Abstract
Minimal residual disease (MRD) assessment is standard in many hematologic malignancies but is considered investigational in multiple myeloma (MM). We report a prospective analysis of the prognostic importance of MRD detection by multiparameter flow cytometry (MFC) in 295 newly diagnosed MM patients uniformly treated in the GEM2000 protocol VBMCP/VBAD induction plus autologous stem cell transplantation [ASCT]). MRD status by MFC was determined at day 100 after ASCT. Progression-free survival (PFS; median 71 vs 37 months, P < .001) and overall survival (OS; median not reached vs 89 months, P = .002) were longer in patients who were MRD negative versus MRD positive at day 100 after ASCT. Similar prognostic differentiation was seen in 147 patients who achieved immunofixation-negative complete response after ASCT. Moreover, MRD− immunofixation-negative (IFx−) patients and MRD− IFx+ patients had significantly longer PFS than MRD+ IFx− patients. Multivariate analysis identified MRD status by MFC at day 100 after ASCT as the most important independent prognostic factor for PFS (HR = 3.64, P = .002) and OS (HR = 2.02, P = .02). Our findings demonstrate the clinical importance of MRD evaluation by MFC, and illustrate the need for further refinement of MM re-sponse criteria. This trial is registered at http://clinicaltrials.gov under identifier NCT00560053.
Introduction
In most hematologic malignancies, response to front-line therapy is a good predictor of prognosis, with the longest survival seen in patients achieving an optimal response. This paradigm is represented by chronic myeloid leukemia (CML), in which hematologic, cytogenetic, and molecular remissions define progressively better response to therapy. In consequence, investigations to define these levels of remission are mandatory in routine clinical practice for treatment stratification and assessment of prognosis.1 The situation is similar for other malignancies such as acute promyelocytic leukemia (APL) or acute lymphoblastic leukemia (ALL).2,3 For this reason, there are continuous efforts to improve the sensitivity of the methods used to assess response to therapy, mainly through the introduction and refinement of both molecular and immunophenotyping approaches, as well as imaging techniques.
Multiple myeloma (MM) should be no exception to this paradigm. For many years, the major goal of MM therapy was to achieve partial response (PR) or disease stabilization.4,5 With the introduction of high-dose therapy plus autologous stem cell transplantation (HDT/ASCT), the new goal became the achievement of complete response (CR), defined as absence of M-protein by immunofixation (IFx) and less than 5% plasma cells (PCs) in bone marrow (BM).6,7 More recently, the International Myeloma Working Group proposed a new response category of “stringent CR,” which requires normalization of the free light chain ratio and the absence of residual clonal cells in the BM by immunohistochemistry or immunofluorescence.8 As noted previously, the assessment of minimal residual disease (MRD), residual tumor cells persisting after therapy, is part of the standard of care in many hematologic malignancies, whereas in MM this is still considered investigational. Thus, MRD studies in MM have involved mainly small series of patients or have been retrospective studies, using molecular techniques such as either polymerase chain reaction (PCR)9-13 or multiparameter flow cytometry (MFC) immunophenotyping.9,14-16
Comparative analysis of PCR- versus MFC-based approaches shows that PCR is a more sensitive technique (10−6 vs 10−4),17,18 although a newer generation of 6- and 8-color MFC approaches may have sensitivities approaching or surpassing PCR.19 Moreover, MFC is more broadly applicable in the MM patient population than PCR. MFC involves the identification of phenotypic aberrancies in myelomatous plasma cells (MM-PCs) that are absent in normal plasma cells (N-PCs)18,20,21 ; aberrant phenotypes are seen in more than 90% of MM patients, whereas allele-specific oligonucleotide (ASO)–PCR is applicable in only approximately 75% of patients.9 Moreover, MFC is less time consuming, as ASO-PCR–based techniques require sequencing of CDR3 to design specific primers for follow up of individual patients.15,18,19,22
Here we report a prospective analysis of the prognostic importance of MRD detection by MFC in BM samples obtained at day 100 after ASCT in 295 MM patients uniformly treated according to the GEM2000 protocol. Our results show that attainment of MFC remission was associated with significantly longer progression-free survival (PFS) and overall survival (OS), and that MFC remission status was the most relevant independent prognostic factor for patient outcome.
Methods
Patients and treatment
The current study was preplanned to evaluate MRD after ASCT in patients uniformly treated according to the GEM2000 protocol.23 The treatment scheme included 6 alternating cycles of vincristine, carmustine, melphalan, cyclophosphamide, prednisone (VBMCP) and vincristine, carmustine, doxorubicin, dexamethasone (VBAD), followed by high-dose melphalan and ASCT. Criteria for entering into the MRD study were partial response (PR) or better following ASCT (N = 577) and BM sample available for immunophenotypic analysis both at diagnosis and at day 100 after ASCT. Nevertheless, it should be noted that for patients in PR with high residual tumor burden (M-component > 1 g/dL and/or BM with more than 5% PCs) clinicians usually did not send samples for MRD investigation. In total, 295 of the 577 patients who were at least in PR after ASCT underwent MRD investigation. To avoid selection bias, we compared the PFS of the 295 patients included in this study with that of the total 577 patients; no significant differences were observed between the 2 groups of patients, with a median PFS of 48 and 47 months, respectively. Written informed consent was obtained in accordance with the Declaration of Helsinki, as well as Institutional Review Board approval from each participating hospital.
Response assessment and immunophenotypic studies
Disease response was assessed at day 100 after HDT/ASCT according to the European Group for Blood and Marrow Transplant (EBMT) criteria,7 modified to include the category of near complete response (nCR; electrophoresis negative for M-protein, but IFx positive).24
BM samples were to be taken at diagnosis (baseline), and at day 100 after transplantation (n = 295). In addition, BM samples were obtained after induction but before HDT/ASCT in a subgroup of patients (n = 157). Erythrocyte-lysed whole BM samples were processed during the first 24 hours following aspiration and stained using a 4-color direct immunofluorescence technique. At diagnosis, the following monoclonal antibody combinations (FITC/PE/PerCP-Cy5.5/APC) were used for identification of PC phenotypic aberrancies to be used as patient-specific probes for assessment of MRD after therapy: CD38/CD56/CD19/CD45, CD138/CD28/CD33/CD38, and CD20/CD117/CD138/CD38.16 In addition, an aliquot stained only with CD38-APC was used as a negative control to evaluate PC autofluorescence levels. For MRD investigation, the CD38/CD56/CD19/CD45 combination differentiated residual MM-PCs from N-PCs in more than 90% of cases. For the remaining patients, 1 or 2 additional monoclonal antibody combinations were used, based on antigens such as CD28, CD117, CD33, or CD20 that were aberrantly expressed at diagnosis.
MFC immunophenotyping was performed in a FACSCalibur flow cytometer (BD Biosciences [BDB], San Jose, CA) with a double-step procedure previously described18 and the CellQUEST software (BDB). In the first step, 2 × 104 cells from the whole BM cellularity were measured. A second acquisition was then performed; a minimum of 3 × 105 BM cells were acquired, and information about PCs contained in a broad “live-gate” drawn to select CD38hi events was recorded and stored. The multiparameter strategy used to differentiate N-PCs from MM-PCs has been previously described by our group,16,18,25,26 in line with the recently reported recommendations of the European Myeloma Network.19 Briefly, PCs were initially identified based on CD38 expression and intermediate side scatter (SSC) signals. N-PCs were then distinguished from MM-PCs based on differential expression of CD38, CD19, CD45, and CD56, and light scatter characteristics. If differentiation could not be achieved, BM samples were further stained with CD28, CD117, CD33, and/or CD20. Data analysis was performed using Paint-A-Gate PRO software (BDB). Patients were considered to be in MFC remission when MM-PCs were undetectable in the BM sample at the MFC sensitivity limit of 10−4 (ie, < 1 MM-PC in 104 N-PCs).
Statistical analysis
PFS was measured from the start of chemotherapy to the date of progression, relapse from CR, or death. Patients who had not progressed or relapsed were censored on the last date they were known to be alive and without progression. OS was measured from the start of chemotherapy to the date of death or last visit. PFS and OS distribution curves were plotted using the Kaplan-Meier method; the log-rank test was used to estimate the statistical significance of differences observed between curves. A univariate analysis was conducted to assess the impact on PFS and OS of various prognostic factors, including age, International Staging System (ISS)27 disease stage, hemoglobin, serum albumin, serum creatinine, serum β2-microglobulin, the percentage of PCs in S-phase, and interphase fluorescent in situ hybridization (FISH) cytogenetics at baseline, plus IFx status and MRD status by MFC at day 100 after transplantation. The Cox regression proportional hazard model (stepwise regression) was used in a multivariate analysis to explore the independent effect of these variables on PFS and OS. Variables were retained in the model if they showed a statistically significant predictive value (P ≤ .05). For all statistical analyses, SPSS software (version 12.0; SPSS, Chicago, IL) was used.
Results
Patient characteristics and response to therapy
A total of 295 patients were included in the present analysis. Baseline demographics and disease characteristics are shown in Table 1. At day 100 after transplantation, 147 (50%) patients had achieved CR; 40 (14%), nCR; and 108 (36%), PR. At data cutoff for this analysis, 173 patients (59%) had relapsed/progressed and 91 (31%) had died; the median follow-up was 57 months (range: 3-127 months). Median PFS and OS for all 295 patients were 48 and 98 months, respectively.
Patient demographics and baseline characteristics
| . | No. . |
|---|---|
| Total no. | 295 |
| Male/female (%) | 158 (54)/137 (46) |
| Median age, y (range) | 59 (29-70) |
| Myeloma subtype (%) | |
| IgG | 161 (55) |
| IgA | 79 (27) |
| IgD | 2 (0.7) |
| IgM | 1 (0.3) |
| Bence Jones protein | 44 (15) |
| Nonsecretory | 8 (2) |
| Durie-Salmon disease stage I/II/III, (%) | 15 (5)/133 (45)/147 (50) |
| International Staging System disease stage I/II/III, (%) | 115 (39)/121 (41)/59 (20) |
| β2-microglobulin, mmol/L, median (range) | 26 (1-347) |
| Hemoglobin, g/L, median (range) | 109 (10-168) |
| Creatinine, mmol/L, median (range) | 97 (44-654) |
| Calcium, mmol/L, median (range) | 2.3 (0.6-4.5) |
| C-reactive protein, mg/dL, median (range) | 1.3 (0-148) |
| Albumin, g/L, median (range) | 37 (17-39) |
| % of plasma cells in S-phase, median (range) | 1.2 (0-11.5) |
| Cytogenetics, n = 97 | |
| IgH translocations, (%) | |
| t(4;14) | 8 (8) |
| t(11;14) | 14 (14) |
| t(14;16) | 5 (5) |
| Others | 5 (5) |
| Del(13q) (%) | 30 (31) |
| Del(17p) (%) | 5 (5) |
| High-risk, any t(4;14), t(14;16), or del(17p) (%) | 16 (16) |
| . | No. . |
|---|---|
| Total no. | 295 |
| Male/female (%) | 158 (54)/137 (46) |
| Median age, y (range) | 59 (29-70) |
| Myeloma subtype (%) | |
| IgG | 161 (55) |
| IgA | 79 (27) |
| IgD | 2 (0.7) |
| IgM | 1 (0.3) |
| Bence Jones protein | 44 (15) |
| Nonsecretory | 8 (2) |
| Durie-Salmon disease stage I/II/III, (%) | 15 (5)/133 (45)/147 (50) |
| International Staging System disease stage I/II/III, (%) | 115 (39)/121 (41)/59 (20) |
| β2-microglobulin, mmol/L, median (range) | 26 (1-347) |
| Hemoglobin, g/L, median (range) | 109 (10-168) |
| Creatinine, mmol/L, median (range) | 97 (44-654) |
| Calcium, mmol/L, median (range) | 2.3 (0.6-4.5) |
| C-reactive protein, mg/dL, median (range) | 1.3 (0-148) |
| Albumin, g/L, median (range) | 37 (17-39) |
| % of plasma cells in S-phase, median (range) | 1.2 (0-11.5) |
| Cytogenetics, n = 97 | |
| IgH translocations, (%) | |
| t(4;14) | 8 (8) |
| t(11;14) | 14 (14) |
| t(14;16) | 5 (5) |
| Others | 5 (5) |
| Del(13q) (%) | 30 (31) |
| Del(17p) (%) | 5 (5) |
| High-risk, any t(4;14), t(14;16), or del(17p) (%) | 16 (16) |
The median overall level of PCs (N-PCs plus MM-PCs) seen in MFC immunophenotypic analyses of BM samples obtained at day 100 after transplantation was 0.26% (mean 0.57% ± 1.33% single standard deviation). Persistent MM-PCs were detected by MFC in 170 patients (58%), who were considered MRD-positive and had a median of 0.14% phenotypically aberrant PCs (range: 0.01%-4%). MM-PCs were absent (< 10−4) and only N-PCs were detected in the remaining 125 patients (42%), who were determined to be MRD negative (ie, in MFC remission). As expected, the percentages of abnormal PCs detected by MFC were significantly different in patients achieving CR, nCR, and PR after transplantation (mean 0.10% vs 0.21% vs 0.76%, respectively; P = .001).
Prognostic impact on PFS and OS of MRD status by MFC at day 100 after ASCT
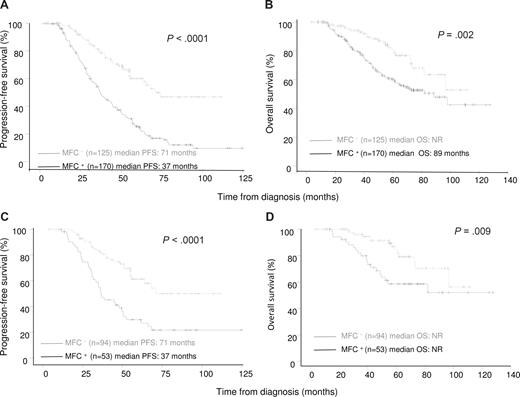
Patients who were MRD negative at day 100 after transplantation had significantly longer PFS (median 71 vs 37 months; P < .001; Figure 1A) and OS (median not reached vs 89 months; P = .002; Figure 1B) compared with patients who were MRD positive. The 5-year PFS rate was 60% in MRD-negative patients versus 22% in MRD-positive patients (P < .001), and the respective 5-year OS rates were 82% and 60% (P = .002).
Progression-free survival and overall survival according to the presence or absence of MM-PCs in the bone marrow at day 100 after ASCT. (A,B) Progression-free survival (PFS) and overall survival (OS) for all patients included in the present analysis (N = 295). (C,D) PFS and OS among the subset of patients achieving CR (n = 147).
Progression-free survival and overall survival according to the presence or absence of MM-PCs in the bone marrow at day 100 after ASCT. (A,B) Progression-free survival (PFS) and overall survival (OS) for all patients included in the present analysis (N = 295). (C,D) PFS and OS among the subset of patients achieving CR (n = 147).
Furthermore, MRD status by MFC at day 100 after ASCT was prognostic for PFS and OS within the subset of 147 patients who achieved CR (IFx negative for M-protein, < 5% PCs in BM by conventional cytomorphology). The 5-year PFS rate was 62% in MRD-negative patients (n = 94) versus 30% in MRD-positive patients (n = 53; P < .001; Figure 1C), and the respective 5-year OS rates were 87% versus 59% (P = .009; Figure 1D). We also analyzed the influence of MRD status on PFS and OS landmarked from day 100 after transplantation, and both PFS and OS remained significantly longer in MRD-negative patients (data not shown).
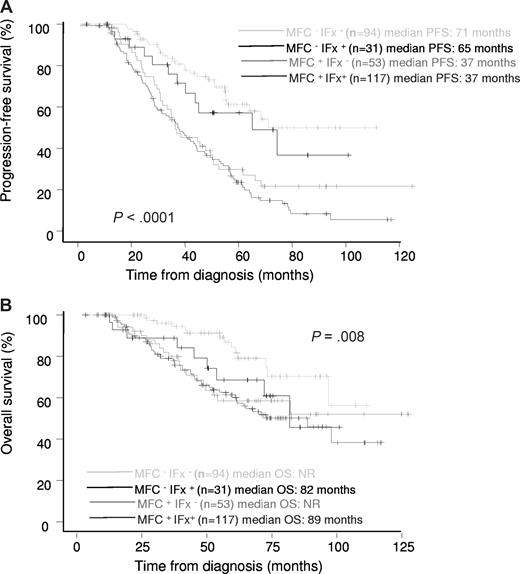
A proportion of patients who were IFx positive for M-protein (response of nCR or less) were nevertheless MRD negative by MFC (n = 31; 21%). Median PFS and OS in these patients were 65 months and 82 months, respectively. PFS was significantly longer in MRD-negative/IFx-negative and MRD-negative/IFx-positive patients versus MRD-positive/IFx-negative patients (median 71, 65, and 37 months, respectively, P = .001; Figure 2A), showing MRD status by MFC to be significantly more important than IFx status. OS among these groups of patients is shown in Figure 2B.
Prognostic influence of MRD status by MFC and IFX status at day 100 after ASCT. Progression-free survival (A) and overall survival (B) among specific risk groups of patients.
Prognostic influence of MRD status by MFC and IFX status at day 100 after ASCT. Progression-free survival (A) and overall survival (B) among specific risk groups of patients.
Prognostic factors for PFS and OS by univariate and multivariate analysis
On univariate analysis, 5 factors in addition to MRD positivity by MFC were identified as having a significant adverse impact on PFS (Table 2): high-risk cytogenetics (any t(4;14), t(14;16), or del (17p); P = .03), a high percentage of S-phase MM-PCs (> 1.5%; P = .004), elevated baseline serum creatinine level (> 176.8 μM [2 mg/dL]; P = .02), low baseline albumin level (< 35 g/L [3.5 g/dL]; P = .04), and IFx-positive status at day 100 after ASCT (P = .001). For OS, 7 factors in addition to MRD positivity by MFC were identified as having a significant adverse impact (Table 2): age (> 60 years; P = .02), elevated baseline serum β2-microglobulin (> 29 nmol/L [3.5 mg/L]; P = .001) and creatinine (> 176.8 μM [2 mg/dL]; P = .005), low baseline hemoglobin (≤ 100 g/L; P = .001) and albumin (< 35 g/L [3.5 g/dL]; P = .002), advanced disease (ISS stage III; P = .005), and IFx-positive status at day 100 after ASCT (P = .015).
Clinical disease features with a significant impact on PFS and/or OS by univariate analysis, plus independent prognostic impact for PFS and/or OS by multivariate analysis
| Prognostic factor . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|
| PFS . | OS . | PFS . | OS . | |||
| Median, mo . | P . | Median, mo . | P . | P . | P . | |
| Age, y | ||||||
| 60 or younger | 48 | NR | ||||
| Older than 60 | 44 | >.1 | 73 | .02 | – | .04 |
| ISS disease stage | ||||||
| I | 54 | NR | ||||
| II | 45 | 98 | ||||
| III | 36 | >.1 | 55 | .005 | – | >.1 |
| Hemoglobin, g/L | ||||||
| More than 100 | 53 | NR | ||||
| 100 or less | 39 | .08 | 73 | <.001 | – | >.1 |
| Serum albumin, g/L | ||||||
| More than 35 | 51 | NR | ||||
| 35 or less | 38 | .04 | 72 | .002 | >.1 | >.1 |
| Serum creatinine, mmol/L | ||||||
| 176.8 or less (2 mg/dL) | 49 | 98 | ||||
| More than 176.8 (2 mg/dL) | 36 | .02 | 54 | .005 | >.1 | >.1 |
| Serum β2-microglobulin, nmol/L | ||||||
| 29 or less (3.5 mg/L) | 54 | NR | ||||
| More than 29 (3.5 mg/L) | 41 | .07 | 71 | <.001 | – | >.1 |
| % plasma cells in S-phase | ||||||
| 1.5 or less | 54 | 98 | ||||
| More than 1.5 | 37 | .004 | 82 | >.1 | >.1 | – |
| Interphase FISH cytogenetics | ||||||
| Standard-risk | 44 | NR | ||||
| High-risk* | 28 | .035 | 54 | .09 | .006 | – |
| MRD status by immunofixation | ||||||
| Negative | 55 | NR | ||||
| Positive | 40 | <.001 | 89 | .015 | >.1 | >.1 |
| MRD status by MFC | ||||||
| CR | 71 | NR | ||||
| No CR | 37 | <.001 | 89 | .002 | .002 | .02 |
| Prognostic factor . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|
| PFS . | OS . | PFS . | OS . | |||
| Median, mo . | P . | Median, mo . | P . | P . | P . | |
| Age, y | ||||||
| 60 or younger | 48 | NR | ||||
| Older than 60 | 44 | >.1 | 73 | .02 | – | .04 |
| ISS disease stage | ||||||
| I | 54 | NR | ||||
| II | 45 | 98 | ||||
| III | 36 | >.1 | 55 | .005 | – | >.1 |
| Hemoglobin, g/L | ||||||
| More than 100 | 53 | NR | ||||
| 100 or less | 39 | .08 | 73 | <.001 | – | >.1 |
| Serum albumin, g/L | ||||||
| More than 35 | 51 | NR | ||||
| 35 or less | 38 | .04 | 72 | .002 | >.1 | >.1 |
| Serum creatinine, mmol/L | ||||||
| 176.8 or less (2 mg/dL) | 49 | 98 | ||||
| More than 176.8 (2 mg/dL) | 36 | .02 | 54 | .005 | >.1 | >.1 |
| Serum β2-microglobulin, nmol/L | ||||||
| 29 or less (3.5 mg/L) | 54 | NR | ||||
| More than 29 (3.5 mg/L) | 41 | .07 | 71 | <.001 | – | >.1 |
| % plasma cells in S-phase | ||||||
| 1.5 or less | 54 | 98 | ||||
| More than 1.5 | 37 | .004 | 82 | >.1 | >.1 | – |
| Interphase FISH cytogenetics | ||||||
| Standard-risk | 44 | NR | ||||
| High-risk* | 28 | .035 | 54 | .09 | .006 | – |
| MRD status by immunofixation | ||||||
| Negative | 55 | NR | ||||
| Positive | 40 | <.001 | 89 | .015 | >.1 | >.1 |
| MRD status by MFC | ||||||
| CR | 71 | NR | ||||
| No CR | 37 | <.001 | 89 | .002 | .002 | .02 |
C-reactive protein level, serum calcium level, and % of bone marrow plasma cells (≥20%) by morphology had no significant impact on PFS and OS.
ISS indicates International Staging System; FISH, fluorescence in situ hybridization; MRD, minimal residual disease; MFC, multiparameter flow cytometry; and CR, complete response.
High-risk cytogenetics includes any t(4;14), t(14;16), and del(17p); standard-risk cytogenetics includes all other cases.
Notably, MRD status by MFC significantly differentiated patients with more favorable (MRD negative) or less favorable (MRD positive) prognosis for PFS within the low-risk groups of patients with less than 1.5% S-phase MM-PCs (P < .001), β2-microglobulin less than 29 nmol/L (3.5 mg/L; P = .001), and ISS stage I disease (P = .013). The prognostic value of MRD status was also evaluated in different cytogenetic risk subgroups, although FISH information was available from only 97 patients. Among standard-risk patients (absence of t(4;14), t(14;16), and del (17p); n = 81), those who were MRD negative had longer PFS and OS compared with MRD-positive patients (median PFS: not reached vs 37 months, P = .001; median OS: not reached for both groups, P = .05). Although the number of high-risk patients (t(4;14), t(14;16), or del(17p)) by FISH analysis was small (n = 16), interestingly those who achieved MRD-negative status also had longer PFS and OS compared with MRD-positive patients (median PFS: 30 vs 19 months, P = .07; median OS: 73 vs 35 months, P = NS).
By multivariate analysis, only MRD status by MFC at day 100 after ASCT and FISH cytogenetics were identified as independent prognostic factors for PFS, and only MRD status by MFC and age were identified for OS (Table 2). The relative risks of progression and death among MRD-positive versus MRD-negative patients were 3.64 (P = .002) and 2.02 (P = .02), respectively. The relative risk of progression among patients with high-risk versus standard-risk cytogenetics was 1.79 (P = .006), and the relative risk of death among patients aged older than 60 years versus younger patients was 1.63 (P = .04).
Prognostic impact on PFS and OS of MRD status before and after ASCT
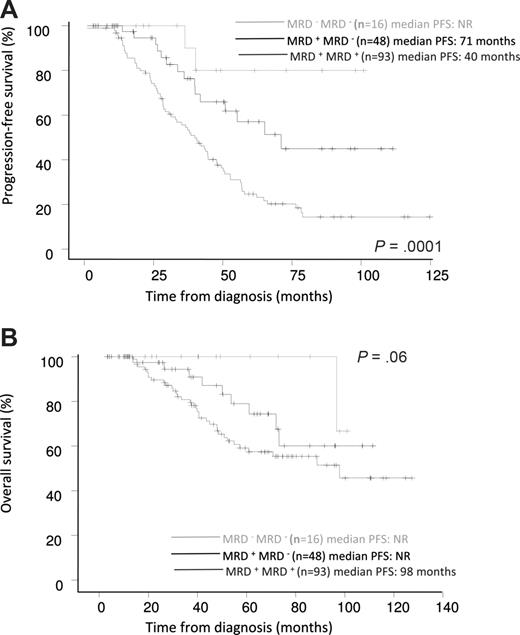
MRD information was available both before and after ASCT in a subgroup of 157 patients. PFS and OS were evaluated in 3 groups of patients defined according to their pattern of MRD status, reflecting their chemosensitivity over the course of treatment. Patients with the worst prognosis were “chemoresistant” patients who were persistently MRD positive (ie, those who were MRD positive both before and after transplantation; n = 93), who had a median PFS of 40 months. Patients who were MRD positive before ASCT but improved to MRD-negative after ASCT (n = 48) had an intermediate prognosis, with a median PFS of 71 months. Patients who achieved optimal response early, who were MRD negative both before and after transplantation (n = 16), had the best prognosis; median PFS was not reached in these patients. The 5-year PFS rates in these 3 prognostic subgroups were 25%, 57%, and 80%, respectively (P = .001; Figure 3A). Similarly, MRD status before and after ASCT determined differential prognosis for OS; the 5-year OS rates in the 3 subgroups were 59%, 78%, and 100%, respectively (P = .06; Figure 3B).
Prognostic influence of sequential MRD status by MFC before and after ASCT. (A) PFS and (B) OS (n = 157).
Prognostic influence of sequential MRD status by MFC before and after ASCT. (A) PFS and (B) OS (n = 157).
Discussion
The clinical relevance of MRD investigation has been well established in several hematologic malignancies such as ALL, acute myeloid leukemia, CML, chronic lymphocytic leukemia, and APL,1,28-34 and it is currently used routinely in clinical practice for risk stratification and treatment monitoring. In contrast, only preliminary results have been reported in MM, typically based on molecular monitoring of very small series of patients who underwent transplantation (N = 13-44).9-13 Despite this, these MM studies have shown that the level of residual clonal PCs in the BM correlates with patient outcome, suggesting that the information provided by MRD analysis in MM could be as valuable as in other hematologic malignancies.
Although molecular techniques such as PCR have greater sensitivity than conventional 4-color MFC, the latter is applicable to a greater proportion of the MM patient population, less time-consuming, and provides a sensitivity below the cutoff levels considered to be clinically relevant (10−4) with molecular techniques.9,15,17-19,22 To our knowledge, as yet only 3 small studies using MFC for investigation of MRD in MM have been reported. The first, by our group, included 87 patients randomized to either receive chemotherapy or undergo ASCT; we showed that the transplant arm was associated with lower levels of residual disease, which correlated with prolonged PFS, although the independent prognostic value of MFC MRD evaluation was not revealed.16 More recently, in a retrospective review of 47 patients, Liu et al14 showed that patients with low levels of MM-PCs prior to ASCT have a better outcome. Similarly, Rawstron et al15 and Davies et al35 showed that among 45 patients who underwent ASCT, those who remained MRD positive at 3 months after transplantation had shorter PFS than patients with undetectable disease. Moreover, these 3 studies show that MFC is more sensitive for MRD evaluation than IFx, probably due to the different kinetics associated with response to therapy.
In the present study, we confirm and extend these preliminary results and show for the first time in a prospective study, involving a large number of uniformly treated MM patients, that MRD status by MFC at day 100 after ASCT is the most relevant prognostic factor for MM patients. Specifically, patients with residual MM-PCs had significantly shorter PFS and OS compared with those with no detectable residual MM-PCs by MFC (ie, those who have attained MFC remission). It should be noted that in patients with poor response to ASCT and high residual tumor burden (M-component > 1 g/dL and/or BM with more than 5% PCs) clinicians usually did not send samples for MRD investigation. This is the reason why a high proportion of the patients included in the present analysis were in CR or nCR, and also why the median OS for the total population in this analysis is longer than typically observed for the overall MM population.
One of the most interesting aspects of the present study was the prognostic importance of MRD investigation in patients who achieved CR by EBMT criteria. The presence or absence of MRD by MFC clearly defined 2 subgroups of patients with significantly different PFS and OS. This indicates that MFC remission is a more sensitive prognostic criterion than CR by IFx, supporting the need for further refinement of MM response criteria to reflect the fact that the better the quality of response, the longer the survival, as reported for other hematologic malignancies. In fact, in a practical clinical approach, MRD investigations by MFC should be restricted to patients in CR or nCR by electrophoresis, since the majority of PR patients will be MRD positive and accordingly the test would not be cost effective.
The superiority of MFC remission over other prognostic factors was confirmed by multivariate analysis, in which MFC remission plus cytogenetics or age at diagnosis represented the best combinations of independent variables for predicting PFS and OS, respectively. Furthermore, based on MRD status by MFC, we could also differentiate 2 prognostic subgroups among patients with standard-risk cytogenetics as well as among the small group of patients displaying high-risk cytogenetics. These findings support the notion that achievement of MFC remission is an important goal even among patients with high-risk cytogenetics.
An interesting question raised by our study is why, despite MFC being more sensitive than IFx, a small proportion of patients who were MRD negative by MFC remained IFx positive. Two possible hypotheses are that: (1) the long half-life of some immunoglobulins may result in a positive biochemical marker being maintained on IFx despite the absence of MM-PCs producing M-protein; or (2) either the BM sample obtained for MFC is not representative of the overall BM status, or there are residual PCs outside of the BM. Whatever the explanation, it is important to note that these MFC-negative/IFx-positive patients had a very good outcome, similar to MFC-negative/IFx-negative patients and significantly better than MFC-positive/IFx-negative patients.
An additional important finding of our study was the influence on PFS and OS of MRD status by MFC over the course of therapy, reflecting the concept of treatment chemosensitivity. Our results show that early responders, who were already MRD negative after induction and prior to HDT/ASCT, as well as patients with continued chemosensitivity, who improved from being MRD positive before transplantation to MRD negative after ASCT, had significantly better outcomes than “MFC chemoresistant patients,” who remained MRD positive before and after ASCT, independent of IFx status. Such chemoresistant patients should be considered a high-risk subgroup and candidates for experimental therapies.
Finally, a comment should be made about the sensitivity of the new techniques used for evaluating response to therapy in MM. The International Myeloma Working Group recently proposed the addition of the “stringent CR” response category, which requires normalization of the free light chain ratio and the absence of residual clonal cells in BM by immunofluorescence or immunohistochemistry. These latter techniques have lower sensitivity (10−2-10−3) compared with MFC (10−4),19 and therefore should be considered suboptimal for MRD assessment. Moreover, with the introduction of 8-color clinical flow cytometers, the sensitivity of immunophenotyping may reach a level similar to, if not greater than, that of ASO-PCR (10−5-10−7), and MFC will represent the method of choice for routine MRD investigations in MM, due to its greater applicability, sensitivity, and speed, with results typically obtained within a few hours. The limitations of MFC evaluation of MRD in follow-up BM samples in MM are the patchy pattern of BM infiltration and the frequency of extramedullary relapses, which cannot be assessed by this approach, and for which imaging techniques may be of value. The recent availability of new imaging tools, such as magnetic resonance imaging (MRI) and positron emission tomography (PET), has shown that MM patients frequently present with macrofocal disease, either with or without a diffuse component, that could harbor nonsecretory MM cells.36 Accordingly, future MRD investigation should probably also include imaging-defined CR. In this context, it would be very important to investigate the clonality of the cells present in these residual focal lesions by fine-needle aspiration.
In summary, our results show that MRD evaluation by MFC is a very useful technique to identify patients at different risk of progression. These patients may benefit from different therapeutic approaches. This type of analysis, particularly when performed after ASCT, may contribute to the design of patient-specific maintenance treatment approaches, as well as the evaluation of the potential benefits of consolidation therapies.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by the Cooperative Research Thematic Network (RTICs; RD06/0020/0006), MM Jevitt, SL firm, and Instituto de Salud Carlos III/Subdirección General de Investigación Sanitaria (FIS: PI060339; 02/0905; 01/0089/01-02; and PI060339; Madrid, Spain).
Authorship
Contribution: J.F.S.M., J.J.L., and A.O. conceived the idea and, together with M.-B.V., designed the study protocol; B.P., M.-B.V., J.C., G.M., J.J.P., M.A.M., M.C.L.-B., and L.M. analyzed the flow cytometry data; A.S., N.C.G., A.G.C., N.H., M.V.M., R.G.-B., J.G., J.H., L.P., D.C., R.M., J.R., A.M., J.J.L., and J.B. contributed with provision of study material or patients; B.P., M.-B.V., J.C., and G.M. collected and assembled data; B.P., M.-B.V., J.C., and J.F.S.M. analyzed and interpreted data; B.P. and M.-B.V. performed statistical analysis; B.P., M.-B.V., and J.F.S.M. wrote the paper; and all authors reviewed and approved the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jesús F. San-Miguel, Hospital Universitario de Salamanca, Paseo de San Vicente 58-182, 37007 Salamanca, Spain; e-mail: sanmigiz@usal.es.
References
Author notes
*B.P. and M.-B.V. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal