Abstract
As B-cell chronic lymphocytic leukemia (B-CLL) progresses, malignant cells extravasate and infiltrate lymphoid tissues. Several molecules, including gelatinase B/MMP-9, contribute to these processes. Although mainly a secreted protease, some MMP-9 is present at the B-CLL cell surface and the function, mode of anchoring, and interactions of this MMP-9 are unknown. Here we show that anti–MMP-9 antibodies immunoprecipitated a 190-kDa CD44v isoform and α4β1 integrin from B-CLL cells, but not from normal B cells. Function-blocking antibodies to α4β1 or CD44, or transfection with specific siRNAs, decreased cell-associated proMMP-9 and increased the secreted form. B-CLL cells attached to and bound proMMP-9 and active MMP-9, and this was inhibited by blocking the expression or function of α4β1 or CD44. The MMP-9 hemopexin domain was critical in these interactions. α4β1 and 190-kDa CD44v (but not CD44H) formed a complex at the cell surface, since they both coimmunoprecipitated with anti-α4, anti-β1, or anti-CD44 antibodies. Immunofluorescence analyses confirmed that α4β1 and CD44v colocalized with MMP-9. Binding of proMMP-9 inhibited B-CLL cell migration, and this required MMP-9 proteolytic activity. Thus, we have identified α4β1 and CD44v as a novel proMMP-9 cell surface docking complex and show that cell-associated MMP-9 may regulate B-CLL cell migration and arrest.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) is characterized by the accumulation of CD5+ B lymphocytes in the peripheral blood.1,2 As the disease progresses, these cells infiltrate the bone marrow and secondary lymphoid tissue, resulting in poor prognosis.1,2 Several molecules that participate in B-CLL cell migration and invasion have been identified. These include the CCL21, CCL19, and CXCL12 chemokines,3,4 the vascular endothelial growth factor (VEGF),5 and the αLβ2 and α4β1 integrins.5,6
Other molecules known to play a role in cell migration are matrix metalloproteinases (MMPs).7-9 B-CLL cells produce and secrete progelatinase-B/proMMP-9 (92 kDa),10 and elevated intracellular levels of this MMP correlate with advanced stage and poor patient survival.11 We and others have shown that MMP-9 plays an important role in B-CLL transendothelial migration and invasion through basement membranes.6,11 We also recently showed that the constitutive levels of proMMP-9 in B-CLL cells are significantly higher than in normal B cells, and that secretion of proMMP-9 by B-CLL cells is up-regulated by α4β1 integrin, CXCR4, or CCR7 (the receptors for CXCL12 and CCL19/CCL21, respectively) engagement.6,12 Besides up-regulating proMMP-9 levels, α4β1 integrin ligation also induces the formation of podosomes in B-CLL cells and the localization of proteolytically active MMP-9 to these invasive structures.6
Although mainly present as a secreted soluble form, proMMP-9 has also been detected at the B-CLL cell surface by flow cytometry and cell biotinylation analyses.11 Indeed, we recently showed that MMP-9 is present at the B-CLL cell membrane fraction in apparently both the proform and the active form.6 Since MMP-9 does not have a transmembrane region, its association to the cell surface requires binding to cell membrane proteins.13 In other cell types, these proteins include CD44 (murine carcinoma and human melanoma cells),14,15 αLβ2 and αMβ2 integrins (neutrophils),16 the α2 chain of collagen IV (breast epithelial cells),17 and the membrane form of the DNA repair protein Ku (monocytic cells).18 Targeting MMP-9 to the cell surface is thought to localize and enhance its proteolytic activity at the pericellular space, and this has been demonstrated upon MMP-9 binding to CD44.15,19 In contrast, MMP-9 interaction with RECK (reversion-inducing-cysteine-rich protein with Kazal motifs) apparently inhibits the enzyme,20 and binding to LRP (low-density lipoprotein receptor-related protein) leads to MMP-9 internalization.21 These previous findings indicate that localization of MMP-9 at the cell surface is complex and appears to regulate multiple aspects of this enzyme.13
The function and molecular associations of MMP-9 at the B-CLL cell surface are not known. These are important issues to be clarified since MMP-9 may play a crucial role in the progression of B-CLL. In the present study, we show that MMP-9 (the pro and active forms) specifically binds α4β1 integrin and a high-molecular-weight variant of CD44 at the B-CLL cell surface. These 2 proteins thus constitute a novel membrane docking complex for MMP-9 in malignant cells. Moreover, we show that cell-bound MMP-9 may regulate B-CLL cell migration and arrest.
Methods
Patients and cells
Peripheral blood samples were collected after informed consent was obtained in accordance with the Declaration of Helsinki from 8 B-CLL patients (Table 1) and 2 mantle-cell lymphoma patients. CD5+ B lymphocytes were purified by Ficoll-Hypaque (Nycomed, Oslo, Norway) centrifugation and negative selection with anti–CD3-conjugated Dynabeads (Dynal Biotech ASA, Oslo, Norway). The resulting B-cell population was more than 95% CD19+ and more than 90% CD5+ (> 70% CD5+ for mantle B cells), as determined by flow cytometry (Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Tonsillar B lymphocytes were obtained from 2 adult individuals undergoing routine tonsillectomy and purified to more than 95% CD19+ and more than 75% CD5+ with anti-CD19– and anti-CD5–conjugated Dynabeads. B lymphocytes from 3 healthy donors (PB-BLs) were purified from buffy coat cells by Ficoll-Hypaque centrifugation and anti-CD19–conjugated Dynabeads, resulting in more than 92% CD19+ cells. The Epstein-Barr virus (EBV)–transformed HUT112 and BRO168 B-cell lines, established from normal B lymphocytes, have been previously reported.6 Human umbilical vein endothelial cells (HUVECs) were cultured as reported.6
Clinical characteristics of B-CLL patients
| Patient . | Sex/age, y . | Stage* . | CD38/ZAP-70† . | Ig status . |
|---|---|---|---|---|
| 1 | M/59 | B/II | +/+ | ND |
| 2 | M/65 | B/II | +/− | ND |
| 3 | F/84 | A/I | +/+ | ND |
| 4 | F/69 | B/II | +/− | ND |
| 5 | M/62 | B/II | +/+ | ND |
| 6 | M/80 | A/0 | −/− | Mutated |
| 7 | M/62 | A/0 | −/− | Mutated |
| 8 | M/78 | A/0 | +/+ | Unmutated |
| Patient . | Sex/age, y . | Stage* . | CD38/ZAP-70† . | Ig status . |
|---|---|---|---|---|
| 1 | M/59 | B/II | +/+ | ND |
| 2 | M/65 | B/II | +/− | ND |
| 3 | F/84 | A/I | +/+ | ND |
| 4 | F/69 | B/II | +/− | ND |
| 5 | M/62 | B/II | +/+ | ND |
| 6 | M/80 | A/0 | −/− | Mutated |
| 7 | M/62 | A/0 | −/− | Mutated |
| 8 | M/78 | A/0 | +/+ | Unmutated |
No patient received therapy.
ND indicates not determined.
Approval was obtained from the Consejo Superior de Investigaciones Científicas Bioethics Review Board for these studies. Additional materials and methods are available in Document S1.
Results
α4β1 Integrin and a high-molecular-weight CD44 isoform coimmunoprecipitate with proMMP-9 in B-CLL cells
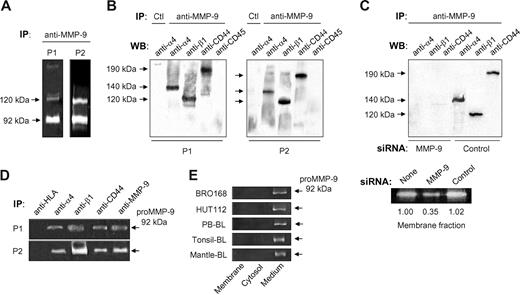
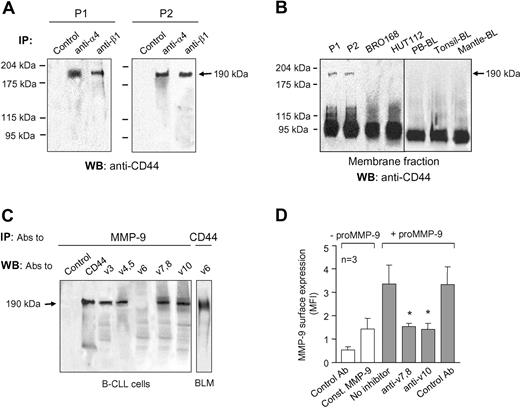
We recently showed that α4β1 integrin regulates proMMP-9 secretion and localization in podosomes in B-CLL cells.6 A fraction of the secreted proMMP-9 may bind to the cell surface as MMP-9 is consistently detected at the B-CLL cell membrane.6,11 We therefore investigated whether α4β1 integrin could interact with surface-associated MMP-9. B-CLL cells from 3 different patients were cultured for 24 hours and lysed, and lysates incubated with an anti–MMP-9 pAb bound to protein A–Sepharose. The presence of MMP-9 in the immunoprecipitates was confirmed by gelatin zymography. This analysis showed a major 92-kDa band, corresponding to proMMP-9 and a minor band of 120 kDa, likely representing the proMMP-9/lipocalin complex previously described in B-CLL cells11 (shown in Figure 1A for 2 representative patients). Further analysis of these immunoprecipitates by Western blotting revealed the presence of 2 bands of 140 kDa and 120 kDa, which corresponded to the α4 and β1 integrin subunits, respectively (Figure 1B). Identical results were obtained for patient 3 (not shown). The α4 (Figure 1B) or β1 (not shown) bands were not present in control immunoprecipitates in which rabbit IgG was incubated with the cell lysates. Although B-CLL cells also express αLβ2 integrin and, in some cases, α3β1, these integrins did not coimmunoprecipitate with proMMP-9 (results not shown).
proMMP-9 interacts with α4β1 integrin and a high-molecular-weight CD44 isoform in B-CLL cells. Lysates from 2 × 107 B-CLL cells cultured for 24 hours in RPMI/0.1% FCS were immunoprecipitated with anti–MMP-9 pAbs or with rabbit IgG (Ctl), and analyzed by gelatin zymography (A) and Western blotting (B). The results from 2 patients of the 3 analyzed with identical results are shown. (C) Lysates from 2 × 107 B-CLL cells transfected with MMP-9 or control siRNA were immunoprecipitated with anti–MMP-9 pAbs and analyzed by Western blotting. The efficiency of the siRNA transfection was confirmed by gelatin zymography. (D) B-CLL cell lysates were immunoprecipitated with the indicated Abs and analyzed by gelatin zymography. Anti-HLA and anti–MMP-9 Abs were used as negative and positive controls, respectively. (E) Gelatin zymographic analysis of the cell fractions and conditioned media of the indicated B-cell types, after 24-hour cell culture.
proMMP-9 interacts with α4β1 integrin and a high-molecular-weight CD44 isoform in B-CLL cells. Lysates from 2 × 107 B-CLL cells cultured for 24 hours in RPMI/0.1% FCS were immunoprecipitated with anti–MMP-9 pAbs or with rabbit IgG (Ctl), and analyzed by gelatin zymography (A) and Western blotting (B). The results from 2 patients of the 3 analyzed with identical results are shown. (C) Lysates from 2 × 107 B-CLL cells transfected with MMP-9 or control siRNA were immunoprecipitated with anti–MMP-9 pAbs and analyzed by Western blotting. The efficiency of the siRNA transfection was confirmed by gelatin zymography. (D) B-CLL cell lysates were immunoprecipitated with the indicated Abs and analyzed by gelatin zymography. Anti-HLA and anti–MMP-9 Abs were used as negative and positive controls, respectively. (E) Gelatin zymographic analysis of the cell fractions and conditioned media of the indicated B-cell types, after 24-hour cell culture.
Because CD44 is known to bind MMP-9 in several cell types,14,15,19 we also analyzed the presence of CD44 in the proMMP-9 immunoprecipitates of B-CLL cells. As shown in Figure 1B, the anti-CD44 Ab recognized a single band of 190 kDa, probably corresponding to a high-molecular-weight CD44 isoform (known as CD44v), while the standard 85/95-kDa CD44 (known as CD44H) was not detected (Figure 1B). Another membrane protein, CD45, was also absent in these immunoprecipitates (Figure 1B), confirming the specificity of the coimmunoprecipitation of proMMP-9 with α4β1 and 190-kDa CD44v. Identical results were obtained when proMMP-9 was immunoprecipitated from lysates of freshly isolated cells, which had not been in culture for 24 hours (Figure S1).
To further establish the specificity of the anti–MMP-9 Ab, we analyzed the lysates of B-CLL cells that had been transfected with either MMP-9 siRNA or a control siRNA. The anti–MMP-9 Ab did not immunoprecipitate the α4 or β1 integrin subunits or 190-kDa CD44v from MMP-9 siRNA–transfected cells, while these proteins were clearly visible in proMMP-9 immunoprecipitates from control siRNA–transfected cells (Figure 1C). Gelatin zymographic analysis confirmed that the MMP-9 siRNA reduced the amount of membrane-bound and secreted proMMP-9 by 3-fold, compared with untransfected or control siRNA–transfected cells (shown in Figure 1C for membrane-bound proMMP-9).
To then determine whether Abs to α4β1 integrin or CD44 pulled down proMMP-9, B-CLL cell lysates were incubated with specific Abs to α4, β1, or CD44, and the immunoprecipitates analyzed by gelatin zymography. As shown in Figure 1D, all 3 Abs pulled down proMMP-9, while anti-HLA Abs did not. The amount of proMMP-9 in these immunoprecipitates was similar to that pulled down by anti–MMP-9 Abs, used as positive control (Figure 1D).
To establish if the observed presence and interactions of surface-bound proMMP-9 also occurred in non–B-CLL B cells, normal peripheral blood B lymphocytes, BRO168 and HUT112 cells, CD5+ tonsillar B cells, and CD5+ mantle lymphoma B cells were cultured for 24 hours. The membrane and cytosolic fractions were then separated and analyzed by gelatin zymography. Figure 1E shows that proMMP-9 was clearly present in the conditioned media of these cells, but absent in their cellular fractions.
Blocking α4β1 or CD44 function with antibodies or peptides significantly reduces the levels of B-CLL cell surface–associated proMMP-9
To determine whether α4β1 and 190-kDa CD44v serve as surface receptors for proMMP-9, we first studied the effect of blocking α4β1 function on cell surface–bound proMMP-9. Equal numbers of cells from 3 different patients were incubated with either the HP2/1 mAb or the CS1 peptide, 2 well-known inhibitors of α4β1 function26-28 ; after 24 hours, the membrane and cytosolic cellular fractions were separated and analyzed by gelatin zymography. In agreement with our previous results,6 no proMMP-9 was detected in the cytosolic fraction (not shown). The levels of membrane-associated proMMP-9 were significantly reduced (P ≤ .01) after cell incubation with HP2/1 or CS1 (Figure S2A left panel). In contrast, the HP1/7 mAb, which does not block α4β1 function26 or the control peptide CS3,27,28 had no effect (Figure S2A).
To determine whether the reduced expression of membrane-bound proMMP-9 correlated with increased secreted levels of this MMP, we analyzed the conditioned media of the same 3 samples by gelatin zymography. As shown in Figure S2A (right panel), cell treatment with HP2/1 or CS1 significantly increased (P ≤ .05) the amount of secreted proMMP-9, while incubation with HP1/7 or CS3 had no effect.
We next performed similar experiments to study the effect of blocking CD44 function on the amount of surface-bound and secreted proMMP-9. As shown in Figure S2B, incubation of B-CLL cells (2 patients) with anti-CD44 mAb significantly reduced (P ≤ .05) membrane-associated proMMP-9 and increased (P ≤ .05) secreted proMMP-9. The control anti-HLA mAb had no effect (Figure S2B). None of the inhibitors of α4β1 or CD44 function affected the levels of MMP-9 mRNA, as confirmed by reverse transcription–polymerase chain reaction (RT-PCR; not shown). These results indicated that both α4β1 integrin and CD44 were important for maintaining proMMP-9 at the B-CLL cell membrane.
Knocking down the expression of the β1 integrin subunit or CD44 in B-CLL cells significantly reduces the amount of surface-bound proMMP-9 and increases the levels of secreted proMMP-9
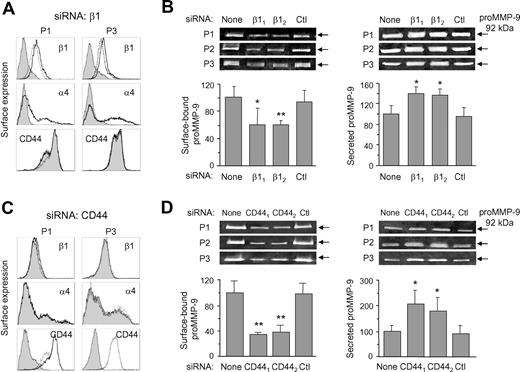
To confirm the results obtained by blocking the function of α4β1 or CD44, we transfected B-CLL cells (3 patients) with 2 different siRNAs for the β1 integrin subunit, since α4β1 is the major integrin in these cells,29,30 or for CD44, or with a control siRNA. siRNA transfection had a minor effect on B-CLL cell viability (shown in Figure S3 for 2 representative samples). Western blotting analyses confirmed that both β1 siRNAs significantly reduced (average 52% and 57% reduction, P ≤ .05, for siRNA1 and siRNA2, respectively, with similar results for all 3 samples) the total levels of β1, with respect to untransfected or control siRNA–transfected cells (Figure S4A). Likewise, both CD44 siRNAs significantly reduced (average 60%, P ≤ .01, and 67% reduction, P ≤ .001, for siRNA1 and siRNA2, respectively) the levels of CD44H, while 190-kDa CD44v was no longer detectable (Figure S4B).
Surface expression of the β1 and α4 integrin subunits was also effectively reduced by both β1 siRNAs (shown in Figure 2A for β1 siRNA2 and for 2 representative samples). This siRNA reduced the MFI average values from 7.8 to 2.2, and from 22.6 to 3.8, for the β1 and α4 subunits, respectively, representing a 71% and 80% reduction on the surface expression of these subunits. The control siRNA had no effect (Figure 2A). All 3 samples analyzed gave similar results. The levels of CD44 on β1 siRNA–transfected cells did not change (Figure 2A), indicating that siRNA transfection did not affect the expression of other membrane proteins. Concomitant with the blockage of α4β1 surface expression, zymographic analyses revealed that proMMP-9 levels were significantly reduced by both β1 siRNA1 and siRNA2 (P ≤ .05 and P ≤ .01, respectively) in the cell membrane fraction, while they were not affected by the control siRNA (Figure 2B). In contrast, and as previously observed by inhibiting α4β1 function, the levels of secreted proMMP-9 were significantly increased (P ≤ .05) on β1 siRNA–transfected cells (Figure 2B).
Effect of β1 integrin subunit or CD44 gene silencing on B-CLL cell membrane-bound and secreted proMMP-9. (A) B-CLL cells were transfected with control or β1 siRNA and analyzed by flow cytometry. Shaded areas represent β1 siRNA-cells; dotted lines, control siRNA cells; and continuous lines, untransfected cells. Representative results from 2 samples of the 3 studied are shown. (B) Gelatin zymographic analysis of proMMP-9 on the membrane fraction and the conditioned media of untransfected or siRNA-transfected B-CLL cells. β11 and β12 indicate the 2 different β1 siRNAs used. Ctl indicates control siRNA. Values represent the average of the 3 samples after normalizing the proMMP-9 levels of untransfected cells to 100. (C) Flow cytometric analysis of B-CLL cells (shown for 2 samples) transfected with control or CD44 siRNA. Line codes are as in panel A. (D) Gelatin zymography of proMMP-9 on the membrane fraction and the conditioned media of untransfected or siRNA-transfected B-CLL cells. CD441 and CD442 indicate the 2 different CD44 siRNAs used. Values represent the average of all 3 patients after normalizing proMMP-9 levels in untransfected cells to 100. *P ≤ .05; **P ≤ .01.
Effect of β1 integrin subunit or CD44 gene silencing on B-CLL cell membrane-bound and secreted proMMP-9. (A) B-CLL cells were transfected with control or β1 siRNA and analyzed by flow cytometry. Shaded areas represent β1 siRNA-cells; dotted lines, control siRNA cells; and continuous lines, untransfected cells. Representative results from 2 samples of the 3 studied are shown. (B) Gelatin zymographic analysis of proMMP-9 on the membrane fraction and the conditioned media of untransfected or siRNA-transfected B-CLL cells. β11 and β12 indicate the 2 different β1 siRNAs used. Ctl indicates control siRNA. Values represent the average of the 3 samples after normalizing the proMMP-9 levels of untransfected cells to 100. (C) Flow cytometric analysis of B-CLL cells (shown for 2 samples) transfected with control or CD44 siRNA. Line codes are as in panel A. (D) Gelatin zymography of proMMP-9 on the membrane fraction and the conditioned media of untransfected or siRNA-transfected B-CLL cells. CD441 and CD442 indicate the 2 different CD44 siRNAs used. Values represent the average of all 3 patients after normalizing proMMP-9 levels in untransfected cells to 100. *P ≤ .05; **P ≤ .01.
CD44 surface expression was also effectively blocked after CD44 siRNA2 (or siRNA1, not shown) transfection, with average MFI values reduced from 19.2 to 2.1, or 91% reduction, while the control siRNA had no effect (Figure 2C). The surface expression of the α4 or β1 integrin subunits was not affected by transfection with CD44 siRNAs (Figure 2C). Zymographic analysis of the cell membrane fraction showed that both CD44 siRNAs, but not the control siRNA, significantly (P ≤ .01) reduced the amount of surface-bound proMMP-9 (Figure 2D). This reduction correlated with a significant (P ≤ .05) increase of secreted proMMP-9 (Figure 2D).
To confirm these results, we studied whether surface-bound proMMP-9 levels were restored upon longer B-CLL cell culture, thus allowing for α4β1 and CD44 re-expression. Figure S5A shows that proMMP-9 levels were nearly fully recovered after 72 hours, in correlation with the surface re-expression of α4β1 and CD44 (Figure S5B).
B-CLL cells bind soluble proMMP-9 and attach to immobilized proMMP-9 via α4β1 and CD44; normal B cells do not bind or adhere to proMMP-9
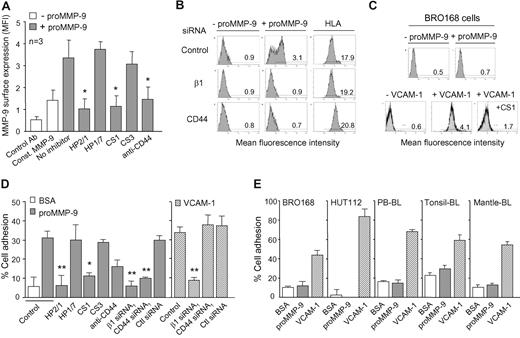
The preceding results indicated that both α4β1 integrin and 190-kDa CD44v coimmunoprecipitated with proMMP-9 and were necessary to localize proMMP-9 at the B-CLL cell surface. To further establish that a direct interaction existed between these 2 proteins and proMMP-9, we analyzed the binding of soluble proMMP-9 to B-CLL cells by flow cytometry. These analyses revealed that the constitutive surface expression of proMMP-9 increased 2.4-fold (average of 3 patients with similar results) upon incubating the cells with soluble proMMP-9 (Figure 3A). This indicated that soluble proMMP-9 could bind to the B-CLL cell surface. To determine whether α4β1 integrin and/or CD44 were involved in this binding, we incubated the cells with the HP2/1 mAb, the CS1 peptide, or an anti-CD44 Ab, prior to adding proMMP-9. Inhibiting the function of α4β1 or CD44 significantly (P ≤ .05) reduced the expression of surface-bound proMMP-9 to constitutive levels, while the control HP1/7 mAb or the inactive CS3 peptide had no effect (Figure 3A).
α4β1 integrin and CD44 mediate soluble proMMP-9 binding to B-CLL cells and adhesion of these cells to immobilized proMMP-9. (A) B-CLL cells, with or without previous incubation with the indicated Abs or peptides, were incubated for 30 minutes with or without proMMP-9 (10 μg/mL) and analyzed by flow cytometry using anti–MMP-9 pAbs. Const MMP-9 indicates constitutive MMP-9. (B) Identical binding experiments were performed with B-CLL cells transfected with the indicated siRNAs. HLA expression was also analyzed as a control for membrane integrity. A representative sample of the 3 studied with identical results is shown. (C) BRO168 cells were incubated with proMMP-9 or VCAM-1 and analyzed by flow cytometry. The binding of VCAM-1 was also measured after cell preincubation with the CS1 peptide. (D) BCECF-AM-labeled B-CLL cells (5 × 105; 3 patients), with or without previous incubation with the indicated Abs or peptides, or transfected with the indicated siRNAs, were added to wells coated with 10 μg/mL proMMP-9 or VCAM-1. After 90 minutes at 37°C, attached cells were quantitated using a fluorescence analyzer. Average values, each with duplicate determinations, represent the percentage of the total number of cells added. (E) The indicated BCECF-AM–labeled B cells were added to proMMP-9– or VCAM-1–coated wells and attached cells measured as explained. All determinations were done in triplicate and values represent the average of 3 (BRO168, HUT112, PBL-BL) or 2 (tonsillar and mantle lymphoma BL) independent experiments. Bar codes are as in panel D. *P ≤ .05; **P ≤ .01.
α4β1 integrin and CD44 mediate soluble proMMP-9 binding to B-CLL cells and adhesion of these cells to immobilized proMMP-9. (A) B-CLL cells, with or without previous incubation with the indicated Abs or peptides, were incubated for 30 minutes with or without proMMP-9 (10 μg/mL) and analyzed by flow cytometry using anti–MMP-9 pAbs. Const MMP-9 indicates constitutive MMP-9. (B) Identical binding experiments were performed with B-CLL cells transfected with the indicated siRNAs. HLA expression was also analyzed as a control for membrane integrity. A representative sample of the 3 studied with identical results is shown. (C) BRO168 cells were incubated with proMMP-9 or VCAM-1 and analyzed by flow cytometry. The binding of VCAM-1 was also measured after cell preincubation with the CS1 peptide. (D) BCECF-AM-labeled B-CLL cells (5 × 105; 3 patients), with or without previous incubation with the indicated Abs or peptides, or transfected with the indicated siRNAs, were added to wells coated with 10 μg/mL proMMP-9 or VCAM-1. After 90 minutes at 37°C, attached cells were quantitated using a fluorescence analyzer. Average values, each with duplicate determinations, represent the percentage of the total number of cells added. (E) The indicated BCECF-AM–labeled B cells were added to proMMP-9– or VCAM-1–coated wells and attached cells measured as explained. All determinations were done in triplicate and values represent the average of 3 (BRO168, HUT112, PBL-BL) or 2 (tonsillar and mantle lymphoma BL) independent experiments. Bar codes are as in panel D. *P ≤ .05; **P ≤ .01.
To confirm the above results, we performed similar binding experiments using B-CLL cells transfected with control, β1, or CD44 siRNAs. Figure 3B shows, for a representative patient, that gene silencing of any of these 2 proteins blocked soluble proMMP-9 binding to B-CLL cells, while the control siRNA had no effect. All 3 patients studied gave identical results, with average MFI values significantly decreasing (P ≤ .05) from 3.4 (untransfected or control siRNA–transfected cells) to basal levels (1.3). As a control for the integrity of the membrane after these transfections, HLA expression was similar for all 3 siRNA conditions (Figure 3B) and for untransfected cells (not shown). These results established that both α4β1 integrin and CD44 are required for soluble proMMP-9 binding to B-CLL cells. Further analyses of these interactions revealed that proMMP-9 remained bound to the cell surface for the 24 hours of the assay (Figure S6A), that binding was saturable (Figure S6B), and that it did not result in internalization of the α4β1/CD44/proMMP-9 complex (Figure S6C).
We then determined if non–B-CLL cells could also bind soluble proMMP-9. In agreement with the immunoprecipitation experiments shown in Figure 1E, BRO168 cells did not express membrane proMMP-9 constitutively (Figure 3C), despite their high expression of α4β1 integrin (not shown). Moreover, BRO168 cells (or PB-BLs, not shown) were unable to bind soluble proMMP-9, even after activation of the integrin with Mn2+ (Figure 3C). In contrast, Mn2+-treated BRO168 cells efficiently bound soluble VCAM-1, and this binding was inhibited by the CS1 peptide (Figure 3C), indicating that α4β1 was fully functional in these cells.
We next studied whether proMMP-9 could serve as an anchoring point for B-CLL cells and whether α4β1 and CD44 mediated this adhesion. B-CLL cells from 3 different patients attached to immobilized proMMP-9 (Figure 3D), and the percentage of adhesion (30%) was similar to that of cells attached to VCAM-1 (33%). Each individual sample gave similar results. Blocking the function of α4β1 integrin with HP2/1 or CS1, abolished cell adhesion to proMMP-9 (and to VCAM-1, not shown). In contrast, the control HP1/7 mAb or the CS3 peptide had no effect. Likewise, cell incubation with the anti-CD44 mAb diminished cell adhesion to proMMP-9 by 53% (Figure 3D). Moreover, knocking down the expression of α4β1 or CD44 by specific siRNA transfection significantly (P ≤ .01) reduced cell adhesion to proMMP-9 to the basal levels of adhesion to BSA (Figure 3D). In contrast, transfection with a control siRNA had no effect (Figure 3D). These results clearly indicated that proMMP-9 supported adhesion of B-CLL cells and that both α4β1 integrin and CD44 were required for this adhesion. Interestingly, this dual requirement appeared to be unique for adhesion to proMMP-9, since B-CLL cell adhesion to VCAM-1 was completely blocked by transfection with β1 siRNA, but was not affected by transfection with CD44 siRNA (Figure 3D).
To determine whether non–B-CLL B cells attached to immobilized proMMP-9, BRO168 and HUT112 cells, PB-BLs, and CD5+ tonsillar or mantle lymphoma B cells were used. All these cells attached well to VCAM-1 (Figure 3E), and adhesion was completely inhibited by HP2/1 or CS1 (not shown). In contrast, these cells did not attach to proMMP-9, and this was particularly striking for HUT112 cells, where adhesion to VCAM-1 was nearly 90% (Figure 3E). Altogether, these results suggested that the ability to use α4β1 and CD44 as receptors for immobilized or soluble proMMP-9 was a unique characteristic of B-CLL cells.
Further characterization of MMP-9 interaction with B-CLL cells
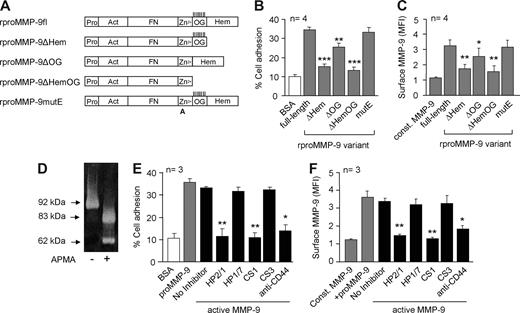
To determine which region of proMMP-9 was important for its interaction with B-CLL cells, we used recombinant proteins31 representing the full-length proMMP-9 (rproMMP-9fl) or several truncated forms (Figure 4A). B-CLL cells attached to and bound rproMMP-9fl efficiently (Figure 4B,C), and this was inhibited by the HP2/1 and anti-CD44 Abs (83% and 69% inhibition, respectively), but not by the HP1/7 Ab (not shown). Removal of the hemopexin domain (rproMMP-9ΔHem) or the hemopexin and O-glycosylated (OG) domains (rproMMP-9ΔHemOG) significantly reduced (P ≤ .001) the ability to support B-CLL cell adhesion (Figure 4B) and the binding to the cell surface (P ≤ .01) (Figure 4C) in all 4 patients studied. Removal of the OG domain alone had a minor although significant effect on cell adhesion (P ≤ .01) and soluble binding (P ≤ .05). Mutating the catalytic E residue (rproMMP-9mutE) did not affect the proMMP-9 cell binding property (Figure 4B,C). These results indicated that proMMP-9 interaction with the B-CLL cell surface involves the hemopexin domain and some contribution of the OG domain.
Further characterization of MMP-9 interaction with B-CLL cells. (A) Schematic domain structure of the recombinant full-length (fl) and proMMP-9 variants used (modified from 31 ). Pro indicates prodomain; Act, active site; FN, gelatin-binding fibronectin-like domain; Zn2+, Zn2+-binding domain; OG, O-glycosylated domain, with vertical lines indicating probable attachment sites for O-linked sugars; and Hem, hemopexin domain. A indicates that the catalytic Glu/E402 residue was substituted by alanine. (B) BCECF-AM–labeled B-CLL cells were added to wells coated with the indicated rproMMP-9 variants (110 nM each) and cell adhesion was measured as explained. (C) Binding of soluble rproMMP-9 variants to B-CLL cells, measured by flow cytometry. Values in panels B and C represent the average data from cells of the 4 patients studied. (D) proMMP-9 was treated with 2 mM APMA for 4 hours at 37°C and activation confirmed by gelatin zymography. (E) BCECF-AM–labeled B-CLL cells were added to wells coated with proMMP-9 or active MMP-9 (110 nM each), and cell adhesion was measured as explained. (F) Flow cytometric analysis of the binding of soluble proMMP-9 or active MMP-9 to B-CLL cells. Values in panels E and F represent the averages obtained with cells of 3 patients studied. MFI indicates mean fluorescence intensity. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
Further characterization of MMP-9 interaction with B-CLL cells. (A) Schematic domain structure of the recombinant full-length (fl) and proMMP-9 variants used (modified from 31 ). Pro indicates prodomain; Act, active site; FN, gelatin-binding fibronectin-like domain; Zn2+, Zn2+-binding domain; OG, O-glycosylated domain, with vertical lines indicating probable attachment sites for O-linked sugars; and Hem, hemopexin domain. A indicates that the catalytic Glu/E402 residue was substituted by alanine. (B) BCECF-AM–labeled B-CLL cells were added to wells coated with the indicated rproMMP-9 variants (110 nM each) and cell adhesion was measured as explained. (C) Binding of soluble rproMMP-9 variants to B-CLL cells, measured by flow cytometry. Values in panels B and C represent the average data from cells of the 4 patients studied. (D) proMMP-9 was treated with 2 mM APMA for 4 hours at 37°C and activation confirmed by gelatin zymography. (E) BCECF-AM–labeled B-CLL cells were added to wells coated with proMMP-9 or active MMP-9 (110 nM each), and cell adhesion was measured as explained. (F) Flow cytometric analysis of the binding of soluble proMMP-9 or active MMP-9 to B-CLL cells. Values in panels E and F represent the averages obtained with cells of 3 patients studied. MFI indicates mean fluorescence intensity. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
To further extend the physiological relevance of proMMP-9 interaction with B-CLL cells, we studied whether mature/active MMP-9 retained this ability. Treatment of proMMP-9 with APMA completely converted the proform into the reported 83/62-kDa MMP-9 active forms8,9 (Figure 4D). These active forms mediated cell adhesion and bound to B-CLL cells as efficiently as proMMP-9 (Figure 4E,F). Both events were significantly inhibited in all 3 samples studied by blocking α4β1 or CD44 with specific Abs or peptides (Figure 4E,F).
The CD44v isoform present in B-CLL cells coimmunoprecipitates with the α4 or β1 integrin subunits and is absent in normal or non-CLL B cells
Since both CD44 and α4β1 integrin appeared to be receptors for proMMP-9, we studied whether these 2 proteins interacted at the B-CLL surface. Fresh B-CLL cells (2 patients) were lysed and lysates immunoprecipitated with either anti-α4 or anti-β1 mAbs, followed by protein G–bound secondary Abs. Western blotting analysis of these immunoprecipitates revealed the presence of 190-kDa CD44v and the absence of 95-kDa CD44H (Figure 5A). Moreover, the anti-CD44 mAb immunoprecipitated the α4 and β1 integrin subunits (Figure S7). These results suggested that the CD44v/α4β1 complex was responsible for the binding of proMMP-9 to the B-CLL cell surface. Because normal or mantle lymphoma B cells did not bind proMMP-9, albeit expressing fully functional α4β1, we analyzed the presence of the CD44v isoform on these cells by Western blotting. Figure 5B shows that while the membrane fraction of B-CLL cells contained both the CD44H and CD44v isoforms, all other B cells analyzed contained only CD44H. Further characterization of the CD44v present on the B-CLL samples, with specific Abs to some of the variable exons, indicated that it contained v3, v4,5, v7,8, and v10 (shown in Figure 5C for a representative sample). In agreement with a recent report,32 v6 was absent, and we established the functionality of the anti-v6 Ab on BLM melanoma cells (Figure 5C). These results were confirmed by fluorescence-activated cell sorting (FACS) analysis of the B-CLL and normal B cells (Figure S8). Moreover, the Abs against the CD44 variable exons blocked the binding of soluble proMMP-9 to B-CLL cells in the 3 patients studied (shown in Figure 5D for anti-v7,8 and anti-v10 Abs), confirming the functional implication of these variable exons in the binding of CD44v to proMMP-9.
Characterization of CD44 isoforms on CLL and non-CLL B cells. (A) Lysates from 2 × 107 B-CLL cells that had been in RPMI/0.1% FCS for 24 hours were immunoprecipitated with the indicated Abs (control: anti-HLA) and analyzed by Western blotting. (B) The membrane fraction from the same B-CLL samples shown in panel A and from the indicated B cells was analyzed by Western blotting using anti-CD44 Abs. The presence of the 190-kDa CD44v isoform on B-CLL cells is indicated. A vertical line has been inserted to indicate a composition of 2 different gels. (C) The cell lysate from a representative B-CLL sample was immunoprecipitated with anti–MMP-9 Abs and analyzed by Western blotting using Abs specific for CD44 variable exons. Lysates from BLM cells were used as control for the anti-v6 mAb. (D) B-CLL cells were incubated with or without proMMP-9 in the presence or absence of the indicated Abs, and binding was analyzed by flow cytometry. Average values are shown. Const MMP-9 indicates constitutive MMP-9. *P ≤ .05.
Characterization of CD44 isoforms on CLL and non-CLL B cells. (A) Lysates from 2 × 107 B-CLL cells that had been in RPMI/0.1% FCS for 24 hours were immunoprecipitated with the indicated Abs (control: anti-HLA) and analyzed by Western blotting. (B) The membrane fraction from the same B-CLL samples shown in panel A and from the indicated B cells was analyzed by Western blotting using anti-CD44 Abs. The presence of the 190-kDa CD44v isoform on B-CLL cells is indicated. A vertical line has been inserted to indicate a composition of 2 different gels. (C) The cell lysate from a representative B-CLL sample was immunoprecipitated with anti–MMP-9 Abs and analyzed by Western blotting using Abs specific for CD44 variable exons. Lysates from BLM cells were used as control for the anti-v6 mAb. (D) B-CLL cells were incubated with or without proMMP-9 in the presence or absence of the indicated Abs, and binding was analyzed by flow cytometry. Average values are shown. Const MMP-9 indicates constitutive MMP-9. *P ≤ .05.
MMP-9 colocalizes with α4β1 integrin and CD44v at the B-CLL cell surface, and both receptors are required for MMP-9 cell surface anchoring
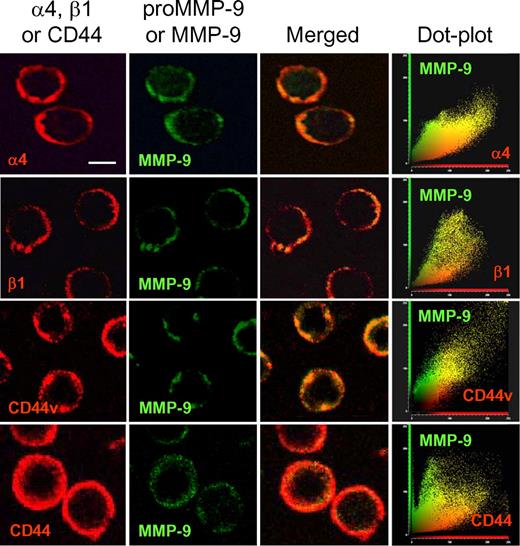
The preceding results indicated that CD44v interacted with α4β1 and that both proteins served as surface docking receptors for proMMP-9. To confirm that CD44v, α4β1, and proMMP-9 (or MMP-9 since the Ab did not distinguish between the pro and activated forms) colocalized at the B-CLL cell surface, we performed immunofluorescence analyses using confocal microscopy and focused on cells with higher expression of MMP-9 for easier visualization. As observed in Figure 6 for a representative sample, the α4 or β1 subunits, as well as CD44v, colocalized with MMP-9 (pro and/or activated) at the cell periphery, as documented by dot-plot analysis. In contrast, CD44H, which did not coimmunoprecipitate with proMMP-9 (Figure 1B) or α4β1 (Figure 5A), did not colocalize with MMP-9 (Figure 6), confirming the specific interaction of this MMP with CD44v.
Colocalization of MMP-9 with CD44v and α4β1 integrin in B-CLL cells. B-CLL cells from a representative sample were added to glass coverslips coated with 5 μg/mL poly-lysine. After 1 hour at 37°C, cells were fixed and analyzed by confocal microscopy using specific Abs for α4, β1, CD44v7,8, and CD44, followed by Texas-Red–labeled secondary Abs. MMP-9 was detected with specific pAbs and Alexa 488–labeled secondary Abs. Colocalization of MMP-9 with CD44v and α4β1 was further demonstrated using dot-plot analyses. Images were acquired using a confocal scanning inverted AOBS/SP2 microscope (Leica Microsystems, Heidelberg, Germany) with a 63×/1.3 NA PL-APO glycerol immersion objective. Leica's LCS 15.37 dye-separation software was used for colocalization studies; when necessary, Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA) was used for image processing. Bar represents 4 μm.
Colocalization of MMP-9 with CD44v and α4β1 integrin in B-CLL cells. B-CLL cells from a representative sample were added to glass coverslips coated with 5 μg/mL poly-lysine. After 1 hour at 37°C, cells were fixed and analyzed by confocal microscopy using specific Abs for α4, β1, CD44v7,8, and CD44, followed by Texas-Red–labeled secondary Abs. MMP-9 was detected with specific pAbs and Alexa 488–labeled secondary Abs. Colocalization of MMP-9 with CD44v and α4β1 was further demonstrated using dot-plot analyses. Images were acquired using a confocal scanning inverted AOBS/SP2 microscope (Leica Microsystems, Heidelberg, Germany) with a 63×/1.3 NA PL-APO glycerol immersion objective. Leica's LCS 15.37 dye-separation software was used for colocalization studies; when necessary, Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA) was used for image processing. Bar represents 4 μm.
We also analyzed by immunofluorescence the expression of MMP-9 on B-CLL cells transfected with CD44, β1, or control siRNAs. Figure S9 shows that knocking down the expression of α4β1 with β1 siRNA completely blocked MMP-9 localization at the B-CLL cell surface, while CD44 expression was not disturbed. Likewise, CD44 gene silencing resulted in the complete loss of surface-associated MMP-9, while the expression of the α4 or β1 subunits was not affected. Transfection with a control siRNA did not affect cell surface–bound MMP-9, which colocalized with the α4 and β1 subunits but not with the CD44H isoform (Figure S9).
Binding of proMMP-9 to the cell surface inhibits B-CLL cell invasion through basement membranes and transendothelial migration
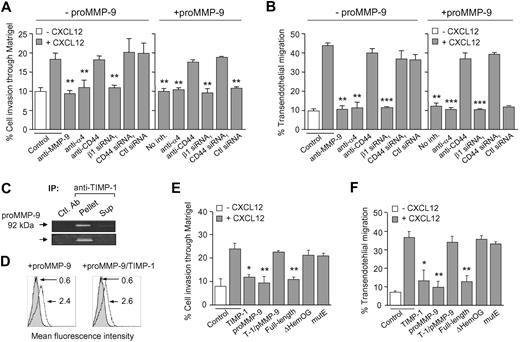
We previously showed6 and have confirmed here (Figure 7A,B) that gene silencing or Ab blocking endogenous MMP-9 inhibits B-CLL cell migration and invasion. To determine whether binding of exogenous proMMP-9 to B-CLL cells had a functional role in these processes, we incubated B-CLL cells with various concentrations of proMMP-9 and measured the migration through Matrigel or HUVECs. Interestingly, a dose-response inhibition of cell migration was observed in both systems (Figure S10A,B). This was not due to a possible toxic effect of proMMP-9 since B-CLL cell viability was not affected in these experiments (shown in Figure S10C for 2 representative samples).
Functional effect of proMMP-9 binding on B-CLL cell migration through Matrigel and HUVECs. B-CLL cells (5 × 105), treated or not with the indicated Abs or transfected with the indicated siRNAs, were incubated for 30 minutes with or without 10 μg/mL proMMP-9. Cells were added to the upper chamber of Transwell filters coated with either Matrigel (A) or TNF-α–activated HUVECs (B), and 150 ng/mL CXCL12 was added to the medium in the bottom chamber. After 24 hours, migrated cells were counted by flow cytometry. Average values (n = 3) represent the percentage of the total number of cells added. (C) proMMP-9 was incubated in solution with TIMP-1 (110 nM each) for 30 minutes and the complex immunoprecipitated with anti–TIMP-1 or anti-HLA (Ctl) mAbs and protein G beads. Immunoprecipitates and supernatants were analyzed by gelatin zymography. (D) B-CLL cells from a representative sample were incubated for 30 minutes with proMMP-9 alone or complexed to TIMP-1, and analyzed by flow cytometry using anti–MMP-9 pAbs. Numbers indicate MFI values for basal MMP-9 (shaded areas) or exogenously added proMMP-9 or proMMP-9/TIMP-1 (continuous lines). (E,F) B-CLL cells (5 × 105) were preincubated with or without the indicated proteins for 30 minutes at 4°C. Cells were washed and added to Transwell filters coated with Matrigel (E) or TNF-α–activated HUVECs (F), and migration was determined after 24 hours by flow cytometry. Average values (n = 3) are expressed as the percentage of the total number of cells added. T-1/pMMP-9 indicates TIMP-1/proMMP-9 complex. *P ≤ .05; **P ≤ .01.
Functional effect of proMMP-9 binding on B-CLL cell migration through Matrigel and HUVECs. B-CLL cells (5 × 105), treated or not with the indicated Abs or transfected with the indicated siRNAs, were incubated for 30 minutes with or without 10 μg/mL proMMP-9. Cells were added to the upper chamber of Transwell filters coated with either Matrigel (A) or TNF-α–activated HUVECs (B), and 150 ng/mL CXCL12 was added to the medium in the bottom chamber. After 24 hours, migrated cells were counted by flow cytometry. Average values (n = 3) represent the percentage of the total number of cells added. (C) proMMP-9 was incubated in solution with TIMP-1 (110 nM each) for 30 minutes and the complex immunoprecipitated with anti–TIMP-1 or anti-HLA (Ctl) mAbs and protein G beads. Immunoprecipitates and supernatants were analyzed by gelatin zymography. (D) B-CLL cells from a representative sample were incubated for 30 minutes with proMMP-9 alone or complexed to TIMP-1, and analyzed by flow cytometry using anti–MMP-9 pAbs. Numbers indicate MFI values for basal MMP-9 (shaded areas) or exogenously added proMMP-9 or proMMP-9/TIMP-1 (continuous lines). (E,F) B-CLL cells (5 × 105) were preincubated with or without the indicated proteins for 30 minutes at 4°C. Cells were washed and added to Transwell filters coated with Matrigel (E) or TNF-α–activated HUVECs (F), and migration was determined after 24 hours by flow cytometry. Average values (n = 3) are expressed as the percentage of the total number of cells added. T-1/pMMP-9 indicates TIMP-1/proMMP-9 complex. *P ≤ .05; **P ≤ .01.
We next studied whether the proMMP-9 functional effect involved binding to α4β1 and CD44. In the absence of exogenous proMMP-9, Ab blocking or gene silencing α4β1 significantly abolished B-CLL cell migration through Matrigel or HUVECs in all samples studied (Figure 7A,B), confirming previous results.3,6 In contrast, inhibiting CD44 did not affect cell migration (Figure 7A,B). Adding proMMP-9 to cells with blocked α4β1 function or expression resulted in inhibition of cell migration, probably due to the effect of α4β1 itself (Figure 7A,B). However, exogenous proMMP-9 was unable to inhibit migration on cells with blocked CD44 expression or function (Figure 7A,B). These results confirmed that proMMP-9 effect on cell migration required the interaction with its specific receptors (CD44 in this case).
To determine whether the inhibitory effect involved the enzymatic activity of MMP-9, we preincubated proMMP-9 with its specific inhibitor TIMP-1 and measured the effect of the complex on B-CLL cell migration. Efficient formation of the proMMP-9/TIMP-1 complex was confirmed by immunoprecipitation with anti–TIMP-1 Abs and subsequent analysis by gelatin zymography (Figure 7C). Moreover, this complex bound equally well to B-CLL cells as proMMP-9 alone (Figure 7D). B-CLL cell migration through Matrigel (Figure 7E) or HUVECs (Figure 7F) was significantly inhibited in all samples analyzed by TIMP-1 (average: 76% and 79% inhibition, respectively) or proMMP-9 (average: 93% and 91% inhibition, respectively). This effect was reverted in both systems by cell incubation with the proMMP-9/TIMP-1 complex (Figure 7E,F). To confirm these results, we tested the effect of the rproMMP-9mutE, which lacks enzymatic activity but binds well to B-CLL cells (Figure 4B,C). Indeed, this mutant had no effect on cell migration, while the parental rproMMP-9fl was as effective as neutrophil-derived proMMP-9 (Figure 7E,F). As expected, the rproMMP-9ΔHemOG variant, which does not bind to B-CLL cells, had no effect on cell migration (Figure 7E,F). These results clearly established that surface-bound MMP-9 may regulate B-CLL cell migration and this requires its proteolytic activity.
Discussion
We have studied the molecular associations and possible function of MMP-9 at the B-CLL cell surface. We show for the first time that (1) B-CLL cells bind soluble proMMP-9 and active MMP-9 and attach to immobilized proMMP-9 and active MMP-9; (2) these interactions are mediated by α4β1 integrin, 190-kDa CD44v, and the MMP-9 hemopexin domain; (3) α4β1 and CD44v constitute a novel cell surface docking complex for MMP-9; (4) MMP-9 colocalizes at the cell surface with α4β1 and CD44v, but not with CD44H; and (5) surface-bound MMP-9 may regulate B-CLL cell arrest and movement.
MMP-9 has previously been shown to interact with αLβ2, αMβ2, αVβ3, and αVβ5 integrins on various normal and tumor cell types.33,34 We show in the present study that α4β1 integrin is a novel docking receptor for proMMP-9 and active MMP-9 in B-CLL cells. Since α4β1 up-regulates proMMP-9,6 and this MMP was shown to be involved in B-CLL cell movement,6,11 α4β1 may constitute a crucial player in the progression of this malignancy. Indeed, elevated expression of α4β1 correlates with the presence of lymphadenopathy,3 and α4β1 is required for chemokine-directed B-CLL cell transendothelial migration.3,5 Moreover, the correlated overexpression of the α4 integrin subunit and CD38 appears to be characteristic of bad B-CLL prognosis.35,36 Our present results support these findings and establish a novel function for α4β1 in B-CLL (ie, anchoring MMP-9).
Our study also shows that the hyaluronan receptor CD4437,38 is another specific receptor for proMMP-9 and active MMP-9 in B-CLL cells. CD44 was previously shown to anchor MMP-9 at the surface of human melanoma cells, and this involved both the standard CD44H form as well as some CD44v isoforms.15 In contrast with this study, proMMP-9 in B-CLL cells specifically interacted and colocalized with a 190-kDa CD44v isoform but not with CD44H, which is highly abundant in these cells. As mentioned for α4β1, overexpression of cellular CD44v,39 in B-CLL or presence of this form in serum,40,41 correlates with a more aggressive disease and bad prognosis. This suggests that CD44v, like α4β1, may also play a role in B-CLL progression. As we show in the present study, one of the CD44v functions is to contribute to and apparently be a determinant of proMMP-9 binding to B-CLL cells. B cells lacking CD44v were unable to bind proMMP-9, albeit expressing fully functional α4β1 integrin. In addition, our results clearly show that α4β1 integrin and CD44v interact at the B-CLL cell surface, indicating that they constitute a docking complex in which both components are necessary for proMMP-9 surface binding. This complex appears to function only when cell associated as, in results not shown, we did not observe proMMP-9 binding to isolated CD44v, α4β1 integrin, or both proteins together, using solid-phase binding assays. One possible explanation for this could be that the docking complex contains another component(s), not yet identified, that regulates binding of proMMP-9, or serves to bridge α4β1, CD44v, and proMMP-9. Such bridging, or/and regulatory function for MMP-9 cell surface binding, has already been described for dentin42 and osteopontin43 in colon and prostate cancer cells, respectively. While these issues deserve further study, the present report is the first to show a specific and apparently exclusive interaction between α4β1 integrin and CD44v and a functional role for this complex.
Using recombinant proMMP-9 variants, we have demonstrated that proMMP-9 interaction with this docking complex involves mainly the hemopexin domain. α4β1 integrin and CD44v are therefore novel ligands for this domain. In several cell types, including B-CLL cells,6 cell-associated MMP-9 is found in membrane-linked cytoskeletal superstructures for tissue invasion,8,9,14,34 suggesting that MMP-9 targeting to the cell membrane (via the hemopexin domain) may be a mechanism of controlling tumor progression. In the present report, we have studied the possible functional role of surface-bound proMMP-9 on B-CLL cell migration. Although we previously showed,6 and confirmed here, that endogenous MMP-9 appears to be required for B-CLL movement, binding of exogenous proMMP-9 to B-CLL cells clearly impaired directed cell migration in a chemotactic gradient. The proteolytic activity of MMP-9 was necessary for this effect, as established from the results with a TIMP-1/proMMP-9 complex and an inactive recombinant proMMP-9 mutant, which did not affect cell migration. As proMMP-9 represents the inactive enzyme, our findings strongly suggest that interaction of its hemopexin domain with α4β1 and CD44v results in activation of proMMP-9 at the cell surface.
The mechanism involved in the observed functional effect is currently unknown. Given the diversity of MMP-9 targets,7,44 it is possible that when present at certain concentrations, surface-bound MMP-9 cleaves substrates that are necessary for B-CLL cell migration or/and induces cell adhesion. Opposite functional effects according to the levels of MMP-9 expression have also been observed in other tumor cell systems.45-48 It is therefore conceivable that B-CLL cells that have migrated to the bone marrow or lymph nodes increase their MMP-9 production, due to the action of chemokines.6 Indeed, immunohistochemical studies have demonstrated that B-CLL cells showing a diffused tissue infiltration pattern (bad prognosis) express high levels of MMP-9.11 MMP-9 from other sources may also be present on these tissues and facilitate B-CLL cell adhesion and arrest, rather than migration, producing the observed accumulation of malignant cells typical of B-CLL progression. The fact that the chemokine CXCL12 is a substrate of MMP-9 might also be relevant in B-CLL biology. Indeed, amino-terminal clipping of 4 residues of CXCL12 inactivates the molecule49 and might destroy the chemotactic gradient in the bone marrow. Because CXCL12 is a major chemoattractant for CXCR4-positive cells, B-CLL clones that have reached the bone marrow might become arrested and resident. The combination of the MMP-9 proteolytic activity and its ability to mediate cell arrest may therefore be crucial for B-CLL cell extravasation and localization in tissues.
In summary, the present report is the first to identify α4β1 integrin and CD44v as a cell surface docking molecular complex for proMMP-9 and active MMP-9. Our results can explain the observed B-CLL cell–associated proMMP-9 and provide evidence for a functional role of this MMP in the regulation of cell arrest and movement in B-CLL. Understanding the regulation and function of molecules involved in these processes is crucial for future therapies aimed to prevent B-CLL progression.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the B-CLL patients who donated samples for this research; Drs Paloma Sánchez-Mateos and Rafael Samaniego (Hospital Gregorio Marañón, Madrid, Spain) for performing the confocal microscopy analyses and for valuable advice on the results; Dr Luisa Botella (Centro de Investigaciones Biológicas, Madrid, Spain) for HUVECs; Dr Ivan Stamenkovic (University of Lausanne, Lausanne, Switzerland) for the CD44 cDNAs; Dr Martin Humphries and Sue Craig (Welcome Trust Center, Manchester, United Kingdom) for the purified α4β1 integrin; Dr Pedro Lastres for help with flow cytometry; and Ilse Van Aelst for excellent technical assistance.
This work was supported by grants PI060400 (A.G.-P.) and PI061637 (M.J.T.) from the Ministerio de Sanidad y Consumo, and by the Fundación de Investigación Médica Mutua Madrileña (A.G.-P.). Research on the human proMMP-9 mutants was supported by the Geconcerteerde OnderzoeksActies (GOA 2007-2011) and the Fund for Scientific Research-Flanders (FWO-Vlaanderen). J.R.-M. was supported by a fellowship from the Fundación Ramón Areces. P.E.V.S. is a postdoctoral fellow of the FWO-Vlaanderen.
Authorship
Contribution: J.R.-M. performed most of the research and designed some experiments; E.U.-B. performed some experiments; J.A.G.-M. and M.J.T. contributed patient samples and clinical data; M.H.C. purified, characterized, and maintained cells; P.E.V.S. and G.O. prepared and characterized the recombinant proMMP-9 variants and reviewed the paper; A.G.-P. designed and supervised research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Angeles García-Pardo, Departamento de Fisiopatología Celular y Molecular, Centro de Investigaciones Biológicas, CSIC, Ramiro de Maeztu 9, 28040 Madrid, Spain; e-mail: agarciapardo@cib.csic.es.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal