WAS is an X-linked immunodeficiency disease caused by mutations in WASp.1 Meyer-Bahlburg and colleagues and Westerberg and colleagues in this issue of Blood have investigated lymphocyte subpopulations in WASp+/- mice and found that WASp confers a selective advantage to the most mature T and B cells.
The Wiskott-Aldrich syndrome protein (WASp) is an important cytoskeletal regulator expressed in hematopoietic cells. WASp and its relative, the ubiquitously expressed neural WASp, participate in the regulation of actin polymerization through activation of the Arp2/3 complex. In patients with Wiskott-Aldrich syndrome (WAS), the WASP gene is mutated, leading to low or no WASp expression and varying degrees of clinical symptoms, such as immunodeficiency, eczema, and thrombocytopenia. Absence of WASp affects migration, adhesion, and activation of neutrophils, platelets, macrophages, dendritic cells, natural killer (NK) cells, and T and B cells.1
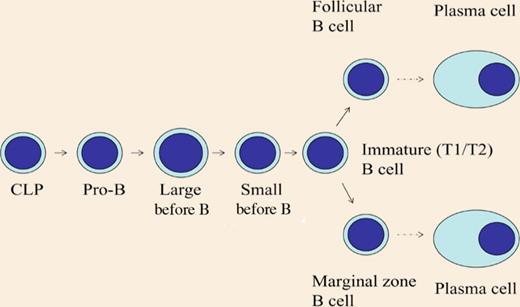
Differentiation of B cells. CLP indicates common lymphoid progenitor. WASp is most important in the more mature B-cell subpopulations.
Differentiation of B cells. CLP indicates common lymphoid progenitor. WASp is most important in the more mature B-cell subpopulations.
The WASP gene is located on the X chromosome. Random X chromosome inactivation in WASp+/- mice would theoretically result in 50% of cells expressing WASp. Westerberg et al show this to be the case in the myeloid compartment, that is, neutrophils, dendritic cells, macrophages, and NK cells. Also, both papers show that in the most immature subsets of T and B cells, such as the double-negative or double-positive thymocytes and the pro-B, pre-B, and immature B cells in the bone marrow, there is no selective advantage for WASp expression. In contrast, in the more mature T and B cells, WASp positive cells had a strong selective advantage (the different stages of B-cell differentiation are shown in the figure). As differentiation proceeds, the advantage of WASp+ cells increases. The strongest advantage for WASp expression was found in regulatory T cells and natural killer T cells in spleen and thymus, and in splenic marginal zone (MZ) B cells, in which at least 80% of the cells expressed WASp. In addition, WASp+ germinal center B cells had a more pronounced selective advantage than nongerminal center cells. In a particular subpopulation of B cells called B1 cells that reside in the peritoneal cavity, WASp expressing cells were dominant. Finally, in a WAS patient with a revertant mutation, there was evidence for selective advantage of mature peripheral B cells.
Meyer-Bahlburg et al investigated the presence of various subpopulations of B cells in wild-type or WASp-deficient mice. No significant differences were observed in the early populations of pro-B cells to immature B cells, whereas the more mature B-cell populations, that is, IgD+ cells in the bone marrow, the follicular and MZ B cells in the spleen were reduced in numbers in mice lacking WASp. In addition, they found that in wild-type mice the mature B-cell subpopulations expressed relatively more WASp as compared with immature B cells.
With reconstitution experiments, the scientists concluded that the relative absence of the WASp negative MZ B cells was due to an intrinsic B-cell deficiency. They went on to analyze the capacity of the cells to divide and found that, surprisingly, the follicular and MZ WASp- B cells had an increased turnover rate as compared with wild-type cells. Thus, the deficiency in the more mature B-cell populations is due to an altered homeostasis and not to a differentiation defect. No evidence was found for an increased rate of apoptosis, but B cells were deficient in the capacity to generate LFA-1-ICAM-1–dependent adhesion complexes. Furthermore, MZ B cells showed impaired migration to sphingosine-1-phosphate. Both LFA-1 - ICAM-1 interactions and sphingosine-1-phosphate have been shown to be important for MZ B-cell positioning.2,3 This suggests that there is an inefficient localization of mature B cells to specific compartments in the absence of WASp. The cells try to compensate for this by increasing their proliferative rate. It is tempting to speculate that this leads to formation of lymphomas or autoimmune reactions, which are both known to develop in WAS patients.
WAS patients have increased susceptibility to bacterial infections, especially encapsulated pathogens.1 WASp-/- mice exhibit impaired responses to T-cell independent antigens.4 The MZ is situated in the outer border of the white pulp of the spleen and consists of B cells and macrophages. It is thought that it provides a first line of defense to blood-borne bacterial antigens. Thus, the papers by both sets of authors give important clues as to how immunodeficiency in WAS develops.
WAS is normally treated with stem cell transplantation. In certain cases, gene therapy might be an alternative. The papers by Meyer-Bahlburg et al and Westerberg et al imply that it is especially important to reconstitute the mature lymphocyte populations.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal