Abstract
Chronic myeloid leukemia (CML) with T315I mutation has been reported to have poor prognosis. We analyzed 27 patients with T315I, including 20 who developed T315I after imatinib failure (representing 11% of 186 patients with imatinib failure), and 7 of 23 who developed new mutations after second tyrosine kinase inhibitor (TKI). Median follow-up from mutation detection was 18 months. At the time of T315I detection, 10 were in chronic phase (CP), 9 in accelerated phase, and 8 in blast phase. Except for the lack of response to second TKIs (P = .002), there was no difference in patient characteristics and outcome between patients with T315I and those with other or no mutations. Patients in CP had a 2-year survival rate of 87%. Although the T315I mutation is resistant to currently available TKIs, survival of patients with T315I remains mostly dependent on the stage of the disease, with many CP patients having an indolent course.
Introduction
T315I affects a contact residue for most available tyrosine kinase inhibitors (TKIs), thus patients with chronic myeloid leukemia (CML) harboring this mutation are resistant to these agents.1-7 It has been suggested that therapy with second-generation TKIs (second TKI) might select for this mutation because of its inherent resistance.8-10 Some studies suggested that patients with T315I have a poor outcome, with median survival of 12.6 months from the start of imatinib therapy.11,12
The objectives of this study were to define the clinical characteristics of patients with T315I, and to assess their outcomes after imatinib failure.
Methods
Between June 2003 and March 2007, 186 patients (112 previously reported, including 13 with T315I) with CML were evaluated for mutations. Mutation analysis was done upon treatment failure as per the definitions of the European LeukemiaNet.13 Definitions of CML phases and responses were as previously described.13-15 All patients were treated following informed consent in accordance with the Declaration of Helsinki on M. D. Anderson Cancer Center institutional review board–approved protocols.
For mutational analysis screening, the entire kinase domain (KD) of the Bcr-Abl fusion transcript was sequenced using nested polymerase chain reaction (PCR). The Bcr-Abl fusion transcript was amplified followed by 2 separate PCR reactions that cover codons 221-390 and codons 350-500 of the Abl kinase domain, respectively.16 Standard dideoxy chain termination cycle sequencing was done using a 3100 or 3130 genetic analyzer (Applied Biosystems, Foster City, CA) with analysis using Seqscape v2.0 software (Applied Biosystems). Mutations were confirmed by sequencing of forward and reverse strands, with sensitivity of 10% to 20% mutation-bearing cells in the analyzed population. For analysis of T315I in follow-up samples, pyrosequencing was performed following this first-round PCR. PCR was performed using one biotin-tagged primer, with single-stranded PCR product isolated on strepavidin-sepharose beads (GE Healthcare, Little Chalfont, United Kingdom) and sequenced using nucleotide dispensation tips and Pyro Gold reagents on a HSQ96 Pyrosequencer (Biotage, Uppsala, Sweden). The sensitivity of pyrosequencing was 1% to 5% mutation-bearing BCR-ABL transcripts, depending on the initial levels of fusion transcript. The percentage of mutated clones by this method represents the ratio of mutated transcripts to unmutated transcripts determined by peak height of the pyrogram ×100.
Results and discussion
Ninety-five (51%) patients had mutations after imatinib failure and 23 after second TKI (ie, new mutations not present after imatinib failure). T315I was detected in 27 patients: 20 after imatinib failure (median 37 months from start of therapy), representing 11% of patients sequenced after imatinib failure and 21% of all mutations, and in 7 of 23 who developed new mutations after a median of 10 months on second TKI (4 dasatinib, 1 nilotinib, 1 bosutinib, 1 INNO-406), representing 5% of patients sequenced after second TKI and 30% of all mutations. Composite mutations occurred in 3 patients: besides T315I, one harbored E255V and G250E, one G250E and F317L, and one Y253F and E255K.
Except for lack of response to a second TKI (P = .002) and shorter time from diagnosis to start of imatinib (P = .017) for those with T315I, there was no significant difference in patient characteristics between the 3 groups (Table 1). The association of a shorter time from diagnosis to T315I could suggest the presence of a preexisting resistant clone.
Characteristics of patients with T315I mutation, other mutations, and no mutations
| Characteristics . | No mutation . | Mutation group . | P . | |
|---|---|---|---|---|
| T315I . | Other mutation . | |||
| Patients, no. (%) | 82 (44) | 27 (15) | 77 (41) | |
| Age, y (range) | 49 (11-96) | 52 (25-60) | 52 (17-80) | .489 |
| Female sex, no. (%) | 44 (54) | 9 (33) | 31 (40) | .097 |
| Prior therapy with interferon alpha, no. (%) | 42 (51) | 13 (48) | 51 (66) | .097 |
| Median time from diagnosis to treatment with imatinib, mo (range) | 11 (1-293) | 4 (1-175) | 24 (1-165) | .017 |
| CML phase at the start of imatinib, no. (%) | ||||
| Chronic | 63 (77) | 21 (78) | 61 (79) | .911 |
| Accelerated | 13 (16) | 4 (15) | 13 (17) | |
| Blastic | 6 (7) | 2 (7) | 3 (4) | |
| Best response to imatinib, no. (%) | ||||
| CHR | 35 (43) | 19 (70) | 39 (51) | .454 |
| MCyR | 34 (41) | 7 (26) | 31 (40) | |
| CCyR | 25 (30) | 5 (19) | 21 (27) | |
| Response duration, mo (range) | 45 (2-69) | 44 (1-60) | 49 (2-70) | .793 |
| CML phase at imatinib failure, no. (%) | ||||
| Chronic | 28 (34) | 10 (37) | 28 (36) | .575 |
| Accelerated | 30 (37) | 9 (33) | 35 (45) | |
| Blastic | 24 (29) | 8 (30) | 14 (18) | |
| Transformation to accelerated–blastic phase, no. (%) | 42 (51) | 15 (56) | 41 (53) | .919 |
| No. treated with second TKI | 76 | 14 | 75 | |
| Response to second TKI, no. (%) | ||||
| Hematologic | 64 (84) | 5 (36) | 61 (81) | .002 |
| Cytogenetic | 33 (43) | 0 (0) | 35 (47) | |
| No. dead (%) | 32 (39) | 11 (41) | 29 (38) | .958 |
| Median follow-up after imatinib failure, mo (range) | 24 (2-68) | 29 (4-55) | 29 (4-73) | .368 |
| Median time on imatinib, mo (range) | 28 (2-70) | 47 (2-60) | 35 (2-71) | .244 |
| Characteristics . | No mutation . | Mutation group . | P . | |
|---|---|---|---|---|
| T315I . | Other mutation . | |||
| Patients, no. (%) | 82 (44) | 27 (15) | 77 (41) | |
| Age, y (range) | 49 (11-96) | 52 (25-60) | 52 (17-80) | .489 |
| Female sex, no. (%) | 44 (54) | 9 (33) | 31 (40) | .097 |
| Prior therapy with interferon alpha, no. (%) | 42 (51) | 13 (48) | 51 (66) | .097 |
| Median time from diagnosis to treatment with imatinib, mo (range) | 11 (1-293) | 4 (1-175) | 24 (1-165) | .017 |
| CML phase at the start of imatinib, no. (%) | ||||
| Chronic | 63 (77) | 21 (78) | 61 (79) | .911 |
| Accelerated | 13 (16) | 4 (15) | 13 (17) | |
| Blastic | 6 (7) | 2 (7) | 3 (4) | |
| Best response to imatinib, no. (%) | ||||
| CHR | 35 (43) | 19 (70) | 39 (51) | .454 |
| MCyR | 34 (41) | 7 (26) | 31 (40) | |
| CCyR | 25 (30) | 5 (19) | 21 (27) | |
| Response duration, mo (range) | 45 (2-69) | 44 (1-60) | 49 (2-70) | .793 |
| CML phase at imatinib failure, no. (%) | ||||
| Chronic | 28 (34) | 10 (37) | 28 (36) | .575 |
| Accelerated | 30 (37) | 9 (33) | 35 (45) | |
| Blastic | 24 (29) | 8 (30) | 14 (18) | |
| Transformation to accelerated–blastic phase, no. (%) | 42 (51) | 15 (56) | 41 (53) | .919 |
| No. treated with second TKI | 76 | 14 | 75 | |
| Response to second TKI, no. (%) | ||||
| Hematologic | 64 (84) | 5 (36) | 61 (81) | .002 |
| Cytogenetic | 33 (43) | 0 (0) | 35 (47) | |
| No. dead (%) | 32 (39) | 11 (41) | 29 (38) | .958 |
| Median follow-up after imatinib failure, mo (range) | 24 (2-68) | 29 (4-55) | 29 (4-73) | .368 |
| Median time on imatinib, mo (range) | 28 (2-70) | 47 (2-60) | 35 (2-71) | .244 |
CP indicates chronic phase; AP, accelerated phase; BP, blastic phase; CHR, complete hematologic response; MCyR, major cytogenetic response; and CCyR, complete cytogenetic response.
The best response to TKI immediately preceding T315I (20 imatinib, 4 dasatinib, 1 nilotinib, 1 bosutinib, 1 INNO-406) was hematologic in 17 (63%) and cytogenetic in 10 (37%; Complete cytogenetic response [CCyR] 7, Partial cytogenetic response [PCyR] 1, minor cytogenetic response [mCyR] 2). The median duration of response was 44 months. The best response to imatinib for patients in CP (n = 21) before the detection of the T315I mutation was complete hematologic response (CHR) only in 13 (62%), Major cytogenetic response (MCyR) in 5 (24%; CCyR in 4). Among the 20 patients with T315I at the time of imatinib failure, 14 received a second TKI and 5 responded, all with hematologic response only (2 CHR, 2 partial hematologic response [PHR], 1 return to CP) with no change in the ratio of the mutated to unmutated transcript. In contrast, all 7 patients without T315I prior to second TKI (ie, T315I developed while on therapy with second TKI) responded to second TKI (1 major molecular response [MMR], 1 CCyR, 1 mCyR, 2 CHR, 2 PHR). Responses were usually transient (median 8 months) but 2 patients had sustained responses despite the presence of T315I. One patient in accelerated phase (AP) with F317L and G250E acquired T315I 5 months after starting nilotinib and achieved MMR sustained for 21 months and was then lost (MCyR at last follow-up). A second patient in AP acquired T315I 6 months after starting bosutinib and achieved mCyR that was sustained for more than 8 months. These patients had a decrease of mutated clone from 92% to 17%, and 75% to 45%, respectively.
Four patients had allogeneic stem cell transplantation (ASCT). Two responded: one (CP) achieved a complete molecular response (CMR) sustained for more than 24 months; the other (AP) achieved CCyR with persistence of residual disease by PCR and recurrence of T315I 9 months after ASCT while maintaining a CCyR. The other 2 patients (both AP) died of progressive disease 4 and 5 months after ASCT. Other patients received hydroxyurea (n = 5), investigational tyrosine kinase inhibitors with anti-T315I activity (T315I-TKI; n = 13), homoharringtonine (n = 7), chemotherapy (n = 3), and decitabine or lonafarnib (n = 1 each). Three patients had a decrease in the T315I clone: 2 treated with homoharringtonine had a decline in T315I clone from 69% to 3%, and from 95% to undetectable, and one treated with T315I-TKI had the T315I clone disappear for 6 months. None achieved a sustained cytogenetic response.
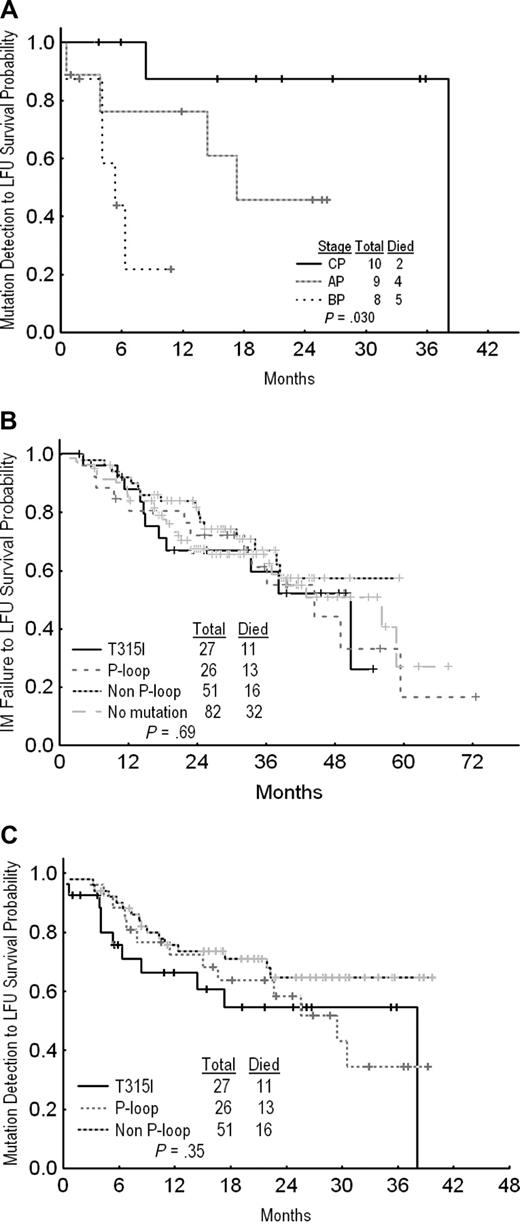
After a median follow-up of 29 months from imatinib failure and 18 months from detection of mutation, 11 of the 27 patients (40%) have died, including 2 of 10 (20%) in CP, 4 of 9 (44%) in AP, and 5 of 8 (68%) in blast phase (BP). In CP the median survival has not been reached, with 87% alive 2 years after T315I detection. In AP and BP, median survivals were 23 and 11 months, respectively, from imatinib failure, and 14 and 4 months, respectively, from detection of mutation (P = .03; Figure 1A). There was no difference in survival between patients with T315I, other mutations, or no mutations (Figure 1B,C). However, the cytogenetic response rate among patients with no mutations or other mutations (43% and 47%, respectively) was higher than for T315I cases, correlating with higher in vitro activity of second TKI against most other mutations.
Overall survival. (A) By CML phase from the time of T315I mutation detection. (B) Patients with T315I mutation versus P-loop mutations versus non–P-loop mutations versus no mutation from the time of imatinib failure. (C) Patients with T315I mutation versus P-loop mutation versus non–P-loop mutation from the time of mutation's detection.
Overall survival. (A) By CML phase from the time of T315I mutation detection. (B) Patients with T315I mutation versus P-loop mutations versus non–P-loop mutations versus no mutation from the time of imatinib failure. (C) Patients with T315I mutation versus P-loop mutation versus non–P-loop mutation from the time of mutation's detection.
In our study, patients with T315I detected in CP usually remained stable or responded to new19,20 and conventional agents such as hydroxyurea (5 patients). Furthermore, 2 patients with T315I detected in AP had a sustained cytogenetic response (1 CCyR, 1 mCyR) while on nilotinib and bosutinib, respectively. To our knowledge, these are the first reported sustained responses of T315I-bearing CML clones to second TKI, indicating that this mutation does not predict for resistance in all cases. Finally, patients with T315I clones had similar survival as patients with other or no mutations.
One other report has suggested a lack of impact of T315I mutation status on survival,21 while others suggested that this mutation confers a shorter survival.11,12 This difference could be explained by patient selection (eg, the proportion of patients in advanced stage). In a recent report, the median survival was 17 months for patients with advanced stage and 42 months for those in CP from the time of initiation of imatinib therapy.11 We show here that some patients with T315I may have an indolent disease and their survival is largely determined by the stage of the disease at the time of T315I.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.J. analyzed data and wrote and approved the manuscript; H.K. wrote and approved the manuscript; D.J. performed sequencing, analyzed data, and approved the manuscript; M.B. performed sequencing and approved the manuscript; G.G.M., S.O., F.R., and G.B. analyzed data and approved the manuscript; J.C. analyzed data and wrote and approved the manuscript.
Conflict-of-interest disclosure: J.C., D.J., and H.K. received research grants from Novartis and Bristol-Myers Squibb. All other authors declare no competing financial interests.
Correspondence: Jorge Cortes, Department of Leukemia, The University of Texas, M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Box 428, Houston, TX 77030; e-mail: jcortes@mdanderson.org.