Abstract
Discoveries during the past decade have revolutionized our understanding of idiopathic thrombotic thrombocytopenic purpura (TTP). Most cases in adults are caused by acquired autoantibodies that inhibit ADAMTS13, a metalloprotease that cleaves von Willebrand factor within nascent platelet-rich thrombi to prevent hemolysis, thrombocytopenia, and tissue infarction. Although approximately 80% of patients respond to plasma exchange, which removes autoantibody and replenishes ADAMTS13, one third to one half of survivors develop refractory or relapsing disease. Intensive immunosuppressive therapy with rituximab appears to be effective as salvage therapy, and ongoing clinical trials should determine whether adjuvant rituximab with plasma exchange also is beneficial at first diagnosis. A major unanswered question is whether plasma exchange is effective for the subset of patients with idiopathic TTP who do not have severe ADAMTS13 deficiency.
Clinical features of idiopathic TTP
In January of 19241 and apparently for a second time in February,2 Moschcowitz presented a case before the New York Pathological Society of “a hitherto undescribed disease (ref. 1 at 21)” that he felt was “remarkable, clinically and anatomically (ref. 2 at 89).” A healthy 16-year-old girl suddenly developed weakness in her arms, pain on moving her wrists and elbows, pallor, and fever (38°C-39°C). Her symptoms worsened and on the tenth day of illness she was admitted to the hospital with anemia, leukocytosis, a few petechiae on one arm, and occult blood in gastric contents and stool. The serum creatinine was normal. Four days later she developed mild left hemiparesis and facial paralysis. The next day she became comatose and died. A limited autopsy showed hyaline thrombi in terminal arterioles and capillaries of the heart, kidney, spleen, and liver; the lungs were spared. Moschcowitz did not obtain a platelet count and did not report schistocytes in the blood film, so we do not have a complete description from him of thrombocytopenia or microangiopathic hemolytic anemia. But based on the pathology at autopsy, we recognize this patient as the first published example of idiopathic thrombotic thrombocytopenic purpura (TTP)
During the next 50 years, the clinical features of TTP became progressively better defined. Most patients were females between the ages of 10 and 39, and they usually exhibited a pentad of signs without obvious alternative causes: microangiopathic hemolytic anemia, thrombocytopenia, neurologic findings, renal damage, and fever. Unfortunately, the prognosis remained grim: mortality exceeded 90%, the average hospital stay was 14 days before death, and 80% of patients died within 3 months after the onset of symptoms.3
Plasma therapy
Moschcowitz also reported that one of his colleagues, Lederer, had seen 4 patients resembling his own case, and all recovered promptly after a single blood transfusion.2 Lederer published his observations4,5 but none of the patients had significant thrombocytopenia, which cast doubt on their diagnosis, and ultimately his papers had no impact. Fewer than a half dozen other reports on transfusion therapy for TTP were published during the next 50 years, and only one of them described a favorable outcome.6
The situation changed dramatically in 1976, when Bukowski et al published their experience with whole blood exchange transfusion. Amazingly, 8 of 14 patients with TTP responded quickly and had remissions lasting from several months to more than 13 years. The effects of exchange transfusion often were dramatic: in 4 cases, profound neurologic deficits—coma, delirium, and hemiparesis—resolved in less than 24 hours, sometimes during the exchange transfusion procedure.7 Very quickly, the active principle in blood was shown to be in the plasma fraction.8 One particularly elegant case report showed that replacement with either plasma or cryosupernatant was effective, whereas albumin was not, and simple plasma infusions could induce prolonged remissions in some patients.9
The value of plasma therapy was demonstrated conclusively in a randomized, prospective comparison of plasma infusion and plasma exchange for the treatment of adults with TTP. Survival at 6 months was 78% with plasma exchange and 63% with plasma infusion, a significant difference in favor of plasma exchange (P = .036).10 Because of this trial, standard treatment for TTP today includes plasma exchange at 40 to 60 mL/kg daily until the patient has a normal platelet count and a normal LDH, and any nonfocal neurologic deficits have resolved. If plasma exchange cannot be performed for some reason, patients may be treated instead with plasma infusion at up to 30 mL/kg daily, provided they can tolerate the fluid load.
Upshaw-Schulman syndrome, a congenital form of TTP
In retrospect, the potential value of plasma therapy had been demonstrated by 1960, when Schulman reported the case of an 8-year-old girl who suffered from infancy with repeated episodes of thrombocytopenia and hemolytic anemia.11 The child responded consistently to plasma with normalization of the platelet count and hemolysis within a few days, and she was maintained in remission by prophylactic plasma infusions every 1 or 2 weeks. Unfortunately, this report did not describe schistocytes as a feature of disease and the thrombocytopenia was attributed to “thrombopoietin” deficiency, which must have delayed the recognition of similarities between this patient's congenital illness and acquired idiopathic TTP.
This connection was made by Upshaw, who saw a 16-year-old girl with a history of relapsing hemolytic anemia and thrombocytopenia since infancy. The patient had always responded dramatically to blood transfusions, becoming clinically well within 48 hours.12 During the next 11 years, Upshaw successfully treated 32 episodes of thrombocytopenia and microangiopathic hemolysis with plasma infusions. Acute episodes almost always were precipitated by some factor such as a minor infection, surgical procedure, pregnancy, fecal impaction, or pancreatitis. Asymptomatic intervals could last from 3 weeks to 20 months, during which the patient had a normal platelet count and a compensated hemolytic state, but shortened intravascular platelet and red cell survival. These abnormalities were normalized after the infusion of 2 units of plasma.
Upshaw proposed that his patient had a congenital TTP-like disorder identical to that described earlier by Schulman. In a prescient final paragraph, he also suggested that the efficacy of plasma therapy for idiopathic TTP in adults and for congenital TTP could be explained if it replaced a single plasma factor that was deficient in both conditions.12
VWF and ADAMTS13 in the pathophysiology of TTP
The deficient plasma factor would not be characterized for another 20 years, but a clue to its function was recognized much earlier by Moake, who published an influential paper in 1982 that linked von Willebrand factor (VWF) to the pathogenesis of TTP.13 He found that patients with relapsing acquired or congenital TTP had circulating “unusually large” or “ultralarge” VWF multimers when they were in remission, and ultralarge VWF was absent from the plasma of healthy persons. Moake proposed that his patients lacked a VWF depolymerase, possibly a protease, that normally cleaved ultralarge VWF to prevent it from causing the intravascular platelet aggregation and thrombosis that characterize TTP. Plasma therapy might replace this depolymerase or remove an inciting cofactor.13 Interestingly, 2 of Moake's 4 subjects were the same patients with congenital TTP who were studied as children by Schulman11 and Upshaw.12 Years later, they became central to deciphering and linking the pathophysiologic mechanisms of both congenital TTP and acquired idiopathic TTP in adults.
In 1996, a candidate VWF-cleaving protease finally was identified in human plasma by Tsai14 and independently by Furlan.15 The protease did not cleave VWF unless the substrate was subjected to fluid shear stress or treated with low concentrations of protein denaturants such as urea or guanidine hydrochloride. The next year, VWF-cleaving protease was shown to be missing from the plasma of patients with congenital TTP.16 Soon after, adults with acquired idiopathic TTP were reported to have severe VWF-cleaving protease deficiency caused by IgG autoantibodies that inhibit the enzyme.17,18
During the next few years, the VWF-cleaving protease was purified,19,20 cloned,21-23 and named ADAMTS13 because it belonged to the recently discovered “a disintegrin-like and metalloprotease with thrombospondin repeats” family of metalloproteases (Figure 1).24 Furthermore, mutations in the ADAMTS13 gene were shown to cause congenital TTP.23 Almost 80 years after Moschcowitz published the first case report, we finally had a molecular mechanism for both congenital and acquired idiopathic TTP (Figure 2).
Structure of ADAMTS13. The primary translation product consists of 1427 amino acid residues. Motifs include a signal peptide (S), propeptide (P), metalloprotease (M), disintegrin (D), Cys-rich, spacer, CUB, and thrombospondin (TSP1) domains (1-8).
Structure of ADAMTS13. The primary translation product consists of 1427 amino acid residues. Motifs include a signal peptide (S), propeptide (P), metalloprotease (M), disintegrin (D), Cys-rich, spacer, CUB, and thrombospondin (TSP1) domains (1-8).
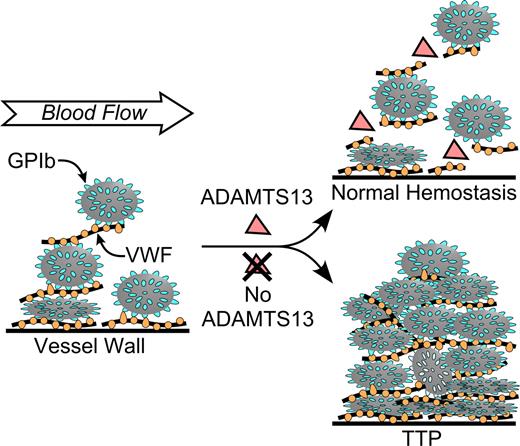
Pathogenesis of idiopathic TTP caused by ADAMTS13 deficiency. Multimeric VWF adheres to endothelial cells or to connective tissue exposed in the vessel wall. Platelets adhere to VWF through platelet membrane GPIb. In flowing blood, VWF in the platelet-rich thrombus is stretched and cleaved by ADAMTS13, limiting thrombus growth. If ADAMTS13 is absent, VWF-dependent platelet accumulation continues, eventually causing microvascular thrombosis and TTP.
Pathogenesis of idiopathic TTP caused by ADAMTS13 deficiency. Multimeric VWF adheres to endothelial cells or to connective tissue exposed in the vessel wall. Platelets adhere to VWF through platelet membrane GPIb. In flowing blood, VWF in the platelet-rich thrombus is stretched and cleaved by ADAMTS13, limiting thrombus growth. If ADAMTS13 is absent, VWF-dependent platelet accumulation continues, eventually causing microvascular thrombosis and TTP.
The last 5 years have seen more progress in understanding how ADAMTS13 regulates the biologic function of VWF, but how to use this knowledge clinically remains uncertain. The major questions revolve around how to use ADAMTS13 test results and how to use intensive immunosuppressive therapy.
Diagnosis and differential diagnosis of idiopathic TTP
The landmark clinical trial that established plasma exchange as the standard of care for idiopathic TTP10 also gave us a case definition that is still generally accepted: microangiopathic hemolytic anemia and thrombocytopenia, without an alternative cause such as autoimmune hemolysis, disseminated intravascular coagulation, cancer, eclampsia, drug toxicity (eg, treatment with calcineurin inhibitors), hematopoietic stem cell transplantation, or malignant hypertension. Patients with certain of these predisposing conditions, particularly treatment with calcineurin inhibitors or stem cell transplants, are considered to have “secondary” TTP rather than idiopathic TTP. Notably, the 3 other features of the classic pentad—renal involvement, neurologic symptoms, and fever—are not necessary. These signs of end organ damage are considered to be relatively late events that should be avoided if possible by prompt diagnosis and treatment.
These diagnostic criteria are not specific for idiopathic TTP caused by ADAMTS13 deficiency. Among patients with apparent idiopathic TTP, the fraction with severe ADAMTS13 deficiency (ADAMTS13 activity < 5%) has varied from 33% to 100% across several studies, with 75% as a rough average.17,18,25-30 The cause of this variation is not known. It may reflect differences in ADAMTS13 assay methods or case definitions, variable attention to secondary causes of thrombotic microangiopathy, or the application of additional selection criteria. For example, exclusion of subjects with serum creatinine level higher than 310 μM (3.5 mg/dL) reportedly increases the frequency of ADAMTS13 deficiency in patients with idiopathic thrombotic microangiopathy to at least 90%.30,31
Conversely, patients with secondary TTP almost never have severe ADAMTS13 deficiency. In addition, ADAMTS13 deficiency rarely if ever occurs in diarrhea-associated hemolytic uremic syndrome (D + HUS) caused by Shiga toxin–producing Escherichia coli, which is characterized by abdominal pain, bloody diarrhea, thrombotic microangiopathy, thrombocytopenia, and acute oliguric renal failure. For example, in the Oklahoma TTP-HUS registry, none of 92 patients with secondary TTP or D + HUS had severe ADAMTS13 deficiency.25 HUS without antecedent diarrhea is referred to as “atypical HUS.” Patients with atypical HUS seldom have ADAMTS13 deficiency and frequently turn out to have abnormalities in the regulation of the alternate complement pathway due to mutations in complement factor H, factor I, factor B, or membrane cofactor protein.32
Thus, severe ADAMTS13 deficiency identifies a large subset of patients with idiopathic TTP who suffer from VWF-dependent microvascular thrombosis. Congenital ADAMTS13 deficiency does not always present during childhood and may be difficult to distinguish from acquired idiopathic TTP in adult patients who do not have a history of prolonged responses to simple plasma infusions. In such cases, the detection of autoantibodies against ADAMTS13 supports a diagnosis of acquired idiopathic TTP. The cause of disease is not known for most patients with idiopathic TTP who do not have severe ADAMTS13 deficiency, and clinical criteria do not reliably identify them. As a practical matter, very few laboratories can perform ADAMTS13 assays rapidly enough, and the clinician must make a diagnosis and initiate therapy without this information.
ADAMTS13 testing at presentation
In general, patients with idiopathic TTP usually respond to plasma exchange and those with secondary TTP do not. Because ADAMTS13 deficiency is extremely rare in secondary TTP, ADAMTS13 deficiency correlates with a good response to plasma exchange when all patients with idiopathic or secondary TTP are lumped together.25,27 However, ADAMTS13 testing is not particularly useful for most patients with secondary TTP because they can be identified without it. For example, severe ADAMTS13 deficiency does not occur in secondary TTP associated with bone marrow transplantation or cyclosporine A, and these disorders also do not respond well to plasma exchange.33-36
For idiopathic TTP, the value of distinguishing patients with and without ADAMTS13 deficiency has been uncertain, but as more data have become available it seems clear that ADAMTS13 assays provide useful prognostic information. Several studies have looked at the relationship between ADAMTS13 levels at diagnosis and the response to plasma exchange, the frequency of relapse, and survival.25,27,37,38 Interestingly, patients with and without severe ADAMTS13 deficiency have had similar response rates and short-term survival (each ∼80%-90%). In contrast, patients with severe ADAMTS13 deficiency have a significantly increased risk of relapsing TTP (∼30% across all studies), whereas patients without severe ADAMTS13 deficiency rarely relapse (∼9% across all studies).25,27,37,38
These comparisons may underestimate the predictive power of severe ADAMTS13 deficiency because the likelihood of relapse increases with time, but many study patients had been followed for only a few months. Our experience at Washington University illustrates this effect. In 2004, we reported that 6 (38%) of 16 patients with idiopathic TTP and severe ADAMTS13 deficiency had relapsed by the time of publication, with a follow-up of 8 to 33 months.27 Three years later, 11 (69%) of these 16 patients have had at least one relapse after disease-free intervals of 8 months to 5 years, and 5 of them (31%) have died.
Assays for autoantibodies to ADAMTS13 provide additional prognostic information. In several studies, the presence of detectable inhibitors at diagnosis has correlated with a higher risk of relapsing disease.25,27,30,39 High-titer antibodies also have been associated with a delayed response to plasma exchange, refractory disease, and early death.39-42 However, these conclusions are based on relatively few patients and some studies have not observed a relationship between anti-ADAMTS13 antibody titer and short-term outcomes.43
ADAMTS13 testing during remission
Because ADAMTS13 activity and autoantibody levels can vary rapidly during the course of idiopathic TTP, it is a pleasant surprise that a single test at diagnosis can predict the risk of subsequent relapse. As one might expect, though, measurements during remission improve the correlation between ADAMTS13 levels and clinical outcomes. Several reports have described patients with idiopathic TTP who responded completely to plasma exchange despite persistent severe ADAMTS13 deficiency,27,28,43,44 but most such patients with ongoing autoimmune responses against ADAMTS13 do relapse eventually. In a recent study of 109 patients achieving a complete response to plasma exchange, approximately one-third of them still had severe ADAMTS13 deficiency at some time during remission. Relapses occurred in 60% of patients with persistent severe ADAMTS13 deficiency compared with only 19% of patients without ADAMTS13 deficiency at the time of testing.44
Concentrating on only those patients known to have ADAMTS13 deficiency before treatment further strengthens the relationship. Pooling the results of 2 relevant studies, the incidence of relapse was 44% for 16 patients presenting with ADAMTS13 deficiency that persisted during remission, compared with 7% for 27 patients without continuing ADAMTS13 deficiency. ADAMTS13 inhibitors exhibited a similar pattern: relapses occurred in 57% of 14 patients with a detectable inhibitor during remission, compared with 4% of 28 patients without inhibitors.27,43
Furthermore, essentially all patients with a prior history of severe ADAMTS13 deficiency will have it again when they relapse with TTP, whether they had normal ADAMTS13 levels at some other time during remission.30,43 An update of our earlier study27 is consistent with this conclusion: patients 1, 7, and 8 responded completely to plasma exchange and subsequently had normal ADAMTS13 activity, but have relapsed with TTP and severe ADAMTS13 deficiency between 4 and 5 years after their first episode.
It seems clear that idiopathic TTP caused by ADAMTS13 deficiency tends to relapse, and relapses are associated with persistent or recurrent severe ADAMTS13 deficiency. Regular laboratory monitoring after treatment with plasma exchange might identify patients with ADAMTS13 deficiency and a high risk of imminent relapse. Of course, monitoring would presuppose the ready availability of rapid assays for ADAMTS13 activity and autoantibodies.
Clinical research questions
Our recent progress in understanding the pathophysiology of TTP has focused attention on several urgent clinical questions for which the answers seem just out of reach. I will discuss 3 of them that seem particularly ripe for study.
Do we need real-time ADAMTS13 assays?
One can argue—and I have supported this position—that rapid ADAMTS13 assays are unnecessary because all patients with idiopathic TTP should be treated with plasma exchange until they achieve a remission, regardless of ADAMTS13 level.45-47 However, this point deserves reexamination.
Severe ADAMTS13 deficiency identifies a specific mechanism of idiopathic TTP that usually responds well to plasma exchange but tends to relapse,25,27,37,38 and high titer antibodies to ADAMTS13 correlate with refractory disease and mortality.39-42 Therefore, rapid ADAMTS13 assays are likely to provide useful prognostic information at diagnosis.
More prosaically, rapid ADAMTS13 testing could speed the institution of plasma exchange therapy for the occasional patient with ADAMTS13 deficiency who presents with neurologic deficits or other signs of tissue ischemia before developing overt thrombotic microangiopathy.48,49 In addition, idiopathic TTP can be misdiagnosed because of symptoms suggesting gastroenteritis, sepsis, or transient cerebral ischemia,45 and rapid ADAMTS13 testing could prevent these errors. Testing can identify patients with severe ADAMTS13 deficiency who are unlucky enough to also have another potential cause of thrombotic microangiopathy, such as a stem cell50 or solid organ transplant.51-53 Patients with autoimmune diseases such as systemic lupus erythematosis can develop thrombocytopenia and neurologic dysfunction as a result of vasculitis, but they also can have autoimmune ADAMTS13 deficiency.37,54-56 Thrombotic microangiopathy during pregnancy may be caused by preeclampsia or HELLP (hemolysis, elevated liver function, and low platelets) syndrome, which are not associated with ADAMTS13 deficiency.57 However, pregnancy can also induce acute TTP in women with congenital or acquired ADAMTS13 deficiency,58 and ADAMTS13 assays may be useful to identify patients who could benefit from prophylactic plasma exchange therapy during pregnancy.59 Thus, ADAMTS13 testing may help to distinguish the different mechanisms of thrombotic microangiopathy in complex clinical situations.
For patients with idiopathic TTP, ADAMTS13 deficiency is a biomarker for a high risk of relapsing disease.25,27,30,39 Experience with congenital TTP also suggests that ADAMTS13 levels of approximately 5% to 10% are sufficient to prevent thrombotic microangiopathy.60-63 The same appears to be true for idiopathic TTP,30,43 so that monitoring ADAMTS13 levels during treatment could be useful to determine whether plasma exchange should be intensified, decreased, or discontinued. In addition, monitoring during remission could identify patients with persistent or recurrent ADAMTS13 deficiency and a high risk of relapse, which might be forestalled by additional immunosuppressive therapy.64
Of course, these hypothetical applications would require the ready availability of rapid assays for ADAMTS13 activity and autoantibodies. Several such assays have been devised65-67 but are available now only in a few specialized laboratories. We need more clinical data concerning the utility of ADAMTS13 levels to determine whether rapid ADAMTS13 assays are useful. If so, they should be deployed more widely.
When should we use immunosuppressive therapy for idiopathic TTP?
Plasma exchange helps most patients with idiopathic TTP survive their acute episode of thrombotic microangiopathy, but does nothing specific to reduce the production of pathogenic anti-ADAMTS13 autoantibodies. Many physicians routinely use corticosteroids for this purpose, and reserve more aggressive immunosuppressive therapy for refractory or relapsing disease.
In this setting, rituximab appears to be effective at normalizing ADAMTS13 levels and inducing durable remissions. Published small series and case reports have described approximately 100 patients with refractory or relapsing idiopathic TTP treated with rituximab, usually at doses of 375 mg/m2 weekly for an average of 4 doses.64,68,69 Approximately 95% of reported patients have had a complete clinical and laboratory responses within 1 to 3 weeks of starting treatment, including a normal ADAMTS13 level and disappearance of anti-ADAMTS13 antibodies. Mild acute reactions to ritixumab infusions were controlled by premedication with steroids, antihistamines, and analgesics. More serious complications have been relatively uncommon. One patient had transient cardiogenic shock70 and another had symptomatic gastrointestinal infection with Strongyloides.64 Relapses have been infrequent, occurring in approximately 10% of patients after intervals of 9 months to 4 years; all but one of these patients had a second prolonged complete remission upon retreatment with rituximab.64,69,71-74
These reports have all the limitations and potential biases of case series, and they should be interpreted cautiously. In particular, judging the efficacy of rituximab is difficult because patients usually receive multiple different treatments. Nevertheless, rituximab seems to rescue most patients with refractory or relapsing idiopathic TTP. Moreover, by abolishing autoantibody production, adjuvant rituximab at first diagnosis (combined with plasma exchange) might further improve outcomes by shortening the duration of plasma exchange, reducing early mortality, and preventing relapses. Therefore, the major questions about rituximab can be reduced to just one: should intensive immunosuppression with rituximab be used as adjuvant or salvage therapy for autoimmune idiopathic TTP?
Consider some of the tradeoffs between adjuvant versus salvage therapy. Using Medicare/Medicaid reimbursement as a standard for comparisons, a typical course of rituximab (∼700 mg × 4 doses, ∼$491/100 mg) is reimbursed at approximately $14 000.75 A typical 10-day course of plasma exchange is at least as costly, amounting to approximately $20 000 for the plasma exchange procedures alone (∼$780 plus ∼4200 mL plasma at ∼$74/250 mL, or ∼$2023/exchange).75 Approximately 30% of patients achieving a complete remission with plasma exchange relapse within 5 years, often several times, and any relapse may cause disability or death. Therefore, if adjuvant rituximab prevented all relapses, with no offsetting toxicity, the number needed to treat (NNT) to prevent one relapse would be less than approximately 3. The NNT would increase if rituximab were less successful, preventing or delaying only some relapses, or merely reducing the duration of plasma exchange needed to achieve a complete response. Even so, whatever your metric—NNT to prevent disability or death, cost per quality-adjusted life-year (QALY), or something else—the cost effectiveness of adjuvant rituximab might prove comparable with that of accepted interventions such as hypertension medication or cholesterol management ($10 000-$60 000 per QALY).76 The relative merits of adjuvant versus salvage immunosuppressive therapy can best be determined through an appropriately designed clinical trial.
How should we treat idiopathic TTP without ADAMTS13 deficiency?
The clinical trial that proved the efficacy of plasma exchange10 was conducted before the discovery of ADAMTS13, and it is impossible to know which participants may have had normal ADAMTS13 levels. Until we learn better, plasma exchange should plausibly remain the standard therapy for idiopathic TTP, regardless of ADAMTS13 levels. As a practical matter, most of us cannot get ADAMTS13 assays performed rapidly enough, so we must start plasma exchange without benefit of these results. In addition, the limited data published on this question suggest that the response rates to plasma exchange are similar for idiopathic TTP with or without severe ADAMTS13 deficiency.25,27,37,38,77
But despite the equivalent early outcomes, the groups differ profoundly in the likelihood of relapse. Approximately one-half of patients with severe ADAMTS13 deficiency suffer at least one relapse within 2 years, whereas patients without severe ADAMTS13 deficiency almost never relapse.25,27,37,38 This difference strongly suggests that distinct pathophysiologic mechanisms are responsible for idiopathic TTP with severe ADAMTS13 deficiency versus idiopathic TTP without severe ADAMTS13 deficiency. In particular, the rarity of relapses among patients without severe ADAMTS13 deficiency suggests that their disease is not caused by an autoimmune response. If there are no autoantibodies (or autoreactive cells) to remove, and therefore no autoimmune target to replenish, then it is difficult to understand how plasma exchange could be beneficial. Perhaps patients with normal ADAMTS13 activity have a self-limited illness that improves independent of plasma exchange.
My point is that we lack convincing data for or against the use of plasma exchange to treat patients with idiopathic TTP who do not have severely decreased plasma ADAMTS13 activity. We prescribe plasma exchange just the same, but with the nagging doubt that the treatment may be irrelevant. Given the high cost,75 inconvenience, invasiveness, and risks78 of plasma exchange, we should learn whether it helps or harms these patients.
We have the tools to do so now, although some issues concerning ADAMTS13 assays need attention. First of all, most ADAMTS13 assay methods require substantial dilution of plasma samples, which also dilutes any autoantibody inhibitors and can lead to overestimation of ADAMTS13 activity and misclassification of patients. This circumstance appears to be uncommon, and different assay methods usually show good agreement,65-67 but improved assays would be welcome. Second, autoantibodies to distal ADAMTS13 domains might theoretically block VWF cleavage in vivo without affecting clinical ADAMTS13 assays that use smaller substrates in vitro,79 leading to misclassification of patients. However, the available data on inhibitor epitope specificity80-82 suggest this problem will be rare, and affected patients should be identifiable with appropriate assays to detect their autoantibodies.
The future
I have outlined several questions that I believe are feasible to address through clinical trials, and the answers could significantly improve patient outcomes. The major obstacle is that patients with idiopathic TTP are rare. The annual incidence of idiopathic TTP is approximately 4 per million,83 so that a large hospital like Barnes-Jewish Hospital in St Louis, Missouri, may see only approximately 10 new patients a year. This number is too low to sustain an adequately powered study to compare treatments directly. However, several promising multicenter trials are in progress or will open soon. A prospective observational study in France is enrolling adult and pediatric patients with TTP for longitudinal measurements of ADAMTS13 activity, antigen, and autoantibodies, and ADAMTS13 gene sequencing (NCT00426686). A single-arm phase 2 study across Canada will evaluate rituximab plus plasma exchange for relapsed or refractory TTP, and enrollment should begin soon (NCT00531089). The French and Canadian trials should yield important insight into the utility of ADAMTS13 testing and the efficacy of rituximab as salvage therapy, respectively.
The Transfusion Medicine/Hemostasis Network in the United States is about to open a phase 3 randomized comparison of plasma exchange with or without rituximab for idiopathic TTP.84 Patients on the plasma exchange arm who fail with refractory or relapsing disease can receive rituximab later. Therefore, this trial will address whether rituximab is best used as adjuvant or salvage therapy. Because severe ADAMTS13 deficiency will not be required for participation, this trial will include some patients with idiopathic TTP who do not have severe ADAMTS13 deficiency, and the results should indicate whether such patients differ fundamentally from those with severe ADAMTS13 deficiency in their response to plasma exchange, incidence of relapse, and response to rituximab. Samples for ADAMTS13 assays will be obtained throughout the course of disease and will be analyzed after the trial is completed. The results should clearly demonstrate whether rapid ADAMTS13 assays could be useful for diagnosis or to guide therapy.
In short, within a few years we should have answers for the clinical research questions that I have posed here. We will know which patients benefit from plasma exchange or intensive immunosuppressive therapy, and whether ADAMTS13 assays can identify them. Furthermore, ADAMTS13 deficiency is not responsible for all cases of TTP, and we can look forward to recognizing and characterizing other causes. The results of ongoing basic and clinical research will place our treatment of idiopathic TTP on an even stronger empiric foundation, with the benefit of understanding the underlying pathophysiologic mechanisms.
Acknowledgments
This work was supported in part by National Institutes of Health grants HL72917 and HL89746.
National Institutes of Health
Howard Hughes Funding
Authorship
Contribution: J.E.S. wrote the paper.
Conflict-of-interest disclosure: J.E.S. is a consultant and member of clinical advisory boards for Baxter BioSciences.
Correspondence: J. Evan Sadler, Howard Hughes Medical Institute, Washington University School of Medicine, 660 S Euclid Ave, Box 8022, St Louis, MO 63110; e-mail: esadler@im.wustl.edu.