (Figure 3A, and Videos S1Video 2. α-GalCer interaction: close up view of NKT (green) + α-GalCer pulsed DC (red) (MOV, 85.3 KB)Video 3. α-GalCer interaction: close up view of NKT (green) + a-GAlCer pulsed CD1d transfectant (red) (MOV, 25.7 KB)Video 4. Autoreactive interaction: close up view of NKT (green) + untreated CD1d transfectant (red) (MOV, 62.0 KB)Video 5. No CD1d interaction: close up view of NKT (green) + CD1d-negative APCs (red) (MOV, 23.4 KB)Video 6. α-GalCer interaction: large field view of NKT cells (green) with α-GalCer pulsed DCs (red) (MOV, 286 KB)–S7). To investigate whether autoreactive activation influenced NKT-cell migration, we compared migration velocity after exposure to CD1d transfectants or the untransfected parent cells. As expected, the NKT cells engaged in long-lasting contact with α-GalCer pulsed CD1d transfectants and thus showed markedly reduced velocity (Figure 3B). However, the NKT cells also showed significantly reduced velocity in the presence of CD1d transfectants compared with the untransfected parent cells, suggesting that autoreactive stimulation resulted in an increased time of interaction with the APCs (Figure 3B).
Dynamics of autoreactive NKT cell interactions with APCs. (A) Time-lapse microscopy of NKT cells interacting with human myeloid DCs. Top rows show fluorescence images taken at the indicated times with NKT cells labeled green and the DCs labeled red, and the bottom rows show phase-contrast views. Data are from 1 representative experiment of 3. Videos S1 through S7 provide kinetic views of NKT cell-APC interactions. (B) Analysis of the velocity of NKT cells in the presence of the indicated APCs. Single-cell tracking analysis was performed using Image J software (NIH), and the average velocity of each T cell was calculated by dividing the distance traveled by the time (20 minutes). Horizontal bars within the datasets show the means with the P values calculated by ANOVA shown at the top. The results are from a representative experiment using clone JC2.4, and similar results were obtained with 1 additional clone. (C) Flow cytometric analysis of tight adherence of NKT cells to APCs. Fluorescently labeled NKT cells and APCs were coincubated for the indicated times, and tightly adhered conjugates were analyzed by flow cytometry. Data are mean plus or minus SD of 3 replicate samples and are from 1 representative experiment of 3 using clone J3N.5. Similar results were observed using 2 other NKT cell clones. (D) Microscopic analysis of carboxyfluorescein diacetate succinimidyl ester-labeled NKT cells (green) conjugated to APCs.
Dynamics of autoreactive NKT cell interactions with APCs. (A) Time-lapse microscopy of NKT cells interacting with human myeloid DCs. Top rows show fluorescence images taken at the indicated times with NKT cells labeled green and the DCs labeled red, and the bottom rows show phase-contrast views. Data are from 1 representative experiment of 3. Videos S1 through S7 provide kinetic views of NKT cell-APC interactions. (B) Analysis of the velocity of NKT cells in the presence of the indicated APCs. Single-cell tracking analysis was performed using Image J software (NIH), and the average velocity of each T cell was calculated by dividing the distance traveled by the time (20 minutes). Horizontal bars within the datasets show the means with the P values calculated by ANOVA shown at the top. The results are from a representative experiment using clone JC2.4, and similar results were obtained with 1 additional clone. (C) Flow cytometric analysis of tight adherence of NKT cells to APCs. Fluorescently labeled NKT cells and APCs were coincubated for the indicated times, and tightly adhered conjugates were analyzed by flow cytometry. Data are mean plus or minus SD of 3 replicate samples and are from 1 representative experiment of 3 using clone J3N.5. Similar results were observed using 2 other NKT cell clones. (D) Microscopic analysis of carboxyfluorescein diacetate succinimidyl ester-labeled NKT cells (green) conjugated to APCs.
To confirm that autoreactive activation results in specific NKT cell interactions with APCs, we tested conjugate formation. T-cell conjugation with APCs is mediated by adhesion molecules, such as the integrin leukocyte function-associated antigen-1. Tight adherence occurs only after TCR signaling induces a conformational change in the extracellular domains of leukocyte function-associated antigen-1 molecules that increases the affinity for ligands, such as ICAM-1 on the APC.42 There was no NKT-cell conjugation to untransfected APCs, confirming that a CD1d-dependent TCR signal was required (Figure 3C). In contrast, NKT cells were able to tightly adhere to both α-GalCer pulsed and untreated CD1d transfectants (Figure 3C). A higher percentage of tight conjugates were formed with α-GalCer pulsed than untreated CD1d transfectants, but the amount of contact time required for the conjugates to form appeared similar (Figure 3C). For both α-GalCer pulsed and untreated CD1d transfectants, an extensive contact interface between the NKT cell and APC was observed (Figure 3D). Thus, although autoreactive activation is characterized by transient interactions with APCs, during the time they contact the APCs, the NKT cells nevertheless form tight conjugates in a CD1d-dependent manner.
ERK signaling during autoreactive activation
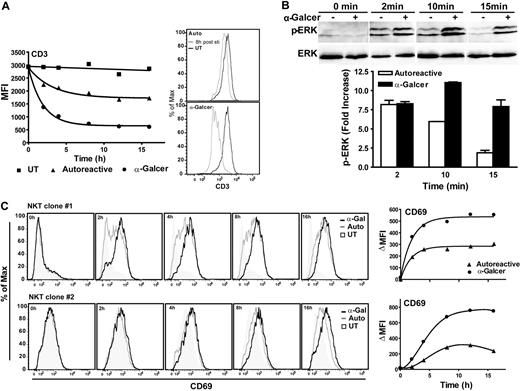
Activation of T cells by TCR-mediated signaling results in internalization of TCR-CD3 complex, and the amount of internalization correlates with the signal strength.43 We therefore assessed NKT-cell CD3 expression levels before and after APC exposure. NKT cells that were exposed to untransfected APCs showed no CD3 down-regulation, whereas exposure to CD1d-transfected APCs resulted in marked CD3 down-regulation within 2 hours (Figure 4A). α-GalCer–pulsed CD1d transfectants induced greater CD3 down-regulation, consistent with stronger TCR signaling (Figure 4A). Both autoreactive and α-GalCer–mediated activation led to CD3 down-regulation by 100% of the NKT cells (Figure 4A), demonstrating that in each case all of the NKT cells were stimulated, but the degree of response varied according to the nature of the stimulus.
ERK signaling during NKT-cell activation. (A) Flow cytometric analysis of CD3 cell surface expression by NKT cells after coincubation with APCs for the indicated times. The results are from 1 representative experiment of 3. Similar results were observed using 2 different clones. (B) NKT cells were exposed to APCs for the indicated times, and then lysed and phospho-ERK was detected by Western blotting (top panels). The same membrane was then stripped and probed with an antibody for total ERK (bottom panels). APCs were pretreated with the U0126 inhibitor before incubation with the NKT cells to eliminate APC phospho-ERK signal. The plot shows densitometric analysis of the phospho-ERK signal from the upper band normalized by the corresponding total ERK signal. Similar results were obtained using 3 different NKT cell clones. (C) Flow cytometric analysis of CD69 expression by NKT cell clones after coincubation with APCs for the indicated times. The plots on the right show the change in mean fluorescence intensity compared with coincubation with untransfected APCs. Similar results were obtained from 2 additional clones.
ERK signaling during NKT-cell activation. (A) Flow cytometric analysis of CD3 cell surface expression by NKT cells after coincubation with APCs for the indicated times. The results are from 1 representative experiment of 3. Similar results were observed using 2 different clones. (B) NKT cells were exposed to APCs for the indicated times, and then lysed and phospho-ERK was detected by Western blotting (top panels). The same membrane was then stripped and probed with an antibody for total ERK (bottom panels). APCs were pretreated with the U0126 inhibitor before incubation with the NKT cells to eliminate APC phospho-ERK signal. The plot shows densitometric analysis of the phospho-ERK signal from the upper band normalized by the corresponding total ERK signal. Similar results were obtained using 3 different NKT cell clones. (C) Flow cytometric analysis of CD69 expression by NKT cell clones after coincubation with APCs for the indicated times. The plots on the right show the change in mean fluorescence intensity compared with coincubation with untransfected APCs. Similar results were obtained from 2 additional clones.
Next, we investigated ERK signaling during NKT-cell activation. ERK phosphorylation was assessed by Western blotting after NKT cells were exposed to CD1d-transfected APCs. To ensure that the phospho-ERK signal was from the NKT cells and not the APCs, the APCs were pretreated for 30 minutes with the MEK inhibitor U0126 to inhibit their ERK phosphorylation. Both autoreactive and α-GalCer activation induced clearly detectable ERK phosphorylation within 2 minutes (Figure 4B). NKT cells that were exposed to untransfected APCs did not show detectable phospho-ERK signal (data not shown). The initial ERK phosphorylation levels induced by autoreactive and α-GalCer activation appeared similar, but the duration of the phospho-ERK signal was shorter for autoreactive activation (Figure 4B).
T-cell up-regulation of CD69 is mainly the result of signaling through the MAPK/ERK pathway.44 Therefore, we analyzed CD69 expression levels as a means to assess the downstream results of ERK signaling. CD69 was up-regulated by both autoreactive and α-GalCer stimulation, whereas there was no increase after exposure to untransfected APCs (Figure 4C). The level of CD69 expression induced by autoreactive activation was lower than that resulting from α-GalCer exposure, but the kinetics appeared similar and both forms of stimulation led to long-lasting CD69 up-regulation (Figure 4C). These results demonstrate that autoreactive stimulation induces ERK signaling that results in durable NKT-cell activation.
Differential effects of calcium signaling
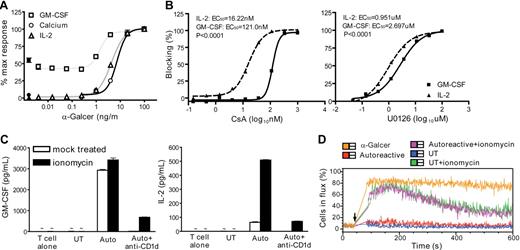
Our results suggested that GM-CSF secretion by NKT cells could be induced at TCR stimulation levels below the threshold required to induce a calcium flux. Using CD1d transfectants pulsed with titrated concentrations of α-GalCer, we observed that GM-CSF secretion remained constant until the calcium flux threshold was crossed, and only then showed a marked increase (Figure 5A). In contrast, IL-2 production correlated closely with the induction of calcium flux (Figure 5A). To further investigate this, we tested GM-CSF and IL-2 sensitivity to the calcineurin inhibitor CsA compared with the MEK1/2 inhibitor U0126, which specifically prevents ERK phosphorylation. Approximately 8-fold lower CsA concentrations were required for half-maximal blocking of IL-2 than GM-CSF, whereas there was less than a 3-fold difference in IL-2 and GM-CSF sensitivity to U0126 (Figure 5B). Conversely, when a calcium flux was induced during NKT cell autoreactive activation by addition of a suboptimal dose of ionomycin, there was only minimal enhancement of GM-CSF secretion (∼ 10%), whereas IL-2 production was increased more than 5-fold (Figure 5C). Flow cytometric analysis showed that the magnitude and duration of the intracellular calcium flux induced by the suboptimal dose of ionomycin were less than those resulting from exposure to CD1d transfectants pulsed with 100 ng/mL α-GalCer (Figure 5D). Together, these results show that NKT cell IL-2 production is highly dependent on calcium signaling, whereas GM-CSF is relatively independent of the calcium pathway. In contrast, GM-CSF and IL-2 are similar in their dependence on ERK signaling. This provides an explanation for the observation that NKT-cell autoreactivity, which clearly activates the ERK pathway but is deficient in calcium signaling, produces a functional response that is biased toward GM-CSF production and includes little or no IL-2 secretion.
Calcium dependence of NKT-cell responses. (A) NKT cells (clone J24L.17) were stimulated by CD1d transfectants that were either untreated (filled symbols) or pulsed with the indicated concentrations of α-GalCer (open symbols). Cytokine secretion and calcium flux responses were normalized by the response to 100 ng/mL α-GalCer. Similar results were observed for 2 additional NKT cell clones. (B) NKT cells were stimulated by α-GalCer–pulsed CD1d transfectants in the presence of titrated doses of cyclosporin A (CsA, left plot), or the MEK1/2 inhibitor U0126 (right plot) and GM-CSF and IL-2 were quantitated by ELISA. The half-maximal effective concentrations (EC50) of CsA for GM-CSF and IL-2 were calculated by nonlinear regression using a sigmoidal dose-response equation. The 95% confidence intervals for inhibition by CsA were as follows: IL-2, 14.07 to 18.71 nM; GM-CSF, 108.8 to 134.6 nM. For inhibition by U0126, they were: IL-2, 0.797 to 1.135 μM; GM-CSF, 2.000 to 3.636 μM. (C) NKT cells alone or in the presence of the indicated APCs were treated with 100 nM of ionomycin (■) or vehicle (□), and cytokine secretion was quantitated by ELISA. Left plot shows GM-CSF; right plot shows IL-2. (D) NKT-cell calcium flux after stimulation by APCs alone or in the presence of 100 nM of ionomycin. The results shown in panels B to D are each from 1 representative experiment of 3 using clone J24N.22. Similar results were observed using 2 different NKT cell clones.
Calcium dependence of NKT-cell responses. (A) NKT cells (clone J24L.17) were stimulated by CD1d transfectants that were either untreated (filled symbols) or pulsed with the indicated concentrations of α-GalCer (open symbols). Cytokine secretion and calcium flux responses were normalized by the response to 100 ng/mL α-GalCer. Similar results were observed for 2 additional NKT cell clones. (B) NKT cells were stimulated by α-GalCer–pulsed CD1d transfectants in the presence of titrated doses of cyclosporin A (CsA, left plot), or the MEK1/2 inhibitor U0126 (right plot) and GM-CSF and IL-2 were quantitated by ELISA. The half-maximal effective concentrations (EC50) of CsA for GM-CSF and IL-2 were calculated by nonlinear regression using a sigmoidal dose-response equation. The 95% confidence intervals for inhibition by CsA were as follows: IL-2, 14.07 to 18.71 nM; GM-CSF, 108.8 to 134.6 nM. For inhibition by U0126, they were: IL-2, 0.797 to 1.135 μM; GM-CSF, 2.000 to 3.636 μM. (C) NKT cells alone or in the presence of the indicated APCs were treated with 100 nM of ionomycin (■) or vehicle (□), and cytokine secretion was quantitated by ELISA. Left plot shows GM-CSF; right plot shows IL-2. (D) NKT-cell calcium flux after stimulation by APCs alone or in the presence of 100 nM of ionomycin. The results shown in panels B to D are each from 1 representative experiment of 3 using clone J24N.22. Similar results were observed using 2 different NKT cell clones.
Classic and innate pathways of NKT-cell activation
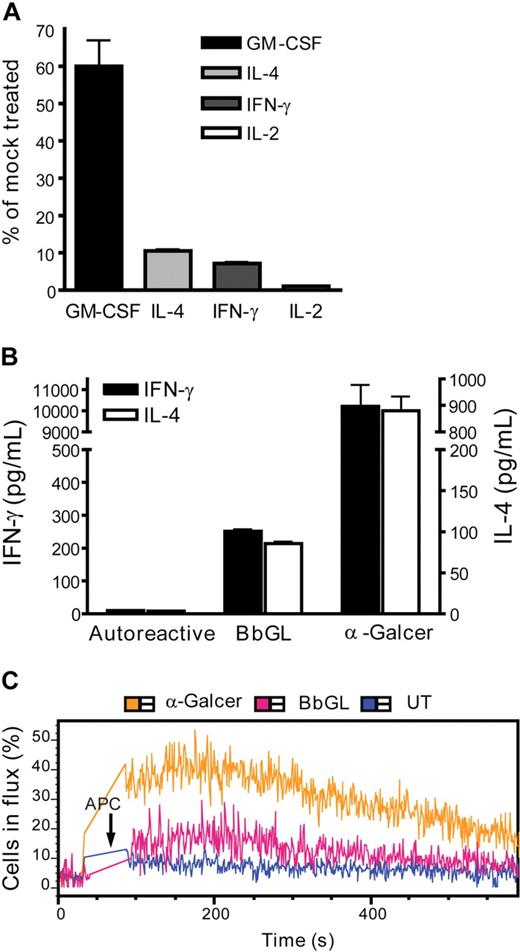
We observed that a concentration of CsA that had only a partial effect on GM-CSF but fully blocked IL-2 resulted in nearly complete inhibition of IFN-γ and IL-4 secretion, suggesting that IFN-γ and IL-4 are more affected by calcium signaling than GM-CSF but are somewhat less dependent than IL-2 (Figure 6A). To further investigate the effect of antigenic stimulation on IFN-γ and IL-4 production, we tested NKT cell activation by other glycolipid compounds. Although our NKT-cell clones reproducibly showed responses to OCH when it was presented by plate-bound recombinant CD1d molecules (Figure 1B), we did not observe any enhanced cytokine secretion in response to CD1d transfectants pulsed with this antigen (data not shown). Similarly, OCH-pulsed APCs failed to stimulate detectable NKT-cell calcium flux (data not shown). Thus, OCH did not appear to activate our human NKT-cell clones beyond the level induced by their autoreactivity to endogenous cellular antigens. In contrast, CD1d transfectants that were pulsed with a glycolipid antigen from B burgdorferi did elicit a modest NKT-cell calcium flux and stimulated enhanced production of both IFN-γ and IL-4 (Figure 6B,C). Thus, a microbial antigen was able to initiate an NKT-cell activation cascade that included a calcium flux response, and that led to enhanced secretion of both IFN-γ and IL-4.
NKT-cell activation by a microbial glycolipid. (A) Calcium dependence of IFN-γ and IL-4 production. NKT cells were stimulated by α-GalCer–pulsed CD1d transfectants in the presence of 111 nM CsA or of vehicle, and the indicated cytokines were measured by ELISA. The plot shows the amount of each cytokine produced in the presence of CsA as a percentage of the amount of the same cytokine induced in the absence of CsA. (B) A microbial antigen induces both IFN-γ and IL-4 secretion. NKT cells were stimulated by CD1d transfectants pulsed with 5 μg/mL BbGL, or 50 ng/mL α-GalCer, or left untreated (Autoreactive), and production of IL-4 (right y-axis) and IFN-γ (left y-axis) was quantitated by ELISA. (C) BbGL-pulsed APCs can stimulate calcium flux by NKT cells. Data are representative of 2 or 3 independent experiments using clone J24N.22 or clone J24L.17.
NKT-cell activation by a microbial glycolipid. (A) Calcium dependence of IFN-γ and IL-4 production. NKT cells were stimulated by α-GalCer–pulsed CD1d transfectants in the presence of 111 nM CsA or of vehicle, and the indicated cytokines were measured by ELISA. The plot shows the amount of each cytokine produced in the presence of CsA as a percentage of the amount of the same cytokine induced in the absence of CsA. (B) A microbial antigen induces both IFN-γ and IL-4 secretion. NKT cells were stimulated by CD1d transfectants pulsed with 5 μg/mL BbGL, or 50 ng/mL α-GalCer, or left untreated (Autoreactive), and production of IL-4 (right y-axis) and IFN-γ (left y-axis) was quantitated by ELISA. (C) BbGL-pulsed APCs can stimulate calcium flux by NKT cells. Data are representative of 2 or 3 independent experiments using clone J24N.22 or clone J24L.17.
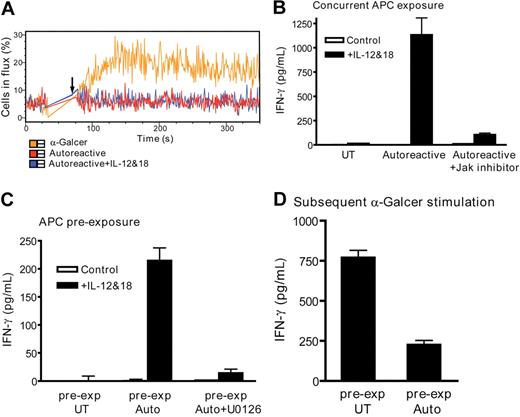
An alternative NKT-cell activation pathway that occurs during microbial infection is mediated by exposure to IL-12 and IL-2 or IL-18.33-35 This pathway was found to stimulate markedly increased secretion of IFN-γ but not to enhance IL-4. In one system, NKT cell IFN-γ secretion appeared to require the presence of CD1d+ APCs, whereas in other systems CD1d was not required.34,35,45 We found that exposure to IL-12 and IL-18 augmented the levels of GM-CSF and IL-13 produced by autoreactively activated NKT cells, and that IFN-γ, but not IL-4, was particularly highly elevated compared with the levels induced by autoreactive activation alone (Figure S3). We investigated the molecular basis for the augmentation of NKT-cell IFN-γ secretion by IL-12 and IL-18. Exposure to IL-12 and IL-18 did not induce NKT-cell calcium flux (Figure 7A). Addition of a specific JAK-STAT pathway inhibitor nearly completely abrogated the enhanced IFN-γ secretion (Figure 7B). Hence, the effect of IL-12 and IL-18 appeared to be mediated through JAK-STAT signaling and did not require calcium flux.
NKT-cell activation by IL-12 and IL-18. (A) Addition of IL-12 and IL-18 does not induce detectable calcium flux by autoreactively activated NKT cells. Similar results were obtained in 4 independent experiments using 2 different NKT-cell clones. (B) IL-12 and IL-18 costimulate IFN-γ secretion by autoreactively activated NKT cells in a JAK-STAT–dependent manner. NKT cells were coincubated with untransfected (UT) or CD1d-transfected APCs (Autoreactive) in the presence of IL-12 and IL-18 (■) or without these cytokines (□), and IFN-γ secretion was quantitated by ELISA. Where indicated, the coincubation was performed in the presence of JAK inhibitor 1. (C) Autoreactive activation potentiates subsequent NKT-cell IFN-γ secretion in response to IL-12 and IL-18. NKT cells were preexposed for 4 hours to the APCs shown on the x-axis; then these APCs were removed and the NKT cells were coincubated with untransfected APCs (ie, CD1d-negative) in the presence of IL-12 and IL-18 (■) or in the absence of these cytokines (□). Where indicated, the CD1d-transfected APC preexposure was performed in the presence of the MEK inhibitor U0126. (D) NKT cells were preexposed to the indicated APCs; these were then removed and the NKT cells were coincubated with α-GalCer–pulsed CD1d-transfected APCs and IFN-γ secretion was quantitated by ELISA. The plots in panels B to D show 1 representative experiment of 5. In each case, similar results were obtained from 3 different NKT-cell clones.
NKT-cell activation by IL-12 and IL-18. (A) Addition of IL-12 and IL-18 does not induce detectable calcium flux by autoreactively activated NKT cells. Similar results were obtained in 4 independent experiments using 2 different NKT-cell clones. (B) IL-12 and IL-18 costimulate IFN-γ secretion by autoreactively activated NKT cells in a JAK-STAT–dependent manner. NKT cells were coincubated with untransfected (UT) or CD1d-transfected APCs (Autoreactive) in the presence of IL-12 and IL-18 (■) or without these cytokines (□), and IFN-γ secretion was quantitated by ELISA. Where indicated, the coincubation was performed in the presence of JAK inhibitor 1. (C) Autoreactive activation potentiates subsequent NKT-cell IFN-γ secretion in response to IL-12 and IL-18. NKT cells were preexposed for 4 hours to the APCs shown on the x-axis; then these APCs were removed and the NKT cells were coincubated with untransfected APCs (ie, CD1d-negative) in the presence of IL-12 and IL-18 (■) or in the absence of these cytokines (□). Where indicated, the CD1d-transfected APC preexposure was performed in the presence of the MEK inhibitor U0126. (D) NKT cells were preexposed to the indicated APCs; these were then removed and the NKT cells were coincubated with α-GalCer–pulsed CD1d-transfected APCs and IFN-γ secretion was quantitated by ELISA. The plots in panels B to D show 1 representative experiment of 5. In each case, similar results were obtained from 3 different NKT-cell clones.
We next investigated whether concurrent exposure to CD1d+ APCs was required. NKT cells were preexposed to CD1d transfectants or the untransfected parent cells, and then these APCs were removed using a protocol that depleted more than 99.5% of the APCs (Figure S4A). After approximately 2 hours, the NKT cells were exposed to untransfected APCs (ie, CD1d-negative APCs) in the presence or absence of IL-12 and IL-18. The NKT cells that were preexposed to untransfected APCs showed no detectable IFN-γ secretion on subsequent stimulation by IL-12 and IL-18; however, those that were preexposed to CD1d-transfected APCs showed significant IFN-γ secretion when IL-12 and IL-18 were added later (Figure 7C). As a control to confirm that the IFN-γ secretion was not the result of a small number of undepleted CD1d+ APCs, we verified that addition of an anti-CD1d monoclonal antibody failed to block the effect (Figure S4B). In addition, we established that approximately 10% CD1d+ APCs were required for concurrent NKT-cell costimulation by IL-12 and IL-18 (Figure S4C). Hence, our flow cytometric analyses suggest that the frequency of undepleted CD1d+ APCs is too low to explain the NKT-cell response.
When the NKT cells were preexposed to CD1d-transfected APCs in the presence of the U0126 inhibitor, the subsequent induction of IFN-γ secretion was not observed (Figure 7C). Pretreatment of unactivated NKT cells with U0126 did not inhibit their responses to subsequent stimulation, indicating that the drug does not have a long-lasting effect (Figure S4C). These results demonstrate that ERK signaling as a result of CD1d-dependent autoreactive activation results in subsequent NKT-cell responsiveness to IL-12 and IL-18, even when CD1d+ APCs are no longer present. In contrast, prior autoreactive activation dampened NKT- cell responses to subsequent α-GalCer activation (Figure 7D). Together, these results show that the partial activation that occurs as a result of autoreactive stimulation makes NKT cells responsive to subsequent cytokine-mediated costimulation but reduces their sensitivity to strong TCR-mediated signals.
Discussion
Our results show, for the first time, that human NKT cells that are activated by weak TCR stimulation produce a cytokine response pattern that is biased toward GM-CSF and IL-13, with little IFN-γ, IL-4, or IL-2. Exposure to CD1d+ APCs that have not been pulsed with specific glycolipid antigens appears to act as a weak TCR stimulus for NKT cells, as the MAPK pathway is engaged but calcium signaling is severely deficient. As a result, mainly cytokines that are relatively independent of calcium signaling are produced, whereas foreign antigenic stimulation induces a full activation cascade, resulting in the secretion of a broad spectrum of cytokines. Thus, NKT-cell autoreactivity leads to a highly restricted functional response that probably promotes a different immunologic outcome than activation by foreign antigens. We have recently shown that GM-CSF and IL-13 secretion by autoreactive NKT cells induces human monocytes to differentiate into immature DCs.36 Hence, we speculate that the particular pattern of cytokine production resulting from NKT-cell autoreactive activation, comprising mainly GM-CSF and IL-13, may serve to constitutively promote monocyte differentiation into DCs.
The identities of the auto-antigens recognized by human NKT cells remain unknown; thus, their TCR affinity is not clear. The activation pattern we have observed is consistent with recognition either of abundant but low affinity antigens or rare but high affinity antigens, but in either case the result is limited TCR signaling. The functional responses of autoreactively activated NKT cells appear similar to those of anergic T cells. Previous studies have demonstrated that altered peptide ligands that induce anergy in major histocompatibility complex-restricted T cells produce weaker and more transient cytoplasmic calcium flux than agonist peptides.46 Conventional T cells that were exposed to low potency ligands in vivo had severely impaired calcium flux but nevertheless activated Ras, and this signaling pattern led to the induction of tolerance.47 In contrast, despite the likelihood that they are repeatedly subject to similar partial activation from exposure to self-antigens, NKT cells do not appear to be anergized in vivo because they clearly retain the ability to produce full functional responses on exposure to a strong agonist, such as α-GalCer.
Our data indicate that autoreactive stimulation of NKT cells potentiates their ability to respond “innately” (ie, without requiring concurrent TCR stimulation) to costimulatory cytokines. NKT cells that were preexposed to CD1d+ but not CD1d− APCs secreted IFN-γ on subsequent treatment with IL-12 and IL-18, and this occurred even when the CD1d+ APCs were no longer present. Importantly, this effect was dependent on ERK activation during exposure to the CD1d+ APCs. These results are consistent with previous studies showing that IL-12 and IL-2 or IL-18 stimulate IFN-γ production by NKT cells and that concurrent recognition of CD1d is not necessarily required.34,35,45 However, our findings indicate that the responsiveness of NKT cells to stimulation by IL-12 and IL-18 is the result of prior TCR-dependent activation that can occur through autoreactive contact with CD1d+ APCs.
We also observed that human NKT cells can form tight conjugates with CD1d+ APCs in the absence of added antigens but do not undergo migration arrest unless a potent TCR agonist is present. This is consistent with recent findings that murine NKT cells actively traffic along liver sinusoids in vivo until α-GalCer is introduced, which rapidly brings about migration arrest.48 Interestingly, NKT cells patrolling liver sinusoids also underwent migration arrest on addition of IL-12 and IL-18, similar to the effect observed from addition of a microbial glycolipid antigen.49 The induction of cytoplasmic calcium flux is thought to be required for T-cell migration arrest; however, although we detected calcium flux in response to a microbial antigen, in our system human NKT cells showed no flux in response to IL-12 and IL-18. Hence, it is possible that certain physiologic APCs, perhaps including those present in liver sinusoids, are better able to induce NKT cell calcium flux than the cells used in our experiments. However, as the migration arrest observed in vivo after addition of IL-12 and IL-18 appeared only partially dependent on recognition of CD1d,49 it is also possible that these cytokines can cause arrest by a different mechanism, such as modulation of the expression levels of adhesion ligands.
In contrast to its potentiating effect on responsiveness to IL-12 and IL-18, we found that preexposure of NKT cells to CD1d+ APCs resulted in reduced responses to subsequent stimulation by α-GalCer. This finding is similar to results from an analysis of transgenic mice that express CD1d only on cortical thymocytes, but not on peripheral APCs.50 In these mice, NKT cells appear to be selected normally in the thymus but do not receive autoreactive stimulation on exiting to the periphery. The NKT cells from these mice showed stronger cytokine responses to α-GalCer–pulsed CD1d+ DCs than those from normal control mice, suggesting that exposure to autoreactive stimulation in vivo had diminished the NKT-cell responses in the control mice.50 Thus, although the autoreactivity of NKT cells may prime them for rapid innate cytokine production, it may also serve to raise the TCR signaling threshold required for responses to antigenic stimulation. This may help to limit the physiologic effects of these highly potent cells during stimulation by microbial antigens. The results presented here provide new insight into how NKT-cell cytokine responses are determined and will serve as a basis for future studies to understand NKT-cell activation defects that occur in human diseases, such as cancer or autoimmune diabetes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Zhu Yiming and Dr Paul Bertics for technical assistance and Dr Lloyd Klickstein for providing reagents.
This work was supported by NIH grants R01 AI060777 (J.E.G.) and AI45889 (R.M.N., A.R.H.), a Royal Society Wolfson Research Merit Award (G.S.B.), and NIH grant AI68062 (A.H.).
National Institutes of Health
Authorship
Contribution: X.W. designed experiments, performed research, and analyzed data; X.C., R.M.N., N.V., D.G., A.R.H., G.S.B., and G.F.P. contributed reagents; L.R., W.S., and S.W. provided technical assistance and expertise; A.H. provided technical expertise; J.E.G. designed experiments, interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jenny E. Gumperz, Department of Medical Microbiology and Immunology, Microbial Sciences Building, 1550 Linden Drive, Madison, WI 53706; e-mail: jegumperz@wisc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal