Abstract
The transcription factor Gata1 is expressed in several hematopoietic lineages and plays essential roles in normal hematopoietic development during embryonic stages. The lethality of Gata1-null embryos has precluded determination of its role in adult erythropoiesis. Here we have examined the effects of Gata1 loss in adult erythropoiesis using conditional Gata1 knockout mice expressing either interferon- or tamoxifen-inducible Cre recombinase (Mx-Cre and Tx-Cre, respectively). Mx-Cre–mediated Gata1 recombination, although incomplete, resulted in maturation arrest of Gata1-null erythroid cells at the proerythroblast stage, thrombocytopenia, and excessive proliferation of megakaryocytes in the spleen. Tx-Cre–mediated Gata1 recombination resulted in depletion of the erythroid compartment in bone marrow and spleen. Formation of the early and late erythroid progenitors in bone marrow was significantly reduced in the absence of Gata1. Furthermore, on treatment with a hemolytic agent, these mice failed to activate a stress erythropoietic response, despite the rising erythropoietin levels. These results indicate that, in addition to the requirement of Gata1 in adult megakaryopoiesis, Gata1 is necessary for steady-state erythropoiesis and for erythroid expansion in response to anemia. Thus, ablation of Gata1 in adult mice results in a condition resembling aplastic crisis in human.
Introduction
Production of mature blood cells from the pluripotent hematopoietic stem cells is continuous throughout life. This process is controlled by combinatorial functions of lineage-specific and ubiquitous transcription factors. The zinc finger transcription factor Gata1 is a prototype of lineage-specific transcription factors, with restricted expression in several myeloid lineages.1,2 Gene ablation studies of Gata1 in mice revealed its essential role in erythroid cell development. Embryos lacking Gata1 die at approximately embryonic day 11.5 because of arrested maturation of primitive erythroid cells.3 A similar phenotype was observed in embryos bearing a Gata1 knockdown mutation (Gata1.05) that causes 95% reduction of Gata1 mRNA level in erythroid cells.4 A critical role for Gata1 in megakaryocyte maturation and platelet formation has been shown in mice with megakaryocyte-specific Gata1 deficiency.5,6 A remarkable phenotype of these mutant mice is that defective maturation of megakaryocytes is associated with excessive proliferation, suggesting that Gata1 inhibits cell proliferation during megakaryocytic maturation
Although roles for Gata1 in postnatal megakaryopoiesis have been thoroughly investigated, the embryonic lethality caused by Gata1 deficiency in erythroid precursors has precluded studies of its role in postnatal erythropoiesis. In adults, the rate of red cell production in the steady-state condition is much lower than that in the fetal and neonatal stages. However, in response to erythropoietic stress, the rate of erythropoiesis is rapidly increased.7 The plasma levels of erythropoietin (Epo), the central regulator of erythroid production, increase because of hypoxia in tissues and are directly proportional to the rate of erythropoiesis.8 It is unknown whether Gata1 contributes to the “erythropoietic reserve,” a characteristic feature of adult erythropoiesis. Recently, we have demonstrated that the expression of a luciferase reporter regulated by the hematopoietic regulatory domain of the mouse Gata1 gene (G1-HRD-luc) is significantly increased in the spleen in response to various erythropoietic stimuli.9 We monitored G1-HRD-luc activity in living mice and found that induction of luciferase activity occurs immediately after an increase in the levels of Epo. The rapid increase of G1-HRD-luc is mainly the result of increased numbers of late erythroid progenitors that express high levels of Gata1. These observations suggest that this factor plays a pivotal role during stress erythropoiesis. However, this has not been demonstrated in vivo yet.
In this study, we have assessed adult megakaryo/erythropoiesis in the absence of Gata1. We have inactivated the Gata1 gene in adult hematopoietic cells using conditional Gata1 knockout mice and 2 inducible Cre transgenic mouse lines. The interferon-inducible Cre system (Mx-Cre) exerts recombination of the target locus in the liver, spleen, and bone marrow (BM).10 The second approach, the tamoxifen-inducible Cre system (Tx-Cre), exerts recombination ubiquitously.11 Our results confirm the requirement of Gata1 in adult megakaryopoiesis, and show a unique role for Gata1 in the homeostatic regulation of adult erythropoiesis.
Methods
Mice
All experiments involving animals were conducted strictly according to the legal and ethical requirements demanded by law and have been approved by the independent local ethical committees for animal experiments.
Conditional Gata1 knockout mice were generated previously2,12,13 (Figure 1A). Because the Gata1 gene is X-linked, the Gata1 knockout phenotype was examined in hemizygous male mice (Gata1fl/y) expressing a Cre transgene. Transgenic mice expressing an interferon-inducible Cre recombinase (Mx-Cre)10 were kindly provided by Drs H. Hirai and M. Kurokawa, University of Tokyo. Mice expressing a tamoxifen (Tx) inducible Cre recombinase under the Rosa26 promoter (Tx-Cre) were generated previously.11
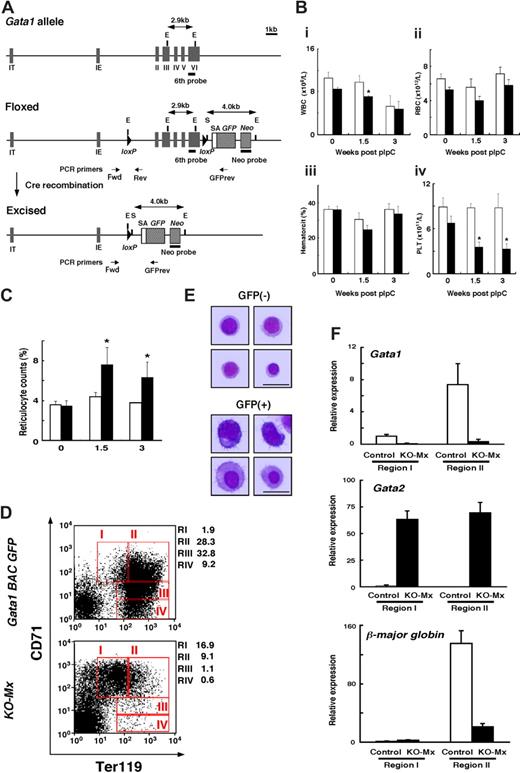
Mx-Cre–mediated deletion of the Gata1 gene in adult mice affects erythropoiesis. (A) Top, the mouse Gata1 locus. Middle, floxed Gata1 allele generated by inserting 2 loxP sites and the splice acceptor (SA)/GFP cDNA cassette. Bottom, Gata1 null allele obtained after Cre-mediated recombination. IT, IE, and II-VI indicate exons of Gata1 gene. E and S indicate EcoRI and SalI restriction sites, respectively. Positions of probes used for Southern blot analysis and PCR primers (forward, reverse, reverse GFP) are indicated. (B) Peripheral blood counts of Gata1fl/y::Mx-Cre(+) mice (KO-Mx, ■) and controls (□) at 0, 1.5, and 3 weeks after pIpC injection. Results are shown as mean plus or minus SD from 4 mice: (i) white blood cell counts, (ii) red blood cell counts, (iii) hematocrit, and (iv) platelet counts (*P < .05 for KO-Mx mice compared with controls). (C) Frequency (%) of reticulocyte counts in peripheral blood from KO-Mx mice (■) and controls (□) at 0, 1.5, and 3 weeks after pIpC injection. Results are shown as mean plus or minus SD (error bars) from 3 mice (*P < .05 for KO-Mx mice compared with controls). (D) FACS analysis of BM cells prepared from the Gata1 BAC GFP (top) and the KO-Mx mice (bottom) at 3 weeks after pIpC injection. Expression of the erythroid surface markers Ter119 and CD71. Expression was examined in cell fractions gated for GFP+ cells. RI, RII, RIII, and RIV represent Ter119lowCD71high, Ter119highCD71high, Ter119highCD71med, and Ter119high-CD71low populations, respectively.24 The relative number in each region as a percentage of gated cells is indicated. (E) Morphology of GFP+ and GFP− cells isolated from the KO-Mx BM. GFP+ and GFP− cells were sorted from RI, RII, and RIII fractions of the KO-Mx BM. Representative examples of Wright-Giemsa-stained cytospins are shown. High-resolution images were taken from 4 separate fields of view to show cellular morphology. Scale bar represents 100 μm. (F) Real-time RT-PCR analyses of Gata1, Gata2, and β-major globin mRNAs in regions I and II cells prepared from the GFP+ fraction of KO-Mx or control BM. BM cells from 3 mice/group were pooled and sorted by FACS. Data were normalized with glyceraldehyde-3 phosphate dehydrogenase (GAPDH) mRNA levels. The expression level of region I from control mice was shown as 1.0. Results are shown as mean plus or minus SD (error bars) from 3 samples.
Mx-Cre–mediated deletion of the Gata1 gene in adult mice affects erythropoiesis. (A) Top, the mouse Gata1 locus. Middle, floxed Gata1 allele generated by inserting 2 loxP sites and the splice acceptor (SA)/GFP cDNA cassette. Bottom, Gata1 null allele obtained after Cre-mediated recombination. IT, IE, and II-VI indicate exons of Gata1 gene. E and S indicate EcoRI and SalI restriction sites, respectively. Positions of probes used for Southern blot analysis and PCR primers (forward, reverse, reverse GFP) are indicated. (B) Peripheral blood counts of Gata1fl/y::Mx-Cre(+) mice (KO-Mx, ■) and controls (□) at 0, 1.5, and 3 weeks after pIpC injection. Results are shown as mean plus or minus SD from 4 mice: (i) white blood cell counts, (ii) red blood cell counts, (iii) hematocrit, and (iv) platelet counts (*P < .05 for KO-Mx mice compared with controls). (C) Frequency (%) of reticulocyte counts in peripheral blood from KO-Mx mice (■) and controls (□) at 0, 1.5, and 3 weeks after pIpC injection. Results are shown as mean plus or minus SD (error bars) from 3 mice (*P < .05 for KO-Mx mice compared with controls). (D) FACS analysis of BM cells prepared from the Gata1 BAC GFP (top) and the KO-Mx mice (bottom) at 3 weeks after pIpC injection. Expression of the erythroid surface markers Ter119 and CD71. Expression was examined in cell fractions gated for GFP+ cells. RI, RII, RIII, and RIV represent Ter119lowCD71high, Ter119highCD71high, Ter119highCD71med, and Ter119high-CD71low populations, respectively.24 The relative number in each region as a percentage of gated cells is indicated. (E) Morphology of GFP+ and GFP− cells isolated from the KO-Mx BM. GFP+ and GFP− cells were sorted from RI, RII, and RIII fractions of the KO-Mx BM. Representative examples of Wright-Giemsa-stained cytospins are shown. High-resolution images were taken from 4 separate fields of view to show cellular morphology. Scale bar represents 100 μm. (F) Real-time RT-PCR analyses of Gata1, Gata2, and β-major globin mRNAs in regions I and II cells prepared from the GFP+ fraction of KO-Mx or control BM. BM cells from 3 mice/group were pooled and sorted by FACS. Data were normalized with glyceraldehyde-3 phosphate dehydrogenase (GAPDH) mRNA levels. The expression level of region I from control mice was shown as 1.0. Results are shown as mean plus or minus SD (error bars) from 3 samples.
Induction of the Cre transgenes in vivo
To induce the Mx-Cre transgene, animals (8-10 weeks of age) were injected intraperitoneally with 250 μg polyinosinic-polycytidylic acid (pIpC) (Sigma-Aldrich, St Louis, MO) dissolved in saline on experimental days 0, 2, and 4. Mice were killed 3 weeks after the first pIpC injection for the analysis. Induction of the Tx-Cre transgene was done as described previously.2 Gata1fl/y mice were used as controls in most experiments. However, wild-type (WT) and Cre transgenic mice were also used as controls in some experiments; phenotypes were indistinguishable among these mice.
Induction of hemolytic anemia with phenylhydrazine
After the last week of Tx treatment, mice were injected subcutaneously with 0.4% phenylhydrazine (PHZ; Sigma-Aldrich) in saline (12 μL/g body weight) for 2 consecutive days (day 1 and 2). Mice were collected at day 5 for analysis.
Recombination analysis
To determine the efficiency of recombination, Southern blot analysis was performed using genomic DNAs. DNAs were digested with EcoRI and SalI and probed with the Gata1 6th exon gene fragment (6th ex probe) and the Neo coding sequence (Neo probe) simultaneously. A 4 kb band hybridizing with the Neo probe appears from both the excised and nonexcised alleles, whereas a 2.9 kb band hybridized with 6th ex probe appeared only from the nonexcised allele. The band intensity was determined by densitometry, and recombination efficiency was estimated by the reduction of the intensity of the 2.9 kb band relative to that of the 4 kb band. Alternatively, Cre-mediated recombination was determined by PCR. To amplify the floxed (nondeleted) allele product, the forward primer 5′-cgccgagctctgtctagtaa-3′ between IE exon and the 5′ loxP site and the reverse primer 5′-ttcctctttctcctcctccg-3′ at 3′-adjacent sequence of the 5′ loxP site were used. To amplify the recombined allele product, the forward primer and reverse GFP primer 5′-ggtgctcaggtagtggtt-3′ were used.
Hematologic analysis and cell culture
Peripheral blood samples were taken from the retro-orbital venous plexus using heparin-coated microtubes. Blood counts were determined with an automated hemocytometer (Nihon Kohden, Tokyo, Japan) or a Vet ABC counter (SCIL, Viernheim, Germany). The analysis of Epo levels in plasma was performed using the Quantikine mouse/rat Epo immunoassay (BD Biosciences, Franklin Lakes, NJ). Colony forming unit- and burst forming unit-erythroid (CFUe and BFUe) assays were performed by culturing 2 × 105 cells per 35-mm dish in methylcellulose media (MethoCult M3234; StemCell Technologies, Vancouver, BC) supplemented with 4 U/mL human recombinant Epo (Janssen-Cilag, Issy-les-Moulineaux, France), 100 ng/mL murine recombinant stem-cell factor, 20 μg/mL iron-saturated human transferrin (kind gifts of Dr M. von Lindern), 2 × 10−4M hemin (Sigma-Aldrich) and 1% penicillin/streptomycin/l-glutamine solution (Invitrogen, Carlsbad, CA). Colony numbers were determined after 3 days (CFUe) or 8 days (BFUe) of culture on triplicate dishes. Hanging drop cultures were done as described14 with freshly isolated BM single cell suspension (HD ST) either in the presence or absence of 0.5 μmol carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen) (HD CFSE).
Cytologic and histologic analysis
Single cell suspensions prepared from BM and spleen were analyzed in a CASY counter (Schärfe Systems, Reutlingen, Germany) for number and cell size distribution. For histologic examination, sections prepared from BM or spleen were stained with hematoxylin or acetylthiocholiniodine as described previously.14 Cytospins from BM and spleen single cell suspensions and spleen cryosections were stained with 1% (w/v) O-dianisidine and Diff-Quik (Medion Diagnostics, Düdingen, Switzerland) as described previously.13 Gata1 immunostaining was performed with the anti-Gata1 monoclonal (N6) antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Reticulocyte counts were examined manually using blood smears stained with new methylene blue. Proplatelet formation assays were performed as described.15
Light microscopy was performed on Olympus BX40F4 Research Microscope (Olympus, Tokyo, Japan) with either PlanApo (2×/0.08) or UPlanFl objective lens (10×/0.30, 20×/0.50, 40×/0.75) or Olympus system biological microscope (CX41) with PLCN objective lens (10×/0.25, 20×/0.40, 40×/0.65). Original magnification of each micrograph is indicated in each figure legend. The imaging medium was air for all subjects. Data acquisition was done with DP70 digital camera attached to the microscopes and DP-controller software (Olympus). Images were processed using Adobe Photoshop (Adobe Systems, San Jose, CA).
Megakaryocyte ploidy analysis
To determine megakaryocyte ploidy class, BM cells were harvested from mice and washed in CATCH medium16 containing 2% FBS and 0.1% tri-sodium citrate. To label the megakaryocyte population, cell suspensions were incubated first with fluorescein isothiocyanate-conjugated anti-CD41 antibody (BD Biosciences). Megakaryocytes were then fixed in 70% ice-cold ethanol and stained with propidium iodide for 30 minutes. After filtration, 5 μL RNaseA (50 μg/mL) was added to each sample. DNA content was determined by flow cytometry (FACSCalibur or Vantage). Data were analyzed using CellQuest 3.3 software (BD Biosciences).
Fluorescence-activated cell sorting analysis
Fluorescence-activated cell sorting (FACS) analysis was performed as described17 using the following antibodies: biotinylated or phycoerythrin (PE)-conjugated anti-Ter119, allophycocyanin-conjugated streptavidin, fluorescein isothiocyanate-, biotinylated or PE-conjugated anti-CD71, PE-conjugated anti-c-Kit, and 7-actinomycin-C (7AAD). PE- and allophycocyanin-conjugated rat IgG2b were used as isotype-matched controls. Antibodies were from BD Biosciences.
Real-time reverse-transcribed polymerase chain reaction
Total RNA was extracted from fractionated BM cells with the RNeasy kit (QIAGEN, Alameda, CA). Reverse transcription of the RNA was performed with the Sensiscript RT Kit (QIAGEN). Real-time reverse-transcribed polymerase chain reaction (RT-PCR) was run on ABI7700 (Applied Biosystems, Foster City, CA) using Taqman probes. Sequences for primers and Taqman probes are available on request.
Western blot analysis
Whole cell extracts prepared from BM were run on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and electroblotted on PVDF membranes as described.13 Western blot analysis was performed using anti-Gata1 antibody (N6; Santa Cruz Biotechnology) or anti-Nucleophosmin (Npm1; Abcam, Cambridge, United Kingdom).
Results
Postnatal ablation of Gata1 results in impaired erythroid cell maturation
We used an interferon-inducible Cre transgene10 (Mx-Cre) to excise the floxed-Gata1 allele in adult Gata1fl/y mice (Figure 1A). Previous studies reported that this transgene mediates recombination in almost all hematopoietic cell lineages and in hematopoietic stem cells on injection of the interferon inducer pIpC.18-23 However, Southern blot analyses revealed that the recombined allele was observed approximately 20% of the WT allele in BM at 3 weeks after the pIpC treatment in our study (data not shown). In peripheral blood, white blood cell (WBC) counts were reduced in Gata1fl/y::Mx-Cre (hereafter referred to as KO-Mx) compared with controls at 1.5 weeks, but this difference was no longer observed at 3 weeks after the pIpC treatment (Figure 1Bi). Red blood cell (RBC) counts and hematocrit (HCT) levels did not differ between KO-Mx mice and WT at 3 weeks after the pIpC treatment (Figure 1Bii,iii). In contrast, platelet counts of KO-Mx mice declined to one-third of the control levels (Figure 1Biv).
Remarkably, although anemia was not observed, reticulocyte counts were significantly greater in the KO-Mx peripheral blood compared with controls at 1.5 and 3 weeks after the pIpC injection (Figure 1C). This suggests that ablation of Gata1 affects erythropoiesis and that unrecombined erythroid cells compensate for this defect, resulting in the observed increase in reticulocytes in KO-Mx mice. To test this possibility, we examined GFP-positive cells in the BM of KO-Mx mice. The GFP cDNA is expected to be transcribed from the Gata1 IE promoter after Cre-mediated recombination and hence can be used as a marker of Gata1-null cells (Figure 1A). FACS analysis revealed that nearly 3% of BM mononuclear cells express GFP in the KO-Mx mice 3 weeks after the pIpC injection (data not shown). We examined the maturation status of the GFP-positive cells in the KO-Mx BM by FACS analysis of the erythroid markers CD71 and Ter119 (Figure 1D). As a control, we used a line of transgenic reporter mice bearing a recombinant bacterial artificial chromosome containing the mouse Gata1 locus (Gata1 BAC GFP), in which the Gata1 coding sequence is replaced by a GFP cDNA sequence. The expression of GFP in these mice recapitulates endogenous Gata1 gene expression (M.S., K.O., and M.Y., unpublished observations, October 25, 2005). The CD71highTer119low fraction (region I) consists of proerythroblasts, whereas the CD71highTer119high, CD71lowTer119high, and CD71negTer119high fractions (regions II-IV) correspond to basophilic, polychromatic, and orthochromatic erythroblasts, respectively.24 GFP-positive cells from Gata1 BAC GFP mice were most frequently present in regions II and III (Figure 1D top). In contrast, GFP-positive cells from the KO-Mx BM showed a significant increase of cell number in region I, whereas Ter119high erythroblasts (regions II-IV) were decreased. These data suggest that maturation of erythroid cells from proerythroblasts to basophilic erythroblasts is impaired in the GFP-positive cells. We next sorted GFP-positive and -negative cells from regions I to III from the KO-Mx BM and morphology of the cells was examined (Figure 1E). The GFP-negative fractions represented round, erythroblast-like cells with a mixture of different maturation stages. In contrast, GFP-positive fractions contained large, irregular-shaped cells, suggesting impaired maturation of erythroblasts. To examine the expression of known Gata1 target genes, we performed real-time RT-PCR analysis using BM cells in regions I and II (Figure 1F). First, we confirmed that Gata1 mRNA expression was barely detectable in the GFP-positive cells sorted from the KO-Mx BM. Remarkably, Gata2 mRNA level was significantly increased compared with controls in both regions I and II. Of note, the significant increase of Gata2 mRNA expression in the absence of Gata1 was observed when the KO-Mx and WT BM cells were compared in similar differentiation status. We then examined the expression of β-major globin. As expected, the expression level of β-major globin mRNA was significantly up-regulated with erythroid cell maturation (regions I,II) in the control BM. In contrast, the increase of β-major globin mRNA with differentiation was much smaller in the KO-Mx BM. Collectively, these data suggest that Gata1 plays an essential role for negative and positive gene regulation of Gata2 and β-major globin genes, respectively, in adult erythroid cells.
Deletion of the Gata1 gene in megakaryocytes results in accelerated proliferation and impaired maturation
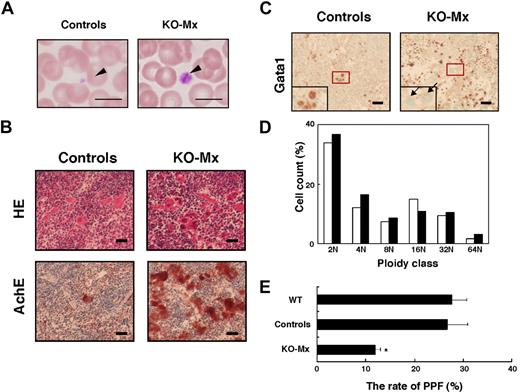
KO-Mx mice exhibited thrombocytopenia at 1.5 and 3 weeks after the pIpC treatment (Figure 1Biv). Microscopic examination of peripheral blood smears revealed that giant platelets were occasionally found in the KO-Mx peripheral blood (Figure 2A). Spleen sections of the KO-Mx mice demonstrated a substantial increase in the number of acetylcholine esterase-positive megakaryocytes (Figure 2B). Immunostaining revealed that Gata1 expression was undetectable in almost all multinuclear megakaryocytes in the KO-Mx spleen, whereas small mononuclear cells in the red pulp were stained with the Gata1 antibody (Figure 2C). These results suggest that megakaryocytes lacking Gata1 are highly proliferative and hence appear dominant over unrecombined megakaryocytes. Of note, the numbers of Gata1-positive small mononuclear cells in the red pulp were increased in the KO-Mx spleen compared with controls. Given that reticulocytosis in the absence of anemia was observed in the KO-Mx mice, these cells are probably unrecombined erythroid cells that compensate for the loss of Gata1 expression in the erythroid compartment.
Mx-Cre–mediated deletion of the Gata1 gene in adult mice affects megakaryopoiesis. (A) Wright-Giemsa staining of peripheral blood smears prepared from the Gata1fl/y::Mx-Cre+ (KO-Mx) and the control mice. The arrowheads indicate platelets. Scale bar represents 10 μm. (B) Histologic examination of spleen sections prepared from the KO-Mx and the control mice. HE indicates hematoxylin and eosin staining; AchE, acetylcholinesterase staining. Original magnification ×100. Scale bar represents 40 μm. (C) Immunohistochemical analysis of spleen using anti-Gata1 antibody (N6). The insets represent higher magnifications of the red square areas. The arrows in the right panel indicate multinuclear megakaryocytes negative for Gata1 immunostaining. Original magnification ×100. Scale bar represents 40 μm. (D) Megakaryocyte ploidy classes in the KO-Mx (■) and control (□) BM cells. (E) Rate (%) of proplatelet formation in cultured megakaryocytes in BM prepared from KO-Mx and control mice. Results are shown as mean plus or minus SD (error bars) from 3 mice (*P < .05 for KO-Mx compared with controls).
Mx-Cre–mediated deletion of the Gata1 gene in adult mice affects megakaryopoiesis. (A) Wright-Giemsa staining of peripheral blood smears prepared from the Gata1fl/y::Mx-Cre+ (KO-Mx) and the control mice. The arrowheads indicate platelets. Scale bar represents 10 μm. (B) Histologic examination of spleen sections prepared from the KO-Mx and the control mice. HE indicates hematoxylin and eosin staining; AchE, acetylcholinesterase staining. Original magnification ×100. Scale bar represents 40 μm. (C) Immunohistochemical analysis of spleen using anti-Gata1 antibody (N6). The insets represent higher magnifications of the red square areas. The arrows in the right panel indicate multinuclear megakaryocytes negative for Gata1 immunostaining. Original magnification ×100. Scale bar represents 40 μm. (D) Megakaryocyte ploidy classes in the KO-Mx (■) and control (□) BM cells. (E) Rate (%) of proplatelet formation in cultured megakaryocytes in BM prepared from KO-Mx and control mice. Results are shown as mean plus or minus SD (error bars) from 3 mice (*P < .05 for KO-Mx compared with controls).
The DNA content of CD41-positive megakaryocytes from BM was similar in the KO-Mx and control cells (Figure 2D), indicating that endomitosis is not affected by the absence of Gata1. In contrast, proplatelet formation, a process that requires cytoplasmic maturation of megakaryocytes, is significantly impaired in the KO-Mx BM (Figure 2E). These results indicate that Gata1 is indispensable for cytoplasmic maturation and subsequent platelet formation in adult megakaryocytes, supporting previous studies in mice with megakaryocyte-specific Gata1 deficiency.5,6
Gata1 deletion by Tx-Cre leads to severe anemia and reduced cellularity in the BM and spleen in adult mice
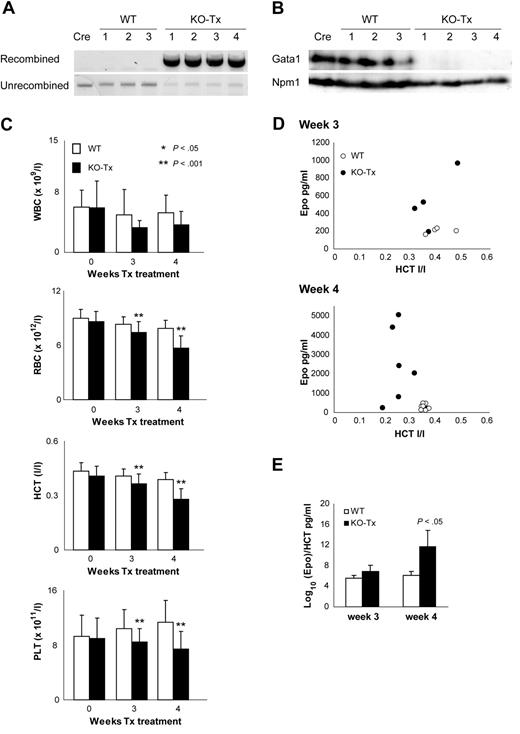
In a second series of experiments, we used a line of mice expressing a tamoxifen-inducible Cre recombinase (Tx-Cre) from the Rosa26 locus to generate compound Gata1fl/y::Tx-Cre mice (referred to as KO-Tx mice). In this study, we wished to examine how complete ablation of Gata1 affects homeostatic regulation of erythropoiesis in adult mice. PCR analysis using genomic DNA revealed that recombination of Gata1 gene in the KO-Tx mice occurred efficiently in BM and spleen at week 4 after the Tx treatment (Figure 3A). Furthermore, whole cell extracts from the KO-Tx BM showed that Gata1 protein was barely detectable at week 4 of the Tx treatment (Figure 3B). The blood of Tx-treated mice was sampled and analyzed at the start (week 0) and during Tx treatment (weeks 3 and 4). A significant decrease of RBC counts and HCT levels was observed in the peripheral blood of KO-Tx mice compared with control at week 4 (Figure 3C). As observed in KO-Mx mice, KO-Tx mice showed a significant reduction in platelet counts at weeks 3 and 4 (Figure 3C). These data suggested that loss of Gata1 in KO-Tx mice resulted in platelet count reduction and anemia. The WBC counts were not significantly altered during the course of Tx treatment (Figure 3C), although in a separate study we found that Gata1 loss affected differentiation and survival of eosinophils and dendritic cells.2
Tx-Cre–mediated Gata1 deletion leads to anemia and thrombocytopenia in adult mice. (A) PCR analysis of recombination in DNA isolated from BM of Gata1fl/y::Tx-Cre (KO-Tx, n = 4), Gata1fl/y (WT, n = 3), and Tx-Cre (Cre, n = 1) mice. (B) Western blotting using anti-Gata1 antibody N6 (top) and Npm1 (bottom). Whole cell extracts were prepared from the BM of KO-Tx (n = 4), WT (n = 3), and Cre (n = 1) mice at week 4 of the Tx treatment. (C) Peripheral blood counts of KO-Tx mice (■) and WT mice (□) at 0, 3, and 4 weeks after the Tx treatment. Samples are collected from 10 to 15 mice per group. Results shown as mean plus or minus SD (error bars). WBC indicates white blood cell counts; RBC, red blood cell counts; HCT, hematocrit; PLT, platelet counts. (D) Erythropoietin (Epo) levels in plasma measured at weeks 3 and 4 after the start of Tx treatment in KO-Tx (●) and WT (○) mice. Epo values are plotted against HCT value for each mouse. (E) Bar graph representing the Log10(Epo)/HCT ratio at weeks 3 and 4 of Tx treatment. Average and SD (error bars) of more than or equal to 4 mice per group are shown.
Tx-Cre–mediated Gata1 deletion leads to anemia and thrombocytopenia in adult mice. (A) PCR analysis of recombination in DNA isolated from BM of Gata1fl/y::Tx-Cre (KO-Tx, n = 4), Gata1fl/y (WT, n = 3), and Tx-Cre (Cre, n = 1) mice. (B) Western blotting using anti-Gata1 antibody N6 (top) and Npm1 (bottom). Whole cell extracts were prepared from the BM of KO-Tx (n = 4), WT (n = 3), and Cre (n = 1) mice at week 4 of the Tx treatment. (C) Peripheral blood counts of KO-Tx mice (■) and WT mice (□) at 0, 3, and 4 weeks after the Tx treatment. Samples are collected from 10 to 15 mice per group. Results shown as mean plus or minus SD (error bars). WBC indicates white blood cell counts; RBC, red blood cell counts; HCT, hematocrit; PLT, platelet counts. (D) Erythropoietin (Epo) levels in plasma measured at weeks 3 and 4 after the start of Tx treatment in KO-Tx (●) and WT (○) mice. Epo values are plotted against HCT value for each mouse. (E) Bar graph representing the Log10(Epo)/HCT ratio at weeks 3 and 4 of Tx treatment. Average and SD (error bars) of more than or equal to 4 mice per group are shown.
To investigate whether the KO-Tx mice respond to the induced anemic condition, we measured Epo levels in plasma (Figure 3D). In KO-Tx mice at week 3, these ranged from 200 to 1000 pg/mL, which was up to 5-fold higher than normal levels. At week 4, levels of Epo in the KO-Tx plasma increased further and ranged from 800 to 5000 pg/mL, which was up to 25-fold higher than the normal levels. WT mice showed normal levels of Epo (150-250 pg/mL25 ) at weeks 3 and 4. The levels of Epo relative to HCT were significantly greater in KO-Tx mice at week 4 (Figure 3E). Remarkably, despite the high Epo/HCT ratios, the total cell number was decreased in the KO-Tx BM at weeks 3 and 4 (Table 1). The cell number of the KO-Tx spleen was also decreased to 65% of the WT level at week 4. These data show that Gata1 ablation leads to a reduction of the cellularity of BM and spleen (Table 1), despite the severe anemia and elevated Epo levels. These results indicate that induction of Epo in response to anemia occurred in KO-Tx mice; however, the erythropoietic reserve failed to respond and Epo levels increased further.
Total number of cells/organ, ×107, ± SD
| Treatment . | Bone marrow . | Spleen . | ||||
|---|---|---|---|---|---|---|
| Week 3 . | Week 4 . | + PHZ . | Week 3 . | Week 4 . | + PHZ . | |
| WT | 7.7 ± 1.2 | 8.1 ± 1.7 | 7.9 ± 1.1 | 23.1 ± 4.1 | 27.2 ± 4.0 | 111.0 ± 24.4 |
| KO-Tx | 5.4 ± 0.6* | 5.5 ± 1.2* | 3.3 ± 2.2* | 22.0 ± 9.6 | 18.3 ± 3.3† | 41.3 ± 14.5† |
| Treatment . | Bone marrow . | Spleen . | ||||
|---|---|---|---|---|---|---|
| Week 3 . | Week 4 . | + PHZ . | Week 3 . | Week 4 . | + PHZ . | |
| WT | 7.7 ± 1.2 | 8.1 ± 1.7 | 7.9 ± 1.1 | 23.1 ± 4.1 | 27.2 ± 4.0 | 111.0 ± 24.4 |
| KO-Tx | 5.4 ± 0.6* | 5.5 ± 1.2* | 3.3 ± 2.2* | 22.0 ± 9.6 | 18.3 ± 3.3† | 41.3 ± 14.5† |
Week 3: KO-Tx mice, n = 4 and WT mice, n = 4; week 4: KO-Tx mice, n = 6 and WT mice, n = 7; + PHZ: KO-Tx mice, n = 3 and WT mice, n = 4.
P < .05 versus WT.
P < .01 versus WT.
Gata1 loss causes deterioration of the erythroid compartment resulting in aplastic crisis
To determine the maturation stage of KO-Tx erythroid cells, we analyzed the expression of CD71 and Ter119 in BM, spleen, and peripheral blood at week 4 of the Tx treatment (Figure 4A). WT mice displayed the expected distribution of the erythroid compartment in the BM and spleen,24 in which early erythroblasts (region II) are more abundant than late erythroblasts (region IV; Figure 4A). CD71+ erythroblasts were observed in the peripheral blood of the WT mice at a low frequency (6.4% ± 1.0%). Strikingly, regions I and II, which correspond to proerythroblasts and basophilic erythroblasts, were almost absent in the KO-Tx BM and spleen. Cells in region IV in the BM and spleen are mature erythroid cells probably derived from nonrecombined progenitors. Furthermore, despite the severe anemia, no CD71+ erythroid cells were detected in the peripheral blood. These data indicate that the reduction in cellularity observed in the BM and spleen of KO-Tx mice was the result of the loss of the erythroid compartment. We conclude that KO-Tx mice display red cell aplasia by week 4 of the Tx treatment.
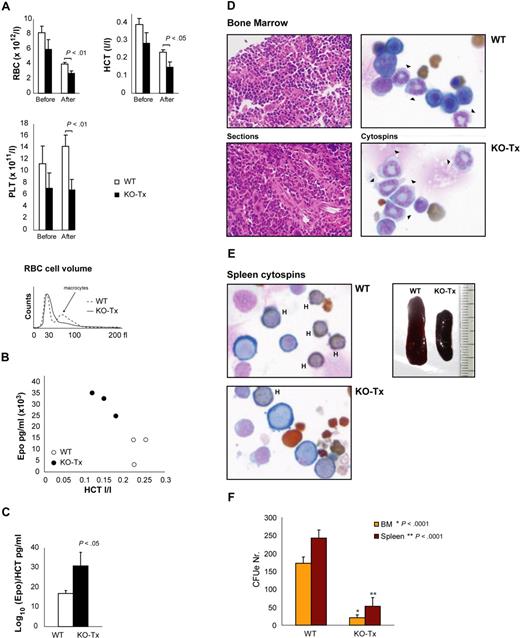
Gata1 is required for the maintenance of the erythroid compartment in adult mice. (A) FACS analysis of the erythroid surface markers Ter119 and CD71. Expression was examined in single cell suspensions prepared from the BM, spleen, and peripheral blood of Gata1fl/y::Tx-Cre (KO-Tx) and WT mice at 4 weeks after Tx treatment. RI, RII, RIII, and RIV represent cell fractions as indicated in Figure 1E and “Results.” The mean plus or minus SD of the percentage of gated live (7AAD−) cells in each region is indicated. (B) BM sections (left, hematoxylin and eosin staining, arrowheads indicate megakaryocytes [M]) and cytospins (right, Wright-Giemsa staining, arrowheads indicate neutrophils) of KO-Tx and WT mice. Original magnification ×100. (C) Spleen sections of KO-Tx and WT mice stained with benzidine (left, erythroid cells stained brown) or acetylcholinesterase (right, megakaryocytes stained brown). (D) CFUe and BFUe assays on BM cells. Average and standard deviation are shown (n ≥ 3). (E) FACS analysis of hanging drop cultures of BM cells using standard conditions (HD ST) and of BM cells labeled with CFSE (HD CFSE). Average and SD are shown (n ≥ 3).
Gata1 is required for the maintenance of the erythroid compartment in adult mice. (A) FACS analysis of the erythroid surface markers Ter119 and CD71. Expression was examined in single cell suspensions prepared from the BM, spleen, and peripheral blood of Gata1fl/y::Tx-Cre (KO-Tx) and WT mice at 4 weeks after Tx treatment. RI, RII, RIII, and RIV represent cell fractions as indicated in Figure 1E and “Results.” The mean plus or minus SD of the percentage of gated live (7AAD−) cells in each region is indicated. (B) BM sections (left, hematoxylin and eosin staining, arrowheads indicate megakaryocytes [M]) and cytospins (right, Wright-Giemsa staining, arrowheads indicate neutrophils) of KO-Tx and WT mice. Original magnification ×100. (C) Spleen sections of KO-Tx and WT mice stained with benzidine (left, erythroid cells stained brown) or acetylcholinesterase (right, megakaryocytes stained brown). (D) CFUe and BFUe assays on BM cells. Average and standard deviation are shown (n ≥ 3). (E) FACS analysis of hanging drop cultures of BM cells using standard conditions (HD ST) and of BM cells labeled with CFSE (HD CFSE). Average and SD are shown (n ≥ 3).
Consistent with the decreased cellularity in the KO-Tx BM, hematopoietic cells were rather dispersed on the KO-Tx BM sections compared with controls (Figure 4B). In addition, megakaryocytes were increased in the KO-Tx BM (Figure 4B arrowheads). Cytologic analysis of BM single cell suspensions revealed an increased proportion of granulocytes (mainly neutrophils) in KO-Tx mice compared with WT (Figure 4B). Spleen sections revealed an almost complete involution of the red pulp in KO-Tx mice, whereas WT mice spleens displayed the expected distribution of red and white pulp, as shown by benzidine staining (Figure 4C). This involution of the red pulp resulted in niche restriction of the megakaryocytes in KO-Tx spleens to the remaining red pulp as opposed to the even distribution of megakaryocytes within the red pulp of WT spleens, as shown by acetylcholine esterase staining (Figure 4C). We performed colony assay to examine whether formation of erythroid progenitors was altered in the KO-Tx BM at week 4 of Tx treatment (Figure 4D). We found that both CFUe and BFUe colony numbers were significantly reduced in the KO-Tx BM compared with that of WT. These results indicate that ablation of Gata1 affects formation of erythroid progenitors in adult BM.
To assess the differentiation potential of BM-derived erythroid progenitors ex vivo, we performed “hanging drop” cultures in standard conditions26 and in the presence of the cell tracker dye CFSE. After 2 days of culture, we analyzed the expression of CD71 and Ter119 by FACS (Figure 4E). The percentage of live cells in KO-Tx cultures was reduced to 50% of that measured in WT samples (data not shown), consistent with previous studies demonstrating that erythroid progenitors derived from Gata1-null ES cells undergo apoptotic cell death after maturation arrest at the proerythroblast stage.27-29 Despite significant cell death in the absence of Gata1 in this culture system, we failed to observe the increased apoptosis of Gata1-null erythroid progenitors in vivo. We assume that in vivo the apoptotic erythroid cells are rapidly cleared in the erythroblastic island, analogous to the rapid clearance of expelled nuclei.30 Erythroid cell differentiation in the absence of Gata1 was impaired in this culture system, with marked reduction of differentiated cells (regions II-IV) compared with that of WT (Figure 4E). Cultures with CFSE revealed a reduced number of cell divisions in KO-Tx cells compared with WT (Figure 4E). These results indicate that Gata1-null erythroid cells display a block in differentiation and an arrest of cell proliferation followed by cell death.
Gata1 is necessary to activate stress erythropoiesis in adult mice
The results described above suggest that Gata1 is required to activate stress erythropoiesis in response to anemia. However, erythropoiesis might be compensated to some extent by functions of other transcription factors on acute exposure to erythropoietic stress.31 To test this possibility, KO-Tx and WT mice were treated with the hemolytic agent PHZ immediately after week 4 of the Tx treatment course. We found that PHZ treatment induced anemia in both KO-Tx and WT mice, although the decrease of RBC counts as well as HCT levels was greater in KO-Tx mice compared with WT (Figure 5A). Platelet counts were not affected by the PHZ treatment. We then examined RBC size distribution (Figure 5A). Erythroid cells from WT showed a second peak of larger cells (macrocytes) after the PHZ treatment. Strikingly, RBCs from KO-Tx mice lacked the peak of macrocytes. The loss of macrocytes in KO-Tx mice is probably the result of the absence of newly generated cells on PHZ treatment. We next measured plasma Epo levels and plotted these against the HCT values. Plasma Epo levels were increased significantly after PHZ treatment in WT mice. Epo levels in KO-Tx mice were already elevated before PHZ treatment and rose even further after PHZ treatment (Figure 5B). The Epo/HCT ratios in KO-Tx mice increased significantly after PHZ treatment compared with those observed at week 4 of Tx treatment (Figures 3E,5C). Histologic analysis revealed that interstitial tissue became prominent in the BM sections of KO-Tx mice (Figure 5D). Most of the hematopoietic cells were multinucleated granulocytes or megakaryocytes (Figure 5D). Consistent with this notion, the cellularity of the BM was reduced to 40% in PHZ-treated KO-Tx mice compared with PHZ-treated WT mice (Table 1). The size and cellularity of the spleen were also significantly reduced (Table 1; Figure 5D). Cytospins revealed that erythroblasts were less abundant, whereas a higher proportion of lymphocytes and macrophages was observed in PHZ-treated KO-Tx spleens compared with PHZ-treated WT spleens (Figure 5D). Finally, we found that CFUe colony numbers were severely reduced both in the BM and spleen of PHZ-treated KO-Tx mice compared with those of PHZ-treated WT mice (Figure 5F). These results indicate that KO-Tx mice fail to activate an effective stress erythropoietic response. Collectively, our data show that Gata1 is indispensable for both steady-state and stress erythropoiesis in adult mice.
Gata1 is essential for the stress erythropoietic response. (A) Peripheral blood counts of the Tx-treated Gata1fl/y::Tx-Cre (KO-Tx) (■) and WT mice (□) after induction of hemolytic anemia with PHZ. Results are shown as mean plus or minus SD (error bars); n ≥ 3. RBC indicates red blood cell counts; HCT, hematocrit; PLT, platelet counts. Bottom graph displays the RBC volume of representative WT and KO-Tx samples. (B) Plasma Epo levels of KO-Tx (●) and WT (○) mice after induction of hemolytic anemia with PHZ. Epo values are plotted against HCT values for each mouse. (C) Log10 (Epo)/HCT ratios after PHZ treatment. Average and SD are shown; n ≥ 4. (D) BM sections (hematoxylin and eosin staining) and cytospins (Wright-Giemsa staining) of KO-Tx and WT mice after induction of hemolytic anemia with PHZ. (E) Spleens and Wright-Giemsa staining of cytospins from splenic cells of KO-Tx and WT mice after PHZ treatment. (F) CFUe assays of BM and spleen cells of KO-Tx and WT mice after induction of hemolytic anemia with PHZ.
Gata1 is essential for the stress erythropoietic response. (A) Peripheral blood counts of the Tx-treated Gata1fl/y::Tx-Cre (KO-Tx) (■) and WT mice (□) after induction of hemolytic anemia with PHZ. Results are shown as mean plus or minus SD (error bars); n ≥ 3. RBC indicates red blood cell counts; HCT, hematocrit; PLT, platelet counts. Bottom graph displays the RBC volume of representative WT and KO-Tx samples. (B) Plasma Epo levels of KO-Tx (●) and WT (○) mice after induction of hemolytic anemia with PHZ. Epo values are plotted against HCT values for each mouse. (C) Log10 (Epo)/HCT ratios after PHZ treatment. Average and SD are shown; n ≥ 4. (D) BM sections (hematoxylin and eosin staining) and cytospins (Wright-Giemsa staining) of KO-Tx and WT mice after induction of hemolytic anemia with PHZ. (E) Spleens and Wright-Giemsa staining of cytospins from splenic cells of KO-Tx and WT mice after PHZ treatment. (F) CFUe assays of BM and spleen cells of KO-Tx and WT mice after induction of hemolytic anemia with PHZ.
Discussion
We present the first loss-of-function study of Gata1 in adult erythropoiesis. Recent studies indicate that the roles of hematopoietic transcription factors at the postnatal stage are not always equivalent to those at the embryonic stage.18-23,32 For example, SCL/Tal1 and Runx1 play critical roles in the maintenance of hematopoietic stem cells in the embryo,33-36 but these factors are dispensable for this compartment in adult hematopoiesis.18,20 Instead, these factors have essential roles in normal erythropoiesis and megakaryopoiesis in adults.18-20,32 Given that Gata1 interacts with these factors and acts cooperatively in the regulation of erythroid-specific genes,37,38 roles for the Gata1 transcription factor network could be different in adult and embryonic hematopoiesis.
Gata1 is dispensable for the onset of megakaryopoiesis
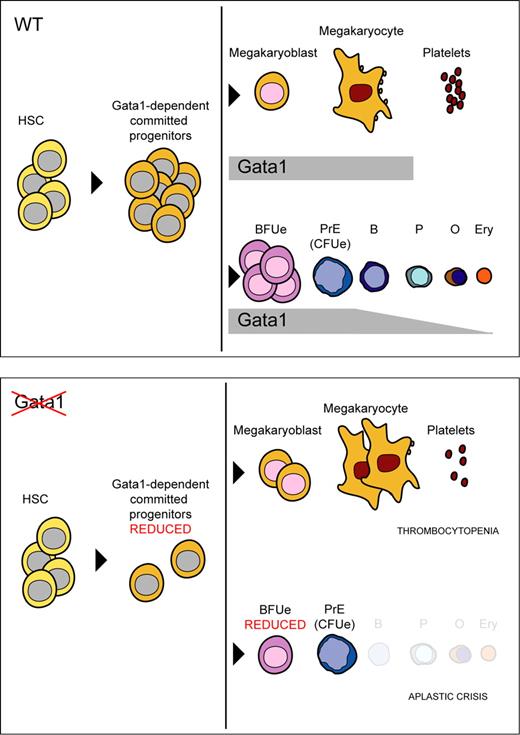
KO-Mx and KO-Tx mice showed overt thrombocytopenia and platelet count reduction, respectively, and displayed increased numbers of megakaryocytes in the spleen. Gata1 null megakaryocytes cultured ex vivo from the KO-Mx BM failed to form proplatelets, whereas the distribution of ploidy classes was not significantly affected. Similar observations have been reported previously in Gata1-deficient megakaryocytes obtained from mutant mice with megakaryocyte-specific Gata1 deficiency.5,6 Together, these results indicate that Gata1 is dispensable for the onset of megakaryopoiesis but required for the maturation of megakaryocytes during adult hematopoiesis (Figure 6).39 The observation that Gata1 null megakaryocytes are able to survive and partially fulfill their hematopoietic fate might be explained by the fact that megakaryocytes retain Gata2 expression.40 It is plausible that Gata2 compensates partially for the loss of Gata1 in megakaryocytes40-42 because the hematopoietic GATA transcription factors are able to take over each other's functions to a large extent.31,43-45
Model for the role of Gata1 in adult erythromegakaryopoiesis. Normal erythromegakaryopoiesis (top panel) and in the absence of Gata1 (bottom panel). Gata1 has a role in myeloid progenitors. Gata1 loss results in reduction of BFUe, CFUe, and defective erythroid differentiation beyond the proerythroblast stage. In megakaryocytes, Gata1 loss might be compensated by Gata2; however, Gata1 regulates megakaryocyte proliferation and is required for proper cytoplasmic maturation.39 HSC indicates hematopoietic stem cell; PrE, proerythroblast; B, basophilic erythroblast; P, polychromatic erythroblast; O, orthochromatic erythroblast; Ery, mature erythrocyte.
Model for the role of Gata1 in adult erythromegakaryopoiesis. Normal erythromegakaryopoiesis (top panel) and in the absence of Gata1 (bottom panel). Gata1 has a role in myeloid progenitors. Gata1 loss results in reduction of BFUe, CFUe, and defective erythroid differentiation beyond the proerythroblast stage. In megakaryocytes, Gata1 loss might be compensated by Gata2; however, Gata1 regulates megakaryocyte proliferation and is required for proper cytoplasmic maturation.39 HSC indicates hematopoietic stem cell; PrE, proerythroblast; B, basophilic erythroblast; P, polychromatic erythroblast; O, orthochromatic erythroblast; Ery, mature erythrocyte.
Multiple stages of erythroid cell differentiation are affected by the absence of Gata1
Although erythropoiesis was supported by the unrecombined cells in the KO-Mx mice, we could trace populations of Gata1-null erythroid cells by GFP expression. We found that maturation of the Gata1 null erythroid cells was arrested at the proerythroblast stage. The stage of maturation arrest is consistent with previous observations in Gata1 knockout embryos3 and in Gata1-null ES cells cultured in vitro,27-29,46 although a recent study demonstrated that some populations of Gata1-null ES cells could survive and continue to proliferate beyond this stage.47 Our data indicate that the maturation from proerythroblasts to erythroblasts is fully dependent on Gata1 in adult erythropoiesis. Interestingly, we found that ablation of Gata1 results in a significant reduction of BFUe and CFUe progenitors in the KO-Tx BM, suggesting additional roles of Gata1 in adult erythropoiesis at earlier maturation stages (Figure 6). We previously obtained evidence that in adult hematopoiesis the GEMM colony-forming potential is affected by the absence of Gata1,2 and loss of Gata1 impairs the maturation of megakaryocyte-erythroid progenitors in in vitro differentiation of ES cells and in fetal liver.48 Therefore, defining the roles of Gata1 in early stage adult hematopoietic progenitors remains an important issue that is currently under investigation in our laboratories.
Gata1 plays a dominant role in the regulation of stress erythropoiesis
WT mice use their erythropoietic reserve to mount a rapid response to tissue hypoxia by expanding erythropoiesis in the spleen.7 The analysis of mouse mutants has revealed that stress erythropoiesis is molecularly distinct from steady-state erythropoiesis in a number of aspects. For instance, it is dependent on intact glucocorticoid receptor49 and Stat524 transcription factors, and Bmp4/Smad5 signaling.50 To elucidate whether Gata1 is involved in the stress erythropoietic response, we induced hemolytic anemia in Gata1fl/y::Tx-Cre mice after Tx treatment. We found that the spleen size was increased approximately 4-fold in PHZ-treated WT mice, whereas cellularity of the BM was maintained. In contrast, PHZ-treated KO-Tx mice display a much more limited approximately 2-fold increase in spleen size and reduced cellularity of the BM, despite the severe anemia and very high Epo levels in plasma. The animals fail to expand erythroid progenitors in the spleen, as shown by the severe reduction of the number of CFUe compared with WT PHZ-treated mice. In contrast, a previous report showed that Stat5 null mutants display a huge approximately 10-fold increase in spleen size on PHZ treatment, but the erythroblasts fail to progress through maturation and display increased apoptotic rates.24 In contrast, Gata1-low mice, which express Gata1 at 5% of normal levels, display an enhanced response to PHZ treatment.51 Sensitivity to Epo increases during erythroid cell development and reaches its maximum at the CFUe and proerythroblast stages.52 Thus, although Gata1 loss blocks development of erythroid progenitors before these cells can fully respond to Epo, these progenitors do develop when low levels of Gata1 are present, as occurs in Gata1-low mice. The spleen harbors distinct stress-responsive progenitors, which are dependent on the glucocorticoid receptor,49 BMP4/Smad5,50 and stem cell factor/c-Kit.53 Our results strongly suggest that this splenic compartment is incapacitated by ablation of Gata1. Collectively, we conclude that Gata1 plays a pivotal role in maintaining the homeostasis of erythropoiesis in adult mice.
Gata1 loss in adult mice resembles pure red cell aplasia in human
The BM and spleen of KO-Tx mice at week 4 showed a significant loss of the erythroid compartment resembling aplastic crisis, an acute form of pure red cell aplasia (PRCA) in human. Abnormally elevated Epo/HCT or Epo/HGB ratios are characteristics of erythrocytic hyperplasia in human. Epo levels in patients with hypoplastic anemia are consistently higher than those observed in patients with nonhypoplastic anemias,54 resembling the abnormally high Epo/HCT ratios observed in KO-Tx mice. The acute form of PRCA is typically associated with parvovirus B19 infection in patients with underlying hemolytic diseases, such as sickle cell anemia.55 The B19 infection also causes chronic PRCA particularly in immunocompromised patients. Many cases of other acquired PRCA are idiopathic and often associated with systemic autoimmune disorders. In addition, severe PRCA resulting from anti-Epo antibodies in patients receiving recombinant human Epo has been reported recently.56 Diamond-Blackfan anemia (DBA) is a congenital form of PRCA.57 Thus far, 2 DBA genes have been identified, encoding ribosomal proteins RPS19 and RPS24. This variety of PRCA etiologies indicates that the causes of erythropoietic failure are heterogeneous, and the molecular basis of various forms of PRCA is still poorly understood. Contribution of Gata1 to PRCA pathology has not been reported yet; our data suggest that functional loss of Gata1 could be involved in some cases of PRCA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sakie Hosoya-Ohmura, Pan Xiaoqing, Reiko Kawai, Mitsuru Okano, Naomi Kaneko, Nynke Gillemans, Sylvia Dekker, Reinier van der Linden, Dubravka Drabek, Tanja Nikolic, and Nanda Vos for technical assistance; Patrick Molenbeek, Iris Janssen, Danielle Zondervan-Bakker, Mariska van Ruiven, and Miranda Buter for assistance with Tx and PHZ treatments; and Drs Mineo Kurokawa and the late Hisamaru Hirai for providing the mice and technical suggestions.
This work was supported by grants from NWO-ALW 815-02-008 (S.P., L.G.), Japan Science and Technology Corporation-ERATO Environmental Response Project (M.Y.), and the Ministry of Education, Science, Sports and Culture (M.Y., K.O.).
Authorship
Contribution: K.O., S.P., and M.Y. designed research; L.G., S.T., M.S., and H.Y-M. performed research; L.G., S.T., M.S., H.Y-M., and K.O. analyzed data; K.O., M.Y., and S.P. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sjaak Philipsen, Erasmus MC-Cell Biology, Room Ee720, PO Box 2040, 3000 CA Rotterdam, The Netherlands; e-mail: j.philipsen@erasmusmc.nl; or Kinuko Ohneda, Department of Pharmacy, Faculty of Pharmacy, Takasaki University of Health and Welfare, 60 Nakaorui-machi, Takasaki-shi, Gunma 370-0033, Japan; e-mail: kohneda@takasaki-u.ac.jp.
References
Author notes
*L.G., S.T., and M.S. contributed equally to this study.

![Figure 4. Gata1 is required for the maintenance of the erythroid compartment in adult mice. (A) FACS analysis of the erythroid surface markers Ter119 and CD71. Expression was examined in single cell suspensions prepared from the BM, spleen, and peripheral blood of Gata1fl/y::Tx-Cre (KO-Tx) and WT mice at 4 weeks after Tx treatment. RI, RII, RIII, and RIV represent cell fractions as indicated in Figure 1E and “Results.” The mean plus or minus SD of the percentage of gated live (7AAD−) cells in each region is indicated. (B) BM sections (left, hematoxylin and eosin staining, arrowheads indicate megakaryocytes [M]) and cytospins (right, Wright-Giemsa staining, arrowheads indicate neutrophils) of KO-Tx and WT mice. Original magnification ×100. (C) Spleen sections of KO-Tx and WT mice stained with benzidine (left, erythroid cells stained brown) or acetylcholinesterase (right, megakaryocytes stained brown). (D) CFUe and BFUe assays on BM cells. Average and standard deviation are shown (n ≥ 3). (E) FACS analysis of hanging drop cultures of BM cells using standard conditions (HD ST) and of BM cells labeled with CFSE (HD CFSE). Average and SD are shown (n ≥ 3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/8/10.1182_blood-2007-09-115121/6/m_zh80080818190004.jpeg?Expires=1770057701&Signature=X4AbEKjH-jJXGNrSaBrmXHDFdwTq9R8qKWIvxJQ2CyBk3CGLSZCulmuivFnJ86JWpX7Q8IxrTHQYoRfcS0cUwiJbJ4c~DFPbTKKHYetuJAiu9AuquRfvIziqOdVtxJ~wzJQ7dzWFxTGv~4KQQJikqEgjHQ-QI9F7iqSVhnjx6mlr9LGnDX60TUYrERM4YMYZTyRfCeA2yQjEd8NOEoD9YbZYCmQ6xwTjye9BDJFF4QJgJQx5uPGYsfPxlvAiE~YhpjW7119d1vvGayjIDDSFTeLk5FL3hQtxm8J6s~wJzg6TROGHWA8LTSGNrHwZdbMeyenvz0Rz5gEUQrcx42gtOw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal