Abstract
Imatinib at 400 mg daily is standard treatment for chronic myeloid leukemia in chronic phase. We here describe the correlation of imatinib trough plasma concentrations (Cmins) with clinical responses, event-free survival (EFS), and adverse events (AEs). Trough level plasma samples were obtained on day 29 (steady state, n = 351). Plasma concentrations of imatinib and its metabolite CGP74588 were determined by liquid chromatography/mass spectrometry. The overall mean (± SD, CV%) steady-state Cmin for imatinib and CGP74588 were 979 ng/mL (± 530 ng/mL, 54.1%) and 242 ng/mL (± 106 ng/mL, 43.6%), respectively. Cumulative estimated complete cytogenetic response (CCyR) and major molecular response (MMR) rates differed among the quartiles of imatinib trough levels (P = .01 for CCyR, P = .02 for MMR). Cmin of imatinib was significantly higher in patients who achieved CCyR (1009 ± 544 ng/mL vs 812 ± 409 ng/mL, P = .01). Patients with high imatinib exposure had better rates of CCyR and MMR and EFS. An exploratory analysis demonstrated that imatinib trough levels were predictive of higher CCyR independently of Sokal risk group. AE rates were similar among the imatinib quartile categories except fluid retention, rash, myalgia, and anemia, which were more common at higher imatinib concentrations. These results suggest that an adequate plasma concentration of imatinib is important for a good clinical response. This study is registered at http://clinicaltrials.gov as NCT00333840.
Introduction
Imatinib mesylate (Gleevec, Glivec; Novartis Oncology, East Hanover, NJ), a selective inhibitor of BCR-ABL kinase activity, has demonstrated significant clinical efficacy in the treatment of chronic myeloid leukemia (CML) and gastrointestinal stromal tumors (GISTs) producing durable responses and prolonged survival such that it has become the standard of care for these diseases.1-5 The International Randomized Study of Interferon and STI571 (IRIS) trial compared the efficacy and safety of imatinib (IM) with that of interferon alpha plus low-dose cytarabine in 1106 patients with newly diagnosed chronic-phase CML.3,6 The initial estimated rate of complete cytogenetic response (CCyR) to imatinib at 18 months was 76%. A recently published, 5-year follow-up analysis indicated an estimated 87% cumulative CCyR rate and an estimated overall survival of 89% among 553 patients who received first-line imatinib.6 Although there was variability in the time that individual patients achieved a CCyR, no patient who achieved CCyR and major molecular response (MMR) by 18 months of therapy had progressed to accelerated or blast-phase at the 5-year follow-up.
Imatinib has favorable pharmacokinetic (PK) characteristics, including rapid and complete oral bioavailability (98%) and a proportional dose-exposure relationship.7,8 Its terminal half-life is approximately 20 hours, allowing for once-daily dosing. Despite significant efficacy and favorable PK properties, there are cases of suboptimal responses and treatment failure with imatinib. The following factors might be associated with these instances of suboptimal response: baseline presence or emergence of BCR-ABL mutations,9,10 drug exposure below the target level due to poor medication adherence,11,12 interpatient exposure variability related in part to metabolizing enzyme activities,13-16 different efflux and uptake transporter activities,17-21 differences in clinical features (Sokal score) at baseline,22 or any other reasons causing a delayed time to CCyR.3,6,23
Imatinib undergoes metabolism through the cytochrome P450 system. CYP3A4 is the major isoenzyme responsible for imatinib metabolism, although CYP1A2, CYP2D6, CYP2C9, and CYP2C19 also contribute to a minor extent.7,8,24 CGP74588 is a major metabolite of imatinib, which has a similar biologic activity and represents approximately 20% of the parent drug plasma level in patients. Due to intrinsic variability of CYP enzyme activity,15,16 high interpatient variability has been reported in imatinib exposure in CML patients.7,8 Drugs that inhibit or induce the CYP3A4 isoenzyme have been shown to alter imatinib PK activity.25-29
Here we report on the clinical significance of imatinib PK, as measured at steady state (day 29 of treatment), and assess how variability in imatinib exposure correlates with cytogenetic and molecular responses, as well as event-free survival (EFS), emergence of specific adverse events, and patient disposition during the 5-year follow-up of the IRIS study.3,6
Methods
Patients included in this analysis were enrolled in the IRIS trial and randomly assigned to initial treatment with imatinib at 400 mg/day. All had Philadelphia chromosome (Ph)–positive CML. The study design and patient demographics for all 553 patients receiving imatinib and outcomes to therapy have been described previously.3,6
Informed consent was obtained in accordance with the Declaration of Helsinki. The study protocol was reviewed and approved by the ethics committee or institutional review boards of all participating centers (a list of participating centers is available on the Blood website; see the Supplemental Materials link at the top of the online article). All 553 patients gave written informed consent to participate in the clinical trial and the PK subanalysis. CCyR and MMR response data have been reported previously.3,6 CCyR was defined as 0% Ph+ metaphase cells of 20 or more examined and MMR (in subjects who achieved CCyR) as 3 or more log reduction in BCR-ABL/BCR ratio by polymerase chain reaction (PCR) assay relative to a standardized baseline derived from the median ratio for 30 untreated patients with chronic-phase CML.30
This paper focuses on 351 patients for whom imatinib plasma concentrations were available on day 29 of treatment and correlates these PK measurements with complete cytogenetic responses achieved during 5 years of therapy. PCR assays were originally done as an exploratory study, and collection of additional samples was interrupted substantially between the second and third years. Thus, due to limited PCR data collection, correlation between PK and achievement of MMR was analyzed only for the first 2 years after initiation of treatment. EFS was evaluated during 5 years of therapy and was measured from enrollment onto the clinical trial until occurrence of any of the following events: death from any cause, loss of a major cytogenetic response (MCyR; 0%-35% Ph+ metaphase cells), loss of a complete hematologic response, or progression to accelerated or blast phase. For patients without an event, EFS was censored at the last available assessment or date of discontinuation from study treatment.
Pharmacokinetic sample analysis
Blood samples were collected at 24 hours (mostly within ± 2 hours) after the first dose (ie, prior to the morning dose on day 2, n = 363), and again on day 29 prior to the morning dose (steady-state trough level, n = 351). Plasma concentrations of imatinib and its metabolite, CGP74588, were measured using liquid chromatography and tandem mass spectrometry (LC/MS/MS) with deuterated imatinib as the internal standard. The limit of quantification was 5 ng/mL for both imatinib and CGP74588; the assay was fully validated.8,31 The accuracy and precision were 104% (± 6%) at the lower limit of quantification and 99% (± 5%) to 108% (± 5%) over the entire concentration range of 4 ng/mL to 10 000 ng/mL.
Data analysis and statistical methods
Trough plasma concentrations of imatinib (Cmin) at steady state (n = 351) were correlated with age, body weight, and body surface area (BSA) at baseline using linear regression analysis (Pearson correlation coefficient provided). To correlate with clinical end points, imatinib plasma trough levels at steady state were grouped into 3 categories based on distribution according to 4 quartiles. As summarized in Table 1, the lower quartile (Q1) includes data on the 25% of patients with the lowest imatinib trough plasma concentration values; quartiles Q2 and Q3 extend 25% below and above the median concentration, respectively; the upper quartile (Q4) includes the 25% of patients with the highest imatinib trough plasma concentration values. The central 50% of the data (interquartile range; ie, excluding Q1 and Q4) were combined for all analyses and are jointly referred to as Q2-Q3. These 3 PK categories (Q1, Q2-Q3, and Q4) were used for stratification as appropriate.
Trough levels of imatinib and CGP74588 at steady state (day 29)
| . | Overall, n=351 . | Quartile 1, n=87 . | Quartiles 2 and 3, n=178 . | Quartile 4, n=86 . |
|---|---|---|---|---|
| Imatinib, ng/mL | 979 (± 530); 153-3910 | 490 (± 120); 153-644 | 889 (± 148); 647-1170 | 1661 (± 602); 1180-3910 |
| CGP74588, ng/mL | 242 (± 106); 50.2-841 | 153 (± 48); 50.2-322 | 236 (± 66); 105-455 | 343 (± 126); 160-841 |
| CGP74588/imatinib | 0.27 (± 0.09); 0.11-0.84 | 0.32 (± 0.11); 0.15-0.84 | 0.27 (± 0.07); 0.11-0.51 | 0.21 (± 0.05); 0.13-0.36 |
| . | Overall, n=351 . | Quartile 1, n=87 . | Quartiles 2 and 3, n=178 . | Quartile 4, n=86 . |
|---|---|---|---|---|
| Imatinib, ng/mL | 979 (± 530); 153-3910 | 490 (± 120); 153-644 | 889 (± 148); 647-1170 | 1661 (± 602); 1180-3910 |
| CGP74588, ng/mL | 242 (± 106); 50.2-841 | 153 (± 48); 50.2-322 | 236 (± 66); 105-455 | 343 (± 126); 160-841 |
| CGP74588/imatinib | 0.27 (± 0.09); 0.11-0.84 | 0.32 (± 0.11); 0.15-0.84 | 0.27 (± 0.07); 0.11-0.51 | 0.21 (± 0.05); 0.13-0.36 |
Data are mean (±SD); range.
The cumulative cytogenetic and molecular response rates were estimated using the Kaplan-Meier method, and strata were compared using the log-rank test. Data for patients without response were censored at the last available assessment unless patients had an event in which case data were censored at maximum follow-up on the study. EFS was plotted based on category of imatinib trough levels using the Kaplan-Meier method. Adverse events (AEs) within the first 3 months of imatinib treatment, as well as AEs documented during the entire 5-year study period, are presented if the occurrence rate was 10% or more of patients. An exception was made for the hematologic events (neutropenia, thrombocytopenia, and anemia when reported as AEs), which were summarized regardless of frequency. In addition, data for patients who discontinued or crossed over to interferon treatment are presented according to their quartile of imatinib trough level.
An exploratory multivariate analysis was performed by stepwise logistic regression to examine overall CCyR rates relative to imatinib trough level, but also baseline patient demographics such as age, sex, body weight, and BSA, as well as disease characteristics including Sokal score, categories of hemoglobin, white blood cells, basophils, absolute neutrophil count, platelet count, and blasts in bone marrow and peripheral blood. Samples with a missing value in any of the variables were excluded from the analysis. Entry criterion was a P value of .15, and the criterion to remain in the model was a P value of .05. Only P values and odds ratios from the final model are reported.
Results
Demographics and plasma trough levels of imatinib and its metabolite, CGP74588
Day 29 PK data were available from a total of 351 patients (221 males and 130 females). The ratio of males to females (1.7) was similar to that in the entire IRIS study (1.6). Median age was 50 years (range, 18-70 years). For the 221 males, the mean body weight was 85.9 plus or minus 16.8 (SD) kg (median, 83.6 kg; range, 52.9-163.3 kg), and the mean BSA was 2.0 plus or minus 0.2 m2 (median, 2.0 m2; range, 1.5-2.8 m2). For the 130 females, the mean body weight was 72.4 plus or minus 18.1 kg (median, 67.8 kg; range, 40.0-133.0 kg), and mean BSA was 1.8 plus or minus 0.2 m2 (median, 1.75 m2; range, 1.35 to 2.54 m2).
Following the first 400-mg dose, the 24-hour mean plus or minus SD (median [range]) plasma concentrations of imatinib and CGP74588 were 526 plus or minus 382 ng/mL (430 ng/mL [0-2920 ng/mL]) and 84 plus or minus 51 ng/mL (70.2 ng/mL [15.0-400 ng/mL]), respectively (n = 363). On day 29, the mean plus or minus SD (median [range]) trough concentrations of imatinib and CGP74588 were 979 plus or minus 530 ng/mL (879 ng/mL [153-3910 ng/mL]) and 242 plus or minus 106 ng/mL (223 ng/mL [50.2-841 ng/mL]), respectively (n = 351) (Table 1). There was a high correlation (0.79) between imatinib and CGP74588 trough levels. The metabolite to parent drug concentration ratio at steady state was 0.268 plus or minus 0.085 overall, but appeared to be slightly lower at higher imatinib levels: 0.32, 0.27, and 0.21 for Q1, Q2-Q3, and Q4 quartiles, respectively.
The distribution of the imatinib trough concentrations at steady state is shown in Figure 1. There were 19 patients with day 29 trough levels more than 2000 ng/mL, all of whom were included in Q4. Based on imatinib trough levels measured on days 2 and 29 for the same subject (n = 321), the mean accumulation ratio (defined as the day 29 to day 2 trough concentration ratio) was estimated to be 2.21 plus or minus 1.15 for imatinib and 3.38 plus or minus 1.54 for CGP74588.
Distribution of imatinib trough levels at 400 mg daily at steady state on day 29 (n = 351). The vertical dashed lines represent 25th, 50th (median), and 75th percentiles (ie, 647, 879, and 1170 ng/mL, respectively).
Distribution of imatinib trough levels at 400 mg daily at steady state on day 29 (n = 351). The vertical dashed lines represent 25th, 50th (median), and 75th percentiles (ie, 647, 879, and 1170 ng/mL, respectively).
Correlation of imatinib trough levels with baseline characteristics
The plasma trough level of imatinib at steady state was slightly higher in females than males (1078 ± 515 ng/mL vs 921 ± 531 ng/mL, respectively). This 17% difference may be attributed to differences in body weight (19%) between sexes. Plasma trough levels of the metabolite CGP74588 followed a similar pattern, while the metabolite/parent drug ratio was the same in males and females. A weak correlation was identified between steady-state trough levels of imatinib and both body weight (r2 = 0.015) and BSA (r2 = 0.038), as shown in Figure 2. There was also a weak correlation between trough levels (or metabolite/parent drug ratio) and the age of patients (r2 = 0.02). However, due to the large interpatient variability in plasma trough concentrations (54%), effects of age, sex, and body weight or BSA on imatinib trough exposure are not likely to be clinically significant.
Steady-state imatinib trough levels by body weight, body surface area, and age at start of study. The predicted imatinib trough levels using linear regression analyses were described by 1763 − 413 × BSA (r2 = 0.038, A), 1266 − 3.5 × body weight (r2 = 0.015, B), and 695 + 5.9 × age (r2 = 0.02, C). Linear regression lines are shown.
Steady-state imatinib trough levels by body weight, body surface area, and age at start of study. The predicted imatinib trough levels using linear regression analyses were described by 1763 − 413 × BSA (r2 = 0.038, A), 1266 − 3.5 × body weight (r2 = 0.015, B), and 695 + 5.9 × age (r2 = 0.02, C). Linear regression lines are shown.
Correlation of imatinib trough levels with patient disposition and adverse event (AE) rates
Of the 351 patients for whom PK samples were evaluable, 246 patients (70.1%) remain on study and 105 (29.9%) patients discontinued imatinib. As summarized in Table 2, of the patients who discontinued, 41.4% were in Q1, 27.5% were in Q2-Q3, and 23% were in Q4. “Unsatisfactory therapeutic effect” was the most frequently cited reason for discontinuation (18.4% of patients in Q1, 15.2% in Q2-Q3, and 8.1% in Q4).
Patient disposition by PK category on day 29 steady-state imatinib trough level
| . | Quartile 1, n=87, no, (%) . | Quartiles 2 and 3, n=178, no. (%) . | Quartile 4, n=86, no. (%) . |
|---|---|---|---|
| Still on imatinib up to 5 y | 51 (58.6) | 129 (72.5) | 66 (76.7) |
| Discontinued imatinib | 36 (41.4) | 49 (27.5) | 20 (23.3) |
| Unsatisfactory effect* | 16 (18.4) | 27 (15.2) | 7 (8.1) |
| Adverse event(s)† | 4 (4.6) | 5 (2.8) | 6 (7.0) |
| Death | 1 (1.1) | 4 (2.2) | 1 (1.2) |
| Transplantation | 5 (5.7) | 5 (2.8) | 0 |
| Other reasons‡ | 10 (11.5) | 8 (4.5) | 6 (7.0) |
| . | Quartile 1, n=87, no, (%) . | Quartiles 2 and 3, n=178, no. (%) . | Quartile 4, n=86, no. (%) . |
|---|---|---|---|
| Still on imatinib up to 5 y | 51 (58.6) | 129 (72.5) | 66 (76.7) |
| Discontinued imatinib | 36 (41.4) | 49 (27.5) | 20 (23.3) |
| Unsatisfactory effect* | 16 (18.4) | 27 (15.2) | 7 (8.1) |
| Adverse event(s)† | 4 (4.6) | 5 (2.8) | 6 (7.0) |
| Death | 1 (1.1) | 4 (2.2) | 1 (1.2) |
| Transplantation | 5 (5.7) | 5 (2.8) | 0 |
| Other reasons‡ | 10 (11.5) | 8 (4.5) | 6 (7.0) |
Including 8 patients who crossed over to interferon and cytarabine (IFN+Ara-C) due to lack of response or loss of response (3, 5, and 0 patients, respectively).
Including 2 patients who crossed over to IFN+Ara-C due to intolerance of imatinib.
Other reasons include the following: subject withdrew consent, protocol violation, loss to follow up, abnormal test procedure, and administrative problems.
Table 3 summarizes frequencies of major AEs for all grades and for grades 3 or 4 observed in the first 3 months, and during the cumulative 5 years of imatinib treatment. The overall frequency of AEs during the first 29 days was lower (data not shown), but the trends with respect to the PK quartiles were similar to those observed within the first 3 months. During the first 3 months of imatinib, the types and grades of emerging AEs were similar among patients in all 3 PK categories, except for fluid retention, nausea, musculoskeletal pain, rash, myalgia, and anemia, which were more frequently reported by patients in Q4 relative to those in Q1. Based on the overall 5-year data, only fluid retention, rash, myalgia, and anemia were more frequently reported by the patients in Q4 on day 29. However, events such as muscle cramps, diarrhea, abdominal pain, headache, and hemorrhage occurred less frequently in these patients compared with those who segregated to Q1.
Frequency of adverse events (all grades, and grades 3 or 4) during the first 3 months and during the 5-year treatment period, by PK category
| Adverse events . | Quartile 1, n=87, % all (% grade 3/4) . | Quartiles 2 and 3, n=178, % all (% grade 3/4) . | Quartile 4, n=86, % all (% grade 3/4) . |
|---|---|---|---|
| AEs within 3 mo | |||
| Fluid retention; superficial edemas | 31.0 (0); 28.7 (0) | 43.8 (0); 43.3 (0) | 52.3 (0); 50.0 (0) |
| Abdominal pain | 28.7 (1.1) | 11.2 (0) | 14.0 (1.2) |
| Nausea | 28.7 (0) | 27.0 (0) | 41.9 (0) |
| Fatigue | 26.4 (0) | 16.9 (0) | 17.4 (1.2) |
| Diarrhea | 24.1 (0) | 26.4 (0) | 16.3 (0) |
| Headache | 21.8 (0) | 20.8 (0) | 17.4 (0) |
| Muscle cramps | 20.7 (0) | 24.2 (0.6) | 17.4 (1.2) |
| Musculoskeletal pain | 20.7 (1.1) | 22.5 (1.7) | 25.6 (2.3) |
| Joint pain | 18.4 (1.1) | 14.6 (1.1) | 19.8 (0) |
| Hemorrhage | 16.1 (1.1) | 9.6 (0.6) | 11.6 (0) |
| Rash and related | 13.8 (1.1) | 20.8 (1.7) | 19.8 (0) |
| Myalgia | 12.6 (0) | 14.6 (1.7) | 24.4 (1.2) |
| Neutropenia | 10.3 (9.2) | 6.7 (5.6) | 7.0 (5.8) |
| Thrombocytopenia | 8.0 (4.6) | 6.2 (2.8) | 9.3 (3.5) |
| Anemia | 2.3 (0) | 4.5 (1.1) | 5.8 (0) |
| AEs within 5 y | |||
| Fluid retention; superficial edema | 52.9 (2.3); 51.7 (1.1) | 61.8 (3.4); 61.2 (1.7) | 75.6 (3.5); 69.8 (1.2) |
| Fatigue | 51.7 (0) | 34.3 (1.1) | 30.2 (2.3) |
| Nausea | 50.6 (1.1) | 43.8 (2.2) | 52.3 (0) |
| Muscle cramps | 49.4 (2.3) | 50.0 (2.2) | 41.9 (1.2) |
| Musculoskeletal pain | 48.3 (3.4) | 47.8 (7.3) | 53.5 (3.5) |
| Diarrhea | 46.0 (3.4) | 57.3 (4.5) | 38.4 (2.3) |
| Abdominal pain | 43.7 (6.9) | 35.4 (4.5) | 31.4 (3.5) |
| Joint pain | 40.2 (2.3) | 27.0 (3.4) | 32.6 (0) |
| Headache | 39.1 (1.1) | 40.4 (0.6) | 32.6 (0) |
| Rash and related | 32.2 (3.4) | 38.8 (2.2) | 51.2 (1.2) |
| Hemorrhage | 31.0 (1.1) | 32.0 (2.2) | 24.4 (1.2) |
| Myalgia | 19.5 (0) | 24.7 (2.2) | 30.2 (1.2) |
| Neutropenia | 14.9 (12.6) | 9.6 (5.6) | 9.3 (8.1) |
| Thrombocytopenia | 13.8 (9.2) | 10.1 (5.1) | 15.1 (4.7) |
| Anemia | 8.0 (0) | 11.8 (3.4) | 19.8 (7.0) |
| Adverse events . | Quartile 1, n=87, % all (% grade 3/4) . | Quartiles 2 and 3, n=178, % all (% grade 3/4) . | Quartile 4, n=86, % all (% grade 3/4) . |
|---|---|---|---|
| AEs within 3 mo | |||
| Fluid retention; superficial edemas | 31.0 (0); 28.7 (0) | 43.8 (0); 43.3 (0) | 52.3 (0); 50.0 (0) |
| Abdominal pain | 28.7 (1.1) | 11.2 (0) | 14.0 (1.2) |
| Nausea | 28.7 (0) | 27.0 (0) | 41.9 (0) |
| Fatigue | 26.4 (0) | 16.9 (0) | 17.4 (1.2) |
| Diarrhea | 24.1 (0) | 26.4 (0) | 16.3 (0) |
| Headache | 21.8 (0) | 20.8 (0) | 17.4 (0) |
| Muscle cramps | 20.7 (0) | 24.2 (0.6) | 17.4 (1.2) |
| Musculoskeletal pain | 20.7 (1.1) | 22.5 (1.7) | 25.6 (2.3) |
| Joint pain | 18.4 (1.1) | 14.6 (1.1) | 19.8 (0) |
| Hemorrhage | 16.1 (1.1) | 9.6 (0.6) | 11.6 (0) |
| Rash and related | 13.8 (1.1) | 20.8 (1.7) | 19.8 (0) |
| Myalgia | 12.6 (0) | 14.6 (1.7) | 24.4 (1.2) |
| Neutropenia | 10.3 (9.2) | 6.7 (5.6) | 7.0 (5.8) |
| Thrombocytopenia | 8.0 (4.6) | 6.2 (2.8) | 9.3 (3.5) |
| Anemia | 2.3 (0) | 4.5 (1.1) | 5.8 (0) |
| AEs within 5 y | |||
| Fluid retention; superficial edema | 52.9 (2.3); 51.7 (1.1) | 61.8 (3.4); 61.2 (1.7) | 75.6 (3.5); 69.8 (1.2) |
| Fatigue | 51.7 (0) | 34.3 (1.1) | 30.2 (2.3) |
| Nausea | 50.6 (1.1) | 43.8 (2.2) | 52.3 (0) |
| Muscle cramps | 49.4 (2.3) | 50.0 (2.2) | 41.9 (1.2) |
| Musculoskeletal pain | 48.3 (3.4) | 47.8 (7.3) | 53.5 (3.5) |
| Diarrhea | 46.0 (3.4) | 57.3 (4.5) | 38.4 (2.3) |
| Abdominal pain | 43.7 (6.9) | 35.4 (4.5) | 31.4 (3.5) |
| Joint pain | 40.2 (2.3) | 27.0 (3.4) | 32.6 (0) |
| Headache | 39.1 (1.1) | 40.4 (0.6) | 32.6 (0) |
| Rash and related | 32.2 (3.4) | 38.8 (2.2) | 51.2 (1.2) |
| Hemorrhage | 31.0 (1.1) | 32.0 (2.2) | 24.4 (1.2) |
| Myalgia | 19.5 (0) | 24.7 (2.2) | 30.2 (1.2) |
| Neutropenia | 14.9 (12.6) | 9.6 (5.6) | 9.3 (8.1) |
| Thrombocytopenia | 13.8 (9.2) | 10.1 (5.1) | 15.1 (4.7) |
| Anemia | 8.0 (0) | 11.8 (3.4) | 19.8 (7.0) |
Correlation of imatinib trough levels with clinical responses
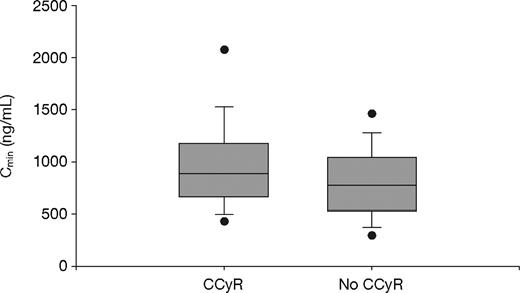
Of the 351 evaluable patients, a total of 297 achieved a CCyR (84.6%): 66 (75.9%) in Q1; 152 (85.4%) in Q2-Q3; and 79 (91.9%) in Q4 (P = .01, Fisher exact test). The imatinib trough levels among patients who achieved a CCyR (n = 297) were significantly higher than those for patients who did not achieve a CCyR (n = 54); the mean values were 1009 plus or minus 544 ng/mL and 812 plus or minus 409 ng/mL, respectively (P = .01, t test; P = .004, Wilcoxon test; Figure 3).
Steady-state imatinib trough levels in chronic-phase CML patients who did and did not achieve CCyR. The mean plasma imatinib trough levels were 1009 plus or minus 544 ng/mL (median, 909 ng/mL) and 812 plus or minus 409 ng/mL (median, 757 ng/mL) for patients who did (n = 297) or did not achieve CCyR (n = 54) during study, respectively (P = .01, t test; P = .004, Wilcoxon test). The figure shows boxplots of Cmin (trough levels) with top and bottom walls of each box representing the 75th and 25th percentiles, respectively. Whiskers above and below the box extend to the 90th and 10th percentiles, respectively, and the dots represent 95th and 5th percentiles.
Steady-state imatinib trough levels in chronic-phase CML patients who did and did not achieve CCyR. The mean plasma imatinib trough levels were 1009 plus or minus 544 ng/mL (median, 909 ng/mL) and 812 plus or minus 409 ng/mL (median, 757 ng/mL) for patients who did (n = 297) or did not achieve CCyR (n = 54) during study, respectively (P = .01, t test; P = .004, Wilcoxon test). The figure shows boxplots of Cmin (trough levels) with top and bottom walls of each box representing the 75th and 25th percentiles, respectively. Whiskers above and below the box extend to the 90th and 10th percentiles, respectively, and the dots represent 95th and 5th percentiles.
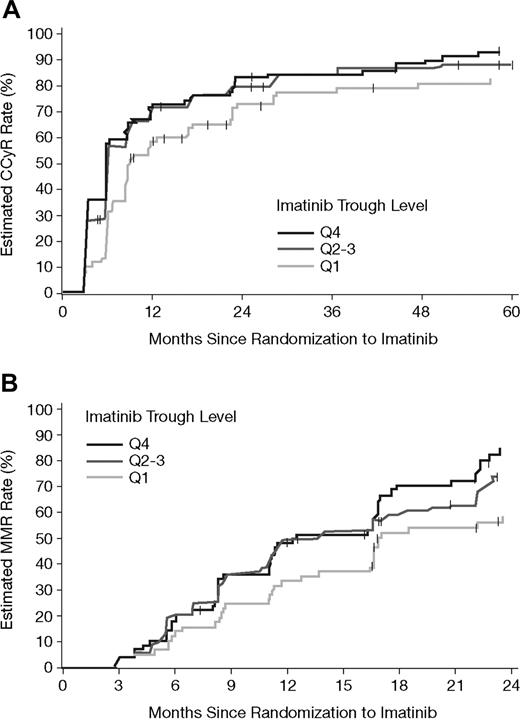
Figure 4 illustrates the estimated cumulative rates of CCyR and MMR relative to time on imatinib therapy for each PK subgroup. Based on Kaplan-Meier analysis, the estimated cumulative rates of CCyR were also statistically significantly different between the imatinib PK trough level categories (P = .01, log-rank test). The difference was attributed mainly to a lower CCyR rate in the Q1 group (P = .005, Q1 vs Q2-Q4). A similar trend was also observed for cumulative MMR rates, as assessed among 265 patients who achieved a CCyR, and for whom a PCR sample was available (P = .02 overall; P = .008 for Q1 vs others). Estimated cumulative response rates at 1, 2, and 4 years are summarized in Table 4. For MMR at 1 and 2 years only, patients who achieved a CCyR within the respective years were included. The durability of CCyR appeared to be lower for patients in the Q1 group. Up to the 5-year cutoff date, for patients who achieved CCyR, more patients lost CCyR from Q1 (16/66 = 24%) than from Q2-Q3 (20/152 = 13%) or Q4 (13/79 = 17%). Due to limited sequential PCR data, the duration of MMR is not reliably accessible and hence cannot be provided.
Estimated cumulative CCyR and MMR rates by PK category of steady-state imatinib trough levels. (A) The estimated cumulative CCyR rates in the 351 patients with available imatinib trough levels at steady state. CCyR rates were significantly lower during the 5-year period for patients in the lowest PK category (Q1 vs others, P = .005, and P = .01 overall). (B) Estimated MMR rates in 265 patients who achieved a CCyR, and for whom PCR data as well as PK samples were available. Among patients with CCyR, lower MMR rates significantly correlated with the lowest imatinib trough levels (Q1 vs others, P = .008, and P = .02 overall).
Estimated cumulative CCyR and MMR rates by PK category of steady-state imatinib trough levels. (A) The estimated cumulative CCyR rates in the 351 patients with available imatinib trough levels at steady state. CCyR rates were significantly lower during the 5-year period for patients in the lowest PK category (Q1 vs others, P = .005, and P = .01 overall). (B) Estimated MMR rates in 265 patients who achieved a CCyR, and for whom PCR data as well as PK samples were available. Among patients with CCyR, lower MMR rates significantly correlated with the lowest imatinib trough levels (Q1 vs others, P = .008, and P = .02 overall).
CCyR and MMR rates by PK category, based on day 29 steady-state imatinib trough levels
| Outcomes . | Quartile 1 (95% CI) . | Quartiles 2 and 3 (95% CI) . | Quartile 4 (95% CI) . |
|---|---|---|---|
| CCyR, n | 87 | 178 | 86 |
| 1 y, % | 59 (48-70) | 71 (64-78) | 73 (63-83) |
| 2 y, % | 73 (63-83) | 80 (73-86) | 84 (75-92) |
| 5 y, % | 83 (73-92) | 88 (83-94) | 93 (87-99) |
| CCyR patients included in analysis: MMR,* n | 57 | 138 | 70 |
| 1 y†, % | 43 (28-59) | 56 (47-66) | 55 (41-68) |
| 2 y‡, % | 63 (49-78) | 78 (69-86) | 86 (76-96) |
| Outcomes . | Quartile 1 (95% CI) . | Quartiles 2 and 3 (95% CI) . | Quartile 4 (95% CI) . |
|---|---|---|---|
| CCyR, n | 87 | 178 | 86 |
| 1 y, % | 59 (48-70) | 71 (64-78) | 73 (63-83) |
| 2 y, % | 73 (63-83) | 80 (73-86) | 84 (75-92) |
| 5 y, % | 83 (73-92) | 88 (83-94) | 93 (87-99) |
| CCyR patients included in analysis: MMR,* n | 57 | 138 | 70 |
| 1 y†, % | 43 (28-59) | 56 (47-66) | 55 (41-68) |
| 2 y‡, % | 63 (49-78) | 78 (69-86) | 86 (76-96) |
Overall, 265 patients who had achieved CCyR had available PCR data as well as PK samples.
Including 209 patients who had achieved CCyR within 1 year and had PCR data and PK samples.
Including 225 patients who had achieved CCyR within 2 years and had PCR data and PK samples.
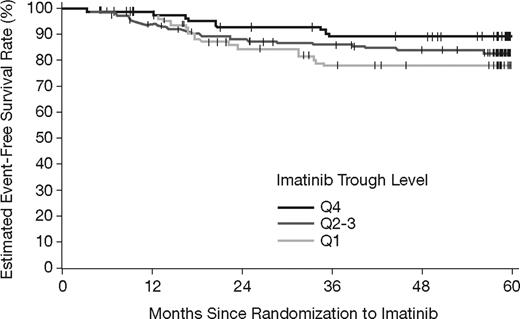
There also appears to be a trend toward lower EFS with lower imatinib trough levels (Figure 5). Estimated EFS rates at 5 years were 78%, 83%, and 89%, for Q1, Q2-Q3, and Q4, respectively. However, no statistically significant difference was observed with the available follow-up (P = .16, log-rank test).
Event-free survival by category of steady-state imatinib trough levels. The estimated EFS rates at 5 years were 78%, 83%, and 89% in the Q1, Q2-Q3, and Q4 groups, respectively (P = .16, log-rank test).
Event-free survival by category of steady-state imatinib trough levels. The estimated EFS rates at 5 years were 78%, 83%, and 89% in the Q1, Q2-Q3, and Q4 groups, respectively (P = .16, log-rank test).
Multivariate analysis of imatinib trough levels as independently prognostic for ability to achieve a CCyR
When including several baseline and disease characteristics into a stepwise logistic regression analysis, only Sokal risk group and imatinib trough level were identified by the final model to be predictive for achievement of a CCyR. Of patients with both Sokal risk and PK samples available, 50% were in the low-risk group, 32% in the intermediate-risk group, and 18% in the high-risk group; their CCyR rates were 94%, 84%, and 73%, respectively (P = .002). Within each Sokal group, the prognostic effect of imatinib trough levels was most apparent in patients of intermediate risk, among whom 64% achieved CCyR in Q1 versus 94% in Q2-Q4 (P = .02). Based on the final multivariate model, for an increase by one Sokal risk level, the odds ratio of achieving a CCyR was 0.55, significantly less than unity (95% CI, 0.32 to 0.93, P = .03), suggesting a decreased likelihood of achieving CCyR with an increase in Sokal score. On the other hand, the odds ratio to achieve a CCyR with respect to an increase in imatinib trough level by 250 ng/mL was significantly greater than unity, 1.77 (1.22 to 2.56, P = .003), suggesting an increased probability of achieving CCyR with an increase in imatinib trough level. These data suggest that in addition to Sokal risk score, imatinib plasma level is a strong predictor of the likelihood of achieving a CCyR.
Discussion
In a recent study by Picard et al, steady-state imatinib plasma trough levels measured after at least 12 months of treatment with standard-dose imatinib correlated with both cytogenetic and molecular responses.14 In the present study, plasma trough levels of imatinib at steady state following the first month of treatment (day 29) also proved to be a significant prognostic covariate for midterm and longer term clinical responses in CML patients. Steady-state levels of imatinib should have been achieved well before day 29. Based on the half-life (20 hours for imatinib and 40 hours for CGP74588), a steady state should have been achieved within 1 week for imatinib and 2 weeks for CGP74588.
The variability of imatinib exposure has clinical implications. Achievement of CCyR is a validated surrogate for clinical benefit in CML and an appropriate measure of antileukemic efficacy. Time to achieving CCyR as well as the overall CCyR rate were significantly different relative to imatinib plasma exposure, as grouped according to quartile (P = .01 for both). Overall, Cmin was significantly higher in patients who achieved a CCyR during the study (mean, 1009 ng/mL vs 812 ng/mL, P = .01). Thus, maintaining an imatinib trough level at or higher than 1000 ng/mL may be important for achieving a CCyR. This result is consistent with the threshold value of 1002 ng/mL reported by Picard et al.14 A similar trend was observed among patients with a CCyR who also achieved an MMR, with patients who segregated into Q4 having a higher probability of achieving an MMR (P = .02). Extending this observation to the whole PK subanalysis population, only an estimated 25% of all patients with imatinib levels less than 647 ng/mL (Q1) achieved an MMR by 1 year of therapy (59% times 43%), whereas 40% of patients with higher imatinib levels achieved an MMR within 1 year (71% times 56% and 73% times 55% for Q2-Q3 and Q4, respectively; Table 4). With emerging evidence that achievement of CCyR with an MMR may be prognostic for long-term treatment efficacy in terms of protection from progression, early dose adjustments for patients with low imatinib steady-state plasma levels may improve clinical response and long-term treatment efficacy.
For this analysis, we assumed that patients maintained adherence to imatinib therapy for the duration of treatment. Based on the dosing records, there were 30 patients who had dose interruptions or reductions during the first month of therapy. However, all had received imatinib on day 28, the day before trough PK sampling, and among the 351 patients evaluated in this subanalysis, the average daily dose during the first month was 393 plus or minus 29 mg. While adherence to therapy is a critical component to maintaining accuracy and validity of the PK analysis, based on imatinib half-life, it was reasonable to assume that the plasma levels of imatinib and CGP74588 were at steady state on day 29. Nonadherence or discontinuation of imatinib has been documented in CML patients and could have impacted clinical responses.11,12,32,33 However, patients enrolled in this study were newly diagnosed with a life-threatening disease, and in the early stages of treatment there was great incentive to take imatinib as prescribed, considering the generally good tolerability of the drug. Of the 553 patients enrolled on the imatinib arm of the IRIS trial, nearly 20% of patients later had a dose escalation to 600- to 800-mg daily dose, by a median of 22 months. No apparent difference in the frequency of dose escalations was observed between PK quartiles (P > .4, Fisher test). Drug-drug interactions are another factor that may affect imatinib plasma concentration. A review of dosing and concomitant medication records showed no evidence of potent CYP3A4 inducers and/or inhibitors administered to these patients during the first 29 days of treatment. The exposure-response relationship may be better defined if long-term measurements of exposure reflecting dose changes and concomitant medications were available.
While better clinical responses (CCyR, MMR, or EFS) were observed in patients with higher imatinib trough concentrations, no major differences were identified between the 3 PK categories in the types or grades of AEs that emerged during therapy, with the exception of fluid retention, rash, myalgia, and anemia; these were all more common among patients with higher imatinib trough levels. Furthermore, the lower frequency of fatigue, abdominal pain, joint pain, and neutropenia occurring among patients with the highest imatinib plasma levels (Q4) suggests that emergence of certain AEs may be more dependent on disease or disease stage, or may be indicative of slower response to therapy, and less a consequence of drug plasma concentrations.
Consistent with the association between imatinib plasma concentrations and ability to achieve a clinically relevant response (CCyR or MMR), as well as a trend toward a longer EFS, the imatinib PK category also correlated with the rate of discontinuation. Patients in the lowest quartile had the highest discontinuation rate, and the highest percentage of patients who discontinued for reason of “unsatisfactory therapeutic effect.” These data suggest that patients are more likely to achieve a satisfactory level of response to therapy, or an improvement in response rates, if an adequate imatinib trough plasma concentration were achieved and maintained.
There appeared to be a weak correlation between imatinib trough level and body weight or BSA. However, these correlations are not likely to be clinically significant due to the large interpatient variability within the same body weight or BSA group. A similarly weak correlation was observed with respect to age. Notably, patients with very high imatinib trough levels were more likely to be 50 years or older, which is probably related to a decreased metabolism capacity or liver function in older patients.15
As shown in Figure 1, of 351 patients for whom PK data were available, 19 had trough levels higher than 2000 ng/mL. A careful review of AEs and dosing records for 6 patients who had trough levels near or higher than 3000 ng/mL revealed no unusual AEs. However, 2 of these 6 patients required a temporary interruption within 1 to 2 months of treatment. None of these patients discontinued imatinib permanently.
A correlation between a high-risk Sokal score and lower probability to achieve a CCyR on imatinib has previously been reported.22 The present multivariate analyses showed that both imatinib trough levels and Sokal risk group were predictive for achievement of CCyR. While Sokal risk reflects the given disease status of individual patients at baseline, imatinib trough level can be increased or decreased through dose adjustment according to the needs of individual patients. Further prospective analyses are warranted to determine definitively the significance of imatinib trough level, Sokal risk, and other biologic parameters in predicting ability to achieve a CCyR response.
In conclusion, imatinib steady-state plasma exposure, measured following the first month of treatment with the standard 400-mg/day dose, correlated with long-term complete cytogenetic and molecular responses, as well as long-term EFS. Maintaining plasma trough levels at or above the mean population concentration of approximately 1000 ng/mL may be important for achieving improved CCyR and MMR rates. Although high imatinib plasma exposure may lead to a higher frequency of certain AEs such as fluid retention, rash, myalgia, and anemia, no increase in discontinuation due to adverse events was observed among patients in Q4 suggesting that AEs remain manageable. In fact, discontinuation rates were highest among patients in the lowest PK category. Factors that affect imatinib exposure, such as drug absorption, metabolism, and interactions between prescribed medications, may impact the ability to achieve a maximal therapeutic benefit. Furthermore, information regarding imatinib blood exposure during therapy has the potential to serve as a valuable tool in evaluating response and optimizing the administered dose, and merits prospective validation in future investigations.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the many patients, nurses, physicians, and IRIS investigators who participated in these studies.
Authorship
Contribution: R.A.L. performed research, analyzed data, and wrote the paper; R.A.L., B.J.D., F.G., and S.G.O. were members of the Study Management Committee for the IRIS study, performed research, and reviewed data; G.J.R., T.K., and I.G. performed research and analyzed data; Y.W. designed and performed PK research, analyzed data, and wrote the paper; all authors approved the final paper.
A complete list of the participating institutions and investigators in the IRIS study can be found in Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article.
Conflict-of-interest disclosure: R.A.L., B.J.D., F.G., and S.G.O. have been consultants for Novartis and received research support. G.J.R., T.K., I.G., and Y.W. are currently employees of Novartis.
Correspondence: Richard A. Larson, University of Chicago, MC-2115, 5841 S Maryland Ave, Chicago, IL 60637; e-mail: rlarson@medicine.bsd.uchicago.edu.