Abstract
To evaluate the prognostic significance of clinicobiologic and pathological features in angioimmunoblastic T-cell lymphoma (AITL), 157 AITL patients were retrieved from the GELA LNH87-LNH93 randomized clinical trials. One hundred forty-seven patients received a cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP)–like regimen with intensified courses in half of them. Histologically, 41 cases were classified as “rich in large cells” and 116 as “classic” (including 19 rich in epithelioid cells, 14 rich in clear cells, and 4 with hyperplastic germinal centers). Sixty-two cases were scored for CD10 and CXCL13 expression according to the abundance of positive lymphoid cells. Median age was 62 years, with 81% advanced stage, 72% B symptoms, 65% anemia, 50% hypergammaglobulinemia, and 66% elevated LDH. Overall 7-year survival was 30%. In multivariate analysis, only male sex (P = .004), mediastinal lymphadenopathy (P = .041), and anemia (P = .042) adversely affected overall survival. Increase in large cells and high level of CD10 and CXCL13 did not affect survival. Intensive regimen did not improve survival. In conclusion, AITL is a morphologically heterogeneous T-cell lymphoma commonly expressing CXCL13 and CD10 and carrying few prognostic factors. It portends a poor prognosis even when treated intensively. However, AITL is not always lethal with 30% of patients alive at 7 years.
Introduction
Angioimmunoblastic T-cell lymphoma (AITL) represents a distinct clinicopathological entity, among nodal peripheral T-cell lymphomas.1 It generally occurs in elderly patients presenting with generalized lymphadenopathy, hepatosplenomegaly, anemia, and hypergammaglobulinemia. Histologic features of AITL include partial or complete effacement of the lymph node architecture by a polymorphous infiltrate that is typically associated with a proliferation of follicular dendritic cells (FDCs) and a prominent arborization of high endothelial venules. The neoplastic cells display minimal cytologic atypia. They are small- to medium-sized cells typically with a clear cytoplasm and they usually aggregate in small clusters. They account for only a fraction of the infiltrate and are admixed with a reactive population of small lymphocytes, eosinophils, plasma cells, histiocytes, and large lymphoid, sometimes Reed-Sternberg–like B cells that are often infected by Epstein-Barr virus (EBV).2 AITL shows a morphologic spectrum and increase in T-cell immunoblasts may be observed.1,3 Although it has not been thoroughly investigated, it was suggested that the increase in T-cell immunoblasts would indicate transformation into a peripheral T-cell lymphoma, unspecified (PTCL/U).3 An increase in EBV-infected B cells may also occur, and, in rare cases, an overt diffuse large B-cell lymphoma develops.4-7 Recent data concerning the identity of the normal cellular counterpart of AITL are emerging. It is now believed that AITL derives from a follicular helper T-cell (TFH) subset.8-14 This subset of T cells would be located at the boundary between the mantle zone and the germinal center light zone and is supposed to provide help to germinal center B cells during their terminal differentiation.8,10 The tumor cells usually express CD4, CD10, Bcl6, and CXCL13, a phenotype that is unique among T-cell lymphomas.11-14
AITL is rare accounting for approximately 2% of all non-Hodgkin lymphomas.15 Due to the rarity of the disease, there have been relatively little data concerning the impact of clinical, biologic, and morphologic features of angioimmunoblastic lymphadenopathy with dysproteinemia (AILD) and/or AITL on survival and outcome.16-23 Randomized clinical trials do not exist and few reports if any included an extended follow up. In this report, we attempted to identify the prognostic significance of different pathological, biologic, and clinical features after a long follow up of consecutive AITL patients treated with chemotherapy according to LNH87 and LNH93 protocols conducted by the Groupe d'Etude des Lymphomes de l'Adulte (GELA). In particular, since rare cases of B-cell lymphomas have been reported to arise after AILD or AITL4-7 and since it was suggested that the increase in T cells could evolve into a PTCL/U,3 we intended to study the prognostic relevance of the increase (> 10%) in large atypical cells.
Methods
Patient selection
Eligibility criteria for this study included patients with confirmed diagnosis of AITL after histopathological and immunohistochemical review. The 157 AITL patients are a subset of 6700 patients enrolled in the LNH87 and LNH93 GELA trials for intermediate- and high-grade lymphomas. Some of the clinical features have been previously reported in 68 patients.24
Histologic analysis
At the time of enrollment in the LNH87 and LNH93 protocols, lymph node biopsies were reviewed by pathologists of the GELA group and initially classified according to the Working Formulation25 and updated Kiel classification,26 based on morphologic examination of slides stained with hematoxylin-eosin and Giemsa and on immunohistochemistry comprising at least CD20 and CD3ϵ. For the purpose of the present study, 4 expert hematopathologists reviewed all T-cell lymphoma cases and completed the phenotype in order to reclassify them according to the WHO classification.1 In AITL cases, the diagnosis was based on the presence of the following 5 criteria: partial or diffuse effacement of the nodal architecture, vascular proliferation with prominent arborization of high endothelial venules, extrafollicular meshwork of FDCs, atypical population of CD3+ T cells, and large CD20+ B cells.
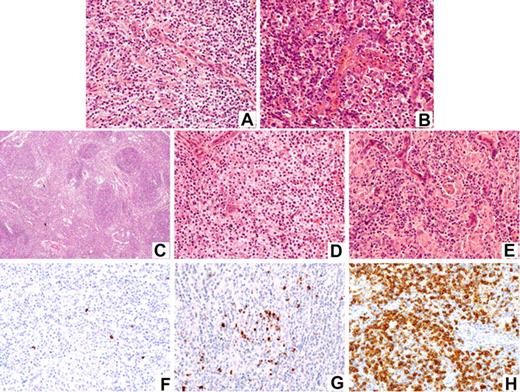
To uncover the prognostic significance of the increase in large cells, cases were assigned to 2 major categories: “rich in large cells” (>10% large B and/or T cells) and “classic,” the latter including cases rich in clear cells, rich in epithelioid cells, and with hyperplastic germinal centers (Figure 1A-E).
Histopathologic spectrum of AITL and patterns of CXCL13 expression. Panels A-E illustrate the large spectrum of angioimmunoblastic lymphoma. Panels F,G illustrate CXCL13 immunostaining patterns: (A) classic-type, (B) rich in large cells, (C) with hyperplastic germinal centers, (D) rich in clear cells, (E) rich in epithelioid cells (hematoxylin eosin stain); (F) score 1, (G) score 2, and (H) score 3. Images were captured by a Zeiss microscope (Carl Zeiss, Heidelberg, Germany); original magnifications ×25 (C) and ×250 (A,B,D-H). The different morphologic subtypes and the level of CXCL13 expression did not influence survival.
Histopathologic spectrum of AITL and patterns of CXCL13 expression. Panels A-E illustrate the large spectrum of angioimmunoblastic lymphoma. Panels F,G illustrate CXCL13 immunostaining patterns: (A) classic-type, (B) rich in large cells, (C) with hyperplastic germinal centers, (D) rich in clear cells, (E) rich in epithelioid cells (hematoxylin eosin stain); (F) score 1, (G) score 2, and (H) score 3. Images were captured by a Zeiss microscope (Carl Zeiss, Heidelberg, Germany); original magnifications ×25 (C) and ×250 (A,B,D-H). The different morphologic subtypes and the level of CXCL13 expression did not influence survival.
Immunohistochemical analysis
Immunohistochemistry was performed on deparaffinized tissue sections using an indirect immunoperoxidase method. After appropriate antigen retrieval, slides were stained for CD20, CD3ϵ, and as far as possible CNA42 and/or CD21 antigens (DakoCytomation, Glostrup, Denmark). CD4, CD2, CD5, and CD7 (DakoCytomation) were performed in 55, 45, 54, and 42 cases, respectively. One-hundred twenty-one cases were tested for the presence of EBV using in situ hybridization with probes specific for the EBV-encoded small RNA (EBER) sequences and/or antibodies to latent membrane protein-1 (LMP-1) (DakoCytomation). Finally, 62 cases, for which additional slides were available, were evaluated for CD10 (56C6; Novocastra, Newcastle, United Kingdom) and CXCL13 (R&D systems, Minneapolis, MN) expression. The abundance of positive lymphoid tumor cells was evaluated semiquantitatively according to a score varying from 0 to 3 as follows (Figure 1F-H): 0 indicates negative/very few positive lymphoid cells; 1, scattered positive lymphoid cells with no or one occasional aggregate; 2, many scattered positive lymphoid cells with more than one aggregate; and 3, sheets of positive lymphoid cells. For detection of CXCL13 expression, an amplification system was used as recently reported.13 Positive internal controls included polymorphonuclear leukocytes and residual germinal center B cells for CD10 and FDCs and TFHs for CXCL13. Cases without positive internal control were excluded from the analysis.
Images were captured with a Zeiss Axioskop2 microscope (Carl Zeiss, Heidelberg, Germany) and Neofluar 100×/0.1 NA optical lenses (Zeiss). Photographs were taken with a DP70 Olympus camera (Olympus, Tokyo, Japan). Image acquisition was performed with Olympus DP Controller 2002, and images were processed with Adobe Photoshop version 7.0 (Adobe Systems, San Jose, CA).
Staging
Patients were clinically staged according to the Ann Arbor classification. Initial investigations included a complete medical history and physical examination; computed tomography of the chest, abdomen, and pelvis; bone marrow biopsy; and biologic evaluation including hemoglobin level, Coombs test, lymphocyte and platelet counts, and gammaglobulin, LDH, albumin, and microglobulin levels (Table 1). Patients were randomized to treatment into one of the 4 groups of LNH87 and 7 groups of LNH93 protocols according to a score taking into account age and a number of prognostic factors of the age-adjusted international prognostic index (aaIPI) score.27 Patients were required to sign an informed consent approved by Hôpital Saint Louis (Paris, France) institutional review board in accordance with the Declaration of Helsinki. They were excluded from the trial if they had positive serologic tests for HIV or human T-lymphotropic virus 1 (HTLV1).
Univariate analysis: survival estimates according to patients' characteristics
| Characteristic . | No. . | % . | 7-y OS, % . | P . | 7-y EFS, % . | P . |
|---|---|---|---|---|---|---|
| Sex | .004 | .094 | ||||
| Male | 95/157 | 60 | 23 | — | 21 | — |
| Female | 62/157 | 40 | 39 | — | 24 | — |
| Age* | .071 | .114 | ||||
| Not older than 60 y | 72/157 | — | 21 | — | 17 | — |
| Older than 60 y | 85/157 | — | 36 | — | 27 | — |
| Performance status | .721 | .613 | ||||
| 0 to 1 | 79/157 | 50 | 27 | — | 17 | — |
| More than 1 | 78/157 | 50 | 31 | — | 27 | |
| B symptoms | 112/155 | 72 | 27 | .125 | 22 | .276 |
| Ann Arbor stage | .425 | .859 | ||||
| I to II | 30/156 | 19 | 32 | — | 14 | — |
| III to IV | 126/156 | 81 | 28 | — | 24 | — |
| Bulky 10 cm or more | 27/102 | 26 | 22 | .130 | 14 | .282 |
| No. of extranodal sites | .094 | .106 | ||||
| 0 to 1 | 75/139 | 54 | 34 | — | 28 | — |
| More than 1 | 64/139 | 46 | 28 | — | 20 | — |
| Extranodal involvement | ||||||
| Liver | 38/154 | 25 | 22 | .130 | 19 | .378 |
| Spleen | 86/155 | 55 | 26 | .143 | 21 | .490 |
| Skin | 46/154 | 30 | 25 | .266 | 19 | .389 |
| Lung | 15/153 | 10 | 18 | .546 | 20 | .858 |
| Bone marrow | 71/151 | 47 | 29 | .775 | 23 | .813 |
| Effusion/edema/ascites | 16/62 | 26 | 25 | .895 | 19 | .525 |
| Polyarthritis/arthralgia | 12/76 | 16 | 25 | .810 | 25 | .263 |
| Skin rash | 36/82 | 44 | 26 | .598 | 19 | .747 |
| IPI score | .799 | .737 | ||||
| 0 to 1 | 15/132 | 11 | 24 | — | 14 | — |
| 2 to 3 | 67/132 | 51 | 26 | — | 20 | — |
| 4 to 5 | 50/132 | 38 | 38 | — | 32 | — |
| PIT score | .106 | .016 | ||||
| 0 to 1 | 38/144 | 26 | 26 | — | 18 | — |
| 2 | 50/144 | 35 | 15 | — | 7 | — |
| 3 to 4 | 56/144 | 39 | 38 | — | 34 | — |
| Anemia†, Hb level no higher than 120 g/L | 101/155 | 65 | 26 | .046* | 22 | .158 |
| Positive Coombs test | 30/92 | 33 | 39 | .299 | 35 | .188 |
| Lymphopenia no higher than 0.7 × 109/L | 77/156 | 49 | 29 | .799 | 19 | .930 |
| Thrombocytopenia no higher than 150 × 109/L | 33/121 | 20 | 26 | .219 | 18 | .363 |
| Hypereosinophilia more than 0.5 × 109/L | 12/38 | 32 | 33 | .324 | 25 | .375 |
| Hypergammaglobulinemia more than 12 g/L | 73/146 | 50 | 36 | .260 | 26 | .294 |
| Increased serum LDH level | 98/149 | 66 | 29 | .794 | 23 | .329 |
| Hypoalbuminemia less than 35 g/L | 71/143 | 50 | 28 | .160 | 24 | .227 |
| Serum β2 microglobulin level more than 250 nM | 71/107 | 66 | 31 | .911 | 22 | .938 |
| Characteristic . | No. . | % . | 7-y OS, % . | P . | 7-y EFS, % . | P . |
|---|---|---|---|---|---|---|
| Sex | .004 | .094 | ||||
| Male | 95/157 | 60 | 23 | — | 21 | — |
| Female | 62/157 | 40 | 39 | — | 24 | — |
| Age* | .071 | .114 | ||||
| Not older than 60 y | 72/157 | — | 21 | — | 17 | — |
| Older than 60 y | 85/157 | — | 36 | — | 27 | — |
| Performance status | .721 | .613 | ||||
| 0 to 1 | 79/157 | 50 | 27 | — | 17 | — |
| More than 1 | 78/157 | 50 | 31 | — | 27 | |
| B symptoms | 112/155 | 72 | 27 | .125 | 22 | .276 |
| Ann Arbor stage | .425 | .859 | ||||
| I to II | 30/156 | 19 | 32 | — | 14 | — |
| III to IV | 126/156 | 81 | 28 | — | 24 | — |
| Bulky 10 cm or more | 27/102 | 26 | 22 | .130 | 14 | .282 |
| No. of extranodal sites | .094 | .106 | ||||
| 0 to 1 | 75/139 | 54 | 34 | — | 28 | — |
| More than 1 | 64/139 | 46 | 28 | — | 20 | — |
| Extranodal involvement | ||||||
| Liver | 38/154 | 25 | 22 | .130 | 19 | .378 |
| Spleen | 86/155 | 55 | 26 | .143 | 21 | .490 |
| Skin | 46/154 | 30 | 25 | .266 | 19 | .389 |
| Lung | 15/153 | 10 | 18 | .546 | 20 | .858 |
| Bone marrow | 71/151 | 47 | 29 | .775 | 23 | .813 |
| Effusion/edema/ascites | 16/62 | 26 | 25 | .895 | 19 | .525 |
| Polyarthritis/arthralgia | 12/76 | 16 | 25 | .810 | 25 | .263 |
| Skin rash | 36/82 | 44 | 26 | .598 | 19 | .747 |
| IPI score | .799 | .737 | ||||
| 0 to 1 | 15/132 | 11 | 24 | — | 14 | — |
| 2 to 3 | 67/132 | 51 | 26 | — | 20 | — |
| 4 to 5 | 50/132 | 38 | 38 | — | 32 | — |
| PIT score | .106 | .016 | ||||
| 0 to 1 | 38/144 | 26 | 26 | — | 18 | — |
| 2 | 50/144 | 35 | 15 | — | 7 | — |
| 3 to 4 | 56/144 | 39 | 38 | — | 34 | — |
| Anemia†, Hb level no higher than 120 g/L | 101/155 | 65 | 26 | .046* | 22 | .158 |
| Positive Coombs test | 30/92 | 33 | 39 | .299 | 35 | .188 |
| Lymphopenia no higher than 0.7 × 109/L | 77/156 | 49 | 29 | .799 | 19 | .930 |
| Thrombocytopenia no higher than 150 × 109/L | 33/121 | 20 | 26 | .219 | 18 | .363 |
| Hypereosinophilia more than 0.5 × 109/L | 12/38 | 32 | 33 | .324 | 25 | .375 |
| Hypergammaglobulinemia more than 12 g/L | 73/146 | 50 | 36 | .260 | 26 | .294 |
| Increased serum LDH level | 98/149 | 66 | 29 | .794 | 23 | .329 |
| Hypoalbuminemia less than 35 g/L | 71/143 | 50 | 28 | .160 | 24 | .227 |
| Serum β2 microglobulin level more than 250 nM | 71/107 | 66 | 31 | .911 | 22 | .938 |
OS indicates overall survival; EFS, event-free survival; IPI, International Prognostic Index; and PIT, prognostic index for peripheral T-cell lymphoma, unspecified.
Median patient age was 62 years (range, 20-89 years).
Median hemoglobin level was 100 g/L (range, 30-120 g/L).
Treatment
The results of both LNH87 and LNH93 protocols have in large part been published.28-36 Briefly, for younger patients, the reference arm was 3 to 4 courses of dose-intensive doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone (ACVBP) followed by sequential consolidation36 (50 patients), and the experimental arm consisted of the following: 8 cycles of methotrexate, bleomycin, doxorubicin, cyclophosphamide, vincristine, and dexamethasone (mBACOD) in patients with no adverse factor28 (LNH 87-1, 11 patients); 4 cycles of ACVBP-like with a second randomization between sequential consolidation and high-dose chemotherapy (CBV) with stem cell rescue for patients younger than 56 years with at least one adverse factor29 (LNH 87-2, 7 patients); 4 alternating induction cycles of teniposide, ifosfamide, mitoxantrone, courses of etoposide, ifosfamide, and mitoxantrone (VIM) and doxorubicin, cyclophosphamide, vindesine, and methotrexate (ACVM) in patients between 56 and 69 years of age with at least one adverse factor30 (LNH 87-3, 14 patients); 3 cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) with involved field radiotherapy for patients with 0 aaIPI factors31 (LNH 93-1, 2 patients); 4 cycles of ACVBP-like followed by a modified consolidation for patients with 1 aaIPI factor (LNH 93-2, 2 patients); and 4 cycles of ACVBP-like followed by high-dose chemotherapy with peripheral blood stem cell rescue for patients younger than 60 years with 2 or more factors32 (LNH 93-3, 8 patients).
For elderly patients, the arms were as follows: 4 cycles of CHOP with or without additional involved field radiotherapy for patients with 0 aaIPI factors33 (LNH 93-4, 4 patients); 8 cycles of CHOP or ACVBP for patients younger than 70 years with 1 to 3 aaIPI factors34 (LNH 93-5, 23 patients); 6 cycles of CHOP-like or reduced CHOP for patients 70 years or older and PS less than 235 (LNH 87-4 and LNH 93-6, 29 patients); oral chemotherapy with etoposide, chlorambucil, and prednisone for patients 70 years or older with a PS 2 or more (LNH 93-7, 7 patients).
Primary treatment failure included change to another therapy including chemotherapy and/or autologous or allogeneic treatment.
Response to treatment was considered as complete response (CR), unconfirmed complete response (CRu), partial response, or failure according to the International Workshop criteria.37
Statistical analysis
Patients' characteristics and remission rates were compared using chi-square test. Event-free survival (EFS) was defined as the time interval between randomization to primary treatment failure, relapse, and death from any cause or last follow up. Overall survival (OS) was defined as the time interval between randomization to last follow up or death from any cause. Estimates of survival were calculated according to the Kaplan-Meier method38 and were compared using the log-rank test.39 Cox proportional hazards regression analysis with OS and EFS as the dependent variables was used to detect potential independent prognostic factors. Differences were considered statistically significant when the 2-sided P value was less than .05. All statistical analyses were performed using the Statistical Application System software (SAS, version 913; SAS Institute, Cary, NC).
Results
Clinical and biologic characteristics of AITL patients at presentation
One-hundred fifty-seven patients were included in the study; they were treated between April 1987 and October 1999. Table 1 summarizes their main clinical and biologic characteristics. The median age was 62 years (range: 20-89 years). Most patients (126/156; 81%) presented with an advanced-stage III-IV, and 21% (29/139) had more than 2 extranodal involved sites. B symptoms were observed in 72% (112/155). Laboratory investigations showed the presence of anemia in 65% (101/155), a positive Coombs test in 33% (30/92), hypergammaglobulinemia more than 12 g/L in 50% (73/146), and elevated serum LDH level in 66% (98/149) of patients. Sixty percent (58/97) and 38% (24/63) of anemic patients had bone marrow involvement and a positive Coombs test, respectively. The international prognostic index (IPI) score was more than 1 in 89% (117/132) of patients, and the prognostic index for PTCL/U (PIT) score40 (scoring system including age > 60 years, performance status [PS] ≥ 2, LDH ≥ normal, and bone marrow involvement) was more than 2 in 39% (56/144) of patients.
Pathological findings
By definition, all cases displayed FDC hyperplasia demonstrated by morphology and/or CNA42/CD21 immunostaining, as well as a variable proportion of CD20 large B cells within an atypical T-cell population. Although clonality could be assessed in only a minority of cases (data not shown), the review process retained cases that disclosed typical histopathological and immunophenotypic features for AITL, and that would not be mistaken for a reactive process, with all but 4 cases showing diffuse effacement of the normal architecture. According to their cytologic aspect, 41 cases were classified as rich in large cells and 116 as classic (Table 2) including 19 rich in epithelioid cells, 14 rich in clear cells, and 4 with hyperplastic germinal centers. As indicated in Table 2, CD3 was positive in 100%, CD4 in 95%, CD2 in 91%, CD5 in 85%, and CD7 in 50% of tested cases. A total of 111 cases were studied for EBV using EBER in situ hybridization. Among the 103 cases with interpretable results, 25 (24%) were negative for EBV, whereas 78 (76%) contained a variable number of EBER-positive cells. In the majority of cases, EBER-positive cells were scattered, with the exception of 19 cases in which there were sheets of EBV-positive cells. Ten additional cases that were not investigated by EBER in situ hybridization disclosed scattered blasts positive for LMP-1. Among the 52 cases interpretable for CD10 expression, 71% disclosed aggregates or sheets of CD10+ cells with atypical features (scored 2 + 3). Scattered CD10+ lymphoid cells (score 1)—the neoplastic nature of which could not be determined—were observed in 12% and CD10 was negative (score 0) in 17% of our cases. Forty-five of 62 sections were adequate for CXCL13 interpretation. CXCL13 staining was found, as a dot reinforcement of the Golgi area, in aggregates or sheets of atypical cells (score 2 + 3) in 73% of cases, and in scattered lymphoid cells (score 1) in the remaining 27%. Overall, at least one marker was scored positive in 86% of the interpretable cases and both markers were negative in 14% of them.
Univariate analysis: survival estimates according to histopathologic and phenotypic* characteristics of AITL patients
| Pathological finding . | No. . | % . | 7-y OS, % . | P . | 7-y EFS, % . | P . |
|---|---|---|---|---|---|---|
| Classic | 116 | 74 | 24 | .444 | 19 | .350 |
| Rich in epithelioid cells | 19 | 13 | — | — | — | — |
| Rich in clear cells | 14 | 9 | — | — | — | — |
| With HGC | 04 | 03 | — | — | — | — |
| Rich in large cells | 41 | 26 | 41 | — | 31 | — |
| CD10 | 52 | .634 | .870 | |||
| Score 0 | 09 | 17 | 27 | — | 14 | — |
| Score 1 | 06 | 12 | 67 | — | 33 | — |
| Score 2 | 25 | 48 | 24 | — | 29 | — |
| Score 3 | 12 | 23 | 17 | — | 8 | — |
| CXCL13 | 45 | .865 | .724 | |||
| Score 0 | 0 | 0 | — | — | — | — |
| Score 1 | 12 | 27 | 33 | — | 17 | — |
| Score 2 | 24 | 53 | 23 | — | 20 | — |
| Score 3 | 09 | 20 | 33 | — | 0 | — |
| EBV (ISH) | 103 | .557 | .386 | |||
| Negative | 25 | 24 | 15 | — | 9 | — |
| Positive | 78 | 76 | 30 | — | 21 | — |
| Pathological finding . | No. . | % . | 7-y OS, % . | P . | 7-y EFS, % . | P . |
|---|---|---|---|---|---|---|
| Classic | 116 | 74 | 24 | .444 | 19 | .350 |
| Rich in epithelioid cells | 19 | 13 | — | — | — | — |
| Rich in clear cells | 14 | 9 | — | — | — | — |
| With HGC | 04 | 03 | — | — | — | — |
| Rich in large cells | 41 | 26 | 41 | — | 31 | — |
| CD10 | 52 | .634 | .870 | |||
| Score 0 | 09 | 17 | 27 | — | 14 | — |
| Score 1 | 06 | 12 | 67 | — | 33 | — |
| Score 2 | 25 | 48 | 24 | — | 29 | — |
| Score 3 | 12 | 23 | 17 | — | 8 | — |
| CXCL13 | 45 | .865 | .724 | |||
| Score 0 | 0 | 0 | — | — | — | — |
| Score 1 | 12 | 27 | 33 | — | 17 | — |
| Score 2 | 24 | 53 | 23 | — | 20 | — |
| Score 3 | 09 | 20 | 33 | — | 0 | — |
| EBV (ISH) | 103 | .557 | .386 | |||
| Negative | 25 | 24 | 15 | — | 9 | — |
| Positive | 78 | 76 | 30 | — | 21 | — |
HGC indicates hyperplastic germinal centers; OS, overall survival; EFS, event-free survival; and ISH, in situ hybridization.
CD3, CD4, CD2, CD5, and CD7 were positive in tumor cells in 100% (157/157), 95% (52/55), 91% (41/45), 85% (46/54), and 50% (21/42), respectively.
Clinical outcome and prognostic parameters in the overall population
All but 10 patients received an anthracycline-based chemotherapy. CR/CRu was achieved in 46% of patients after induction therapy. No difference could be observed between the different chemotherapeutic arms of the randomized studies including stem cell transplantation. An attempt was made to put together all the intensive regimens versus the conventional ones, and it was not possible to discriminate a better or a worse outcome according to treatment.
Thirty-one (20%) of 156 patients developed severe infection and 82 (53%) of 156 developed severe neutropenia during therapy (≥ grade 3 toxicity). Sixty-six (42%) of 156 patients relapsed or progressed under treatment. Forty-five of them received salvage chemotherapy and 12 of them received autologous or allogeneic treatment.
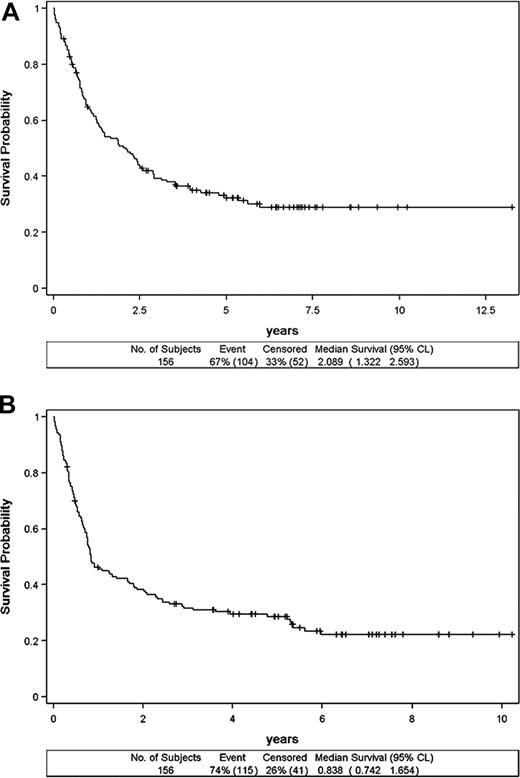
Follow-up could be assessed in 156 patients. The median follow-up was 68 months (range: 3.77-161.47 months). The 2-, 5-, and 7-year OS rates were 51% (CI: 42.85-58.77), 33% (CI: 25.63-40.95), and 29% (CI:, 21.19-36.73) respectively (Figure 2A), reaching an apparent plateau level around 6 years. The 2-, 5-, and 7-year EFS rates were 38% (CI: 30.66-46.06), 29% (CI: 21.54-36.02), and 23% (CI: 15.57-29.94), respectively (Figure 2B). Overall, there were 107 deaths. Patients died from lymphoma (57%), treatment toxicity (7%), infection (7%), lymphoma and toxicity (7%), lymphoma and infection (5%), toxicity and infection (4%), lymphoma and toxicity and infection (1%), other cancer (4%), or from other or unknown reason (8%). Of note, 2 of the 7 patients older than 70 years remained alive and free of disease after 5 and 10 years of follow-up.
Survival of the 156 patients with angioimmunoblastic T-cell lymphoma. Overall survival (A) and event-free survival (B).
Survival of the 156 patients with angioimmunoblastic T-cell lymphoma. Overall survival (A) and event-free survival (B).
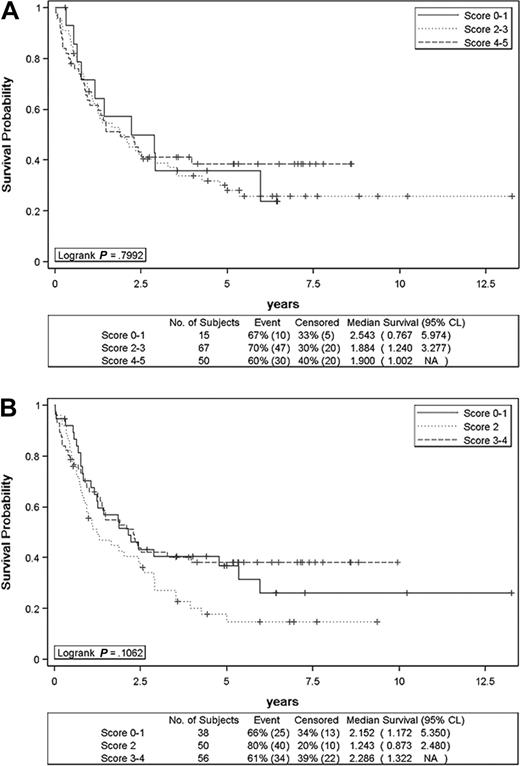
On univariate analysis (Table 1), male sex (P = .004), mediastinal lymphadenopathy (P = .013), and anemia (P = .046) turned out to be poor prognostic factors for OS. Anemia correlated with bone marrow involvement (P < .001) and showed a trend toward an association with a positive Coombs test (P = .098). Bone marrow involvement and positive Coombs test did not influence survival, however. Mediastinal lymphadenopathy (P = .026) and PIT score (P = .016) influenced EFS. However, on careful examination of PIT survival curves according to score, patients with scores 3 to 4 were doing better than those with scores 0 to 1 and 2. The same applied to age (P = .071), where patients older than 60 years were doing better than younger ones with regard to OS. Since it is likely that these unexpected findings represent a false positive selection (ie, alpha = 0.05) due to sampling fluctuations among the numerous parameters tested in our study, we decided not to include age and PIT in the multivariate analysis. On multivariate analysis, male sex (P = .004), mediastinal lymphadenopathy (P = .041), and anemia (P = .042) remained as independent prognostic factors for OS (Table 3). Mediastinal lymphadenopathy (P = .053) and male sex (P = .067) were at the limits of statistical significance for EFS. OS and EFS differed significantly in favor of patients who developed CR/CRu (P < .001). IPI was not predictive of survival (Figure 3A). The different histologic subtypes, the presence of EBV-positive cells, as well as CD10 and CXCL13 scoring did not have any influence on survival (Table 2). Of note, cases with sheets of EBV-positive cells paralleled those that were rich in large cells (P = .0219). The impact of the clinicopathological prognostic index (including age, LDH > normal, nonambulatory PS, Ki67 ≥ 80%) described by Went et al for PTCL/U patients could not be assessed in our AITL series.41
Parameters influencing overall survival of AITL patients on multivariate analysis
| Parameter . | P . | Relative risk . | 95% CI low . | 95% CI high . |
|---|---|---|---|---|
| Male sex | .004 | 1.856 | 1.221 | 2.819 |
| Anemia, Hb no higher than 120 g/dL | .042 | 1.570 | 1.017 | 2.425 |
| Mediastinal lymphadenopathy | .041 | 1.535 | 1.017 | 2.316 |
| Parameter . | P . | Relative risk . | 95% CI low . | 95% CI high . |
|---|---|---|---|---|
| Male sex | .004 | 1.856 | 1.221 | 2.819 |
| Anemia, Hb no higher than 120 g/dL | .042 | 1.570 | 1.017 | 2.425 |
| Mediastinal lymphadenopathy | .041 | 1.535 | 1.017 | 2.316 |
Overall survival of angioimmunoblastic T-cell lymphoma patients. Overall survival figures according to IPI (A) and PIT (B) scores are shown.
Overall survival of angioimmunoblastic T-cell lymphoma patients. Overall survival figures according to IPI (A) and PIT (B) scores are shown.
Comparison between the 2 histologic categories: classic and rich in large cells
Overall, the 2 populations classic and rich in large cells were not statistically different in terms of clinical features, laboratory findings, and treatment response (P = .676) except for a higher frequency of elevated serum LDH (P = .027) and β2 microglobulin levels (P = .046) within the group rich in large cells. The distribution of OS and EFS curves was not statistically different between these 2 groups either.
Discussion
To the best of our knowledge, the present series of AITL patients is the largest reported so far in which all patients were included in prospective randomized clinical trials. In this series, we further extend the peculiar clinical and biologic findings of AITL. We found very few prognostic factors for this disease, and we confirm its poor prognosis despite a first-line intensive CHOP-like chemotherapy with a curative intent in most patients. We also emphasize its morphologic heterogeneity as well as the common expression of CD10 and CXCL13 determined on routinely fixed samples.
From a clinical point of view and in keeping with previous reports,16-23,42-44 we observed that AITL is a disease of the elderly presenting with systemic manifestations and features known to be poor prognostic factors for B-cell lymphomas: 54% of our patients were older than 60 years, 50% had an altered PS, 81% presented with stage III-IV disease, 46% had more than one extranodal site involvement, and 66% had an elevated LDH level. The clinical symptoms and biologic signs are not specific of AITL but their association appears to be very suggestive of the disease. Notably, skin rash (44%), arthritis/arthralgia (16%), pleural effusion/ascites/edema (26%), eosinophilia (32%), hypergammaglobulinemia (50%), and positive Coombs test (33%) appear to represent distinctive manifestations of AITL.
A small number of studies sought to identify prognostic factors in AITL/AILD, yielding controversial results.16-23 Their results might be hampered by the relatively limited number of patients and/or the heterogeneous therapies including prednisolone alone in a proportion of these patients. In the current study, we could not find any pertinent prognostic factor for this disease besides male sex (P = .004), mediastinal lymphadenopathy (P = .041), and anemia (P = .042) that adversely affected OS on multivariate analysis. The impact of IPI score was rarely defined in the literature for AITL, and in agreement with Lee et al,22 it was curiously not predictive of survival at all. And in contrast to what has been originally reported for PTCL/U,40 a high PIT score was not associated with a worse outcome in our AITL series. Even when PIT was included in the multivariate analysis, it did not significantly influence survival (data not shown). Moreover, in the present study where patients received more intensive chemotherapy overall compared with previous reports, it was not possible to demonstrate any survival benefit for one treatment arm over the other even for those submitted to consolidation with autologous stem cell transplantation. This latter subset analysis is in agreement with what had already been reported by Mounier et al on non–anaplastic T-cell lymphomas and front-line autologous stem cell transplantation.45 ACVBP regimen was not superior to standard CHOP and mBACOD regimens with respect to OS. These data should be interpreted with caution however due to the relatively low number of cases assigned to each treatment arm and obviously require further confirmation in prospective randomized trials. In a retrospective study by Schetelig et al, including 29 AITL patients treated with high-dose chemotherapy followed by autologous stem cell transplantation, CR increased from 45% before high-dose chemotherapy to 76% after, with a 44% and 37% 5-year OS and progression-free survival, respectively.46 Interestingly, similar results have been recently reported in a large series of AITL patients treated with high-dose chemotherapy and autologous stem cell transplantation, with a 59% OS and 42% progression-free survival at 4 years after transplantation.47
From a pathologic point of view, the present series describes the morphologic heterogeneity of AITL, which could be a source of misdiagnosis to poorly experienced pathologists, especially concerning cases with hyperplastic germinal centers and very rich in epithelioid cells. The former can be confused with a hyperplastic reactive disorder and the latter with Lennert lymphoma or Hodgkin lymphoma. This is reflected by the large error rate (50%) in the referred cases described by Attygalle et al11 and by our very low recruitment of cases with hyperplastic germinal centers, a pattern that has recently received attention since it was described in 1998.48
Few reports have tried to identify histologic prognostic features, but none of them would be of proven clinical value.18,20,22,49 The current study specifically attempted to investigate the potential prognostic relevance for the increase in large cells, but it failed to show any significance on outcome for this increase. In this respect, the presence of sheets of EBV-infected cells did not affect survival in this series of patients treated with polychemotherapy. Moreover, the number of CD10+ and CXCL13+ atypical cells, which is supposed to represent the neoplastic counterpart, did not show any influence on survival. The different morphologic subtypes, and in particular the abundance of clear cells, were not predictive of survival either.
It has been recently shown that CXCL13, a chemokine critically involved in B-cell migration into germinal centers, was highly up-regulated in the TFH subset.50-52 Subsequently, we and others have shown that CXCL13 was expressed by neoplastic cells of most AITLs and that CD10 as well as TFH markers such as CXCL13 belong to the molecular gene signature of AITL.9,13,14 CD10 and CXCL13 were proposed as sensitive immunohistochemical markers in AITL.11,13,14 This finding was confirmed in the present study where at least one marker stained clusters of atypical lymphocytes in the majority (86%) of cases. However, in the few cases (14%) where it was difficult to assign the neoplastic nature for the rare scattered stained cells on purely morphologic grounds, both markers were considered negative. In such circumstances, the diagnosis of AITL should rely on a combination of classical morphologic criteria together with clinical and biologic features.
It was hypothesized that the clinical effects of AITL are due to marked dysregulation of the immune system rather to direct complications of tumor growth.11,14,53 This hypothesis can be supported by our findings that high IPI and PIT scores—which are supposed to reflect increase in tumor burden—did not correlate with a worse outcome, and by the fact that eosinophilia and hypergammaglobulinemia are more often encountered in AITL than in other subtypes of T-cell lymphomas.24 Moreover, a recent gene expression study of highly enriched tumor samples of AITL showed that nearly 90% of AITL signature was contributed by nonneoplastic cells.9 The AITL profiling portrait included genes involved in the humoral immune response and in the modulation of vasculogenesis and extracellular matrix and genes encoding various chemokines mediating the recruitment of inflammatory cells. These findings are in line with the peculiar histologic features of AITL where tumor cells are greatly outnumbered by the surrounding reactive cells and found in intimate contact with the expanding meshwork of FDCs. As a whole, these results support the idea considering AITL as an immunologically functional disease in which the clinical behavior is determined by the resultant cross talk between the malignant cells and the immunologic micro-environment. An improved understanding of the interactions between neoplastic cells and microenvironment in AITL would offer the possibility of identifying new targets for rationale design of future treatments. In this respect, immunomodulatory approaches54-57 deserve some consideration in future prospective randomized trials.
In conclusion, despite various intensive regimens with an anthracycline-based chemotherapy, AITL, compared with other non-Hodgkin lymphomas, pursues an aggressive clinical course. AITL does not present any pertinent prognostic factor besides, naturally, the achievement of a complete response to therapy. However, after a long follow-up, we observed a trend for a plateau level reaching a survival probability of approximately 30% at 6 years, thus providing a threshold for evaluation of new drugs or strategies including allograft with reduced intensity regimen. A longer follow-up is needed to determine whether these patients are eventually cured of their disease.
The online version of this article contains a data supplement.
This study was presented at the 48th annual meeting of the American Society of Hematology, Orlando, FL, December 2006.58
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Sylvie Sousa, Chafika Coppeaux, Nicolas Nio, and Marion Fournier for their skillful assistance in data management and Nadine Martin-Garcia for her technical assistance.
Authorship
Contribution: C.G. and P.G. designed the study; N. Mourad, C.G., and P.G. wrote the paper; N. Mounier performed the statistical analysis; N. Mourad, N. Mounier, J.B., C.G., and P.G. controlled and analyzed the data; A.F., C.J.L.M.M., J.D., and P.G. reviewed the pathological material; J.-F.E. performed part of the EBER studies; N. Mounier, E.R., A.D., R.B., A.B., C.H., B.C., and C.G. participated in data collection; all authors checked the final version of the paper.
A complete list of the members of the Groupe d'Etude des Lymphomes de l'Adulte appears in Document S1, available on the Blood website (see the Supplemental Materials link at the top of the online article).
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Philippe Gaulard, Département de Pathologie et Inserm U841, Hôpital Henri Mondor, Créteil–F-94000, France; e-mail: philippe.gaulard@hmn.aphp.fr.