Abstract
Some autoimmune disorders are increasingly recognized as risk factors for non-Hodgkin lymphoma (NHL) overall, but large-scale systematic assessments of risk of NHL subtypes are lacking. We performed a pooled analysis of self-reported autoimmune conditions and risk of NHL and subtypes, including 29 423 participants in 12 case-control studies. We computed pooled odds ratios (OR) and 95% confidence intervals (CI) in a joint fixed-effects model. Sjögren syndrome was associated with a 6.5-fold increased risk of NHL, a 1000-fold increased risk of parotid gland marginal zone lymphoma (OR = 996; 95% CI, 216-4596), and with diffuse large B-cell and follicular lymphomas. Systemic lupus erythematosus was associated with a 2.7-fold increased risk of NHL and with diffuse large B-cell and marginal zone lymphomas. Hemolytic anemia was associated with diffuse large B-cell NHL. T-cell NHL risk was increased for patients with celiac disease and psoriasis. Results for rheumatoid arthritis were heterogeneous between studies. Inflammatory bowel disorders, type 1 diabetes, sarcoidosis, pernicious anemia, and multiple sclerosis were not associated with risk of NHL or subtypes. Thus, specific autoimmune disorders are associated with NHL risk beyond the development of rare NHL subtypes in affected organs. The pattern of associations with NHL subtypes may harbor clues to lymphomagenesis.
Introduction
The etiology of non-Hodgkin lymphoma (NHL) remains largely unexplained, despite its dramatic worldwide rise in incidence in recent decades. The heterogeneity of this group of malignancies with regard to histology, molecular biology, and clinical course is well established,1 whereas etiologic variation among subtypes has been recognized only recently. Known risk factors for NHL overall include uncommon states of severe immune suppression, such as hereditary and acquired immunodeficiency syndromes.2 However, growing evidence from studies of the occurrence of specific NHL subtypes in inflammatory and infectious conditions also suggests a role for chronic immune stimulation.3-7
Local antigenic drive is central to NHL development in the parotid gland in Sjögren syndrome and in the small intestine in celiac disease.4,6 Sjögren syndrome8 and celiac disease9 are also associated with an increase in risk of malignant lymphomas overall. However, whether these associations are the result of a much higher risk of lymphomas originating in the affected organs or whether they also reflect specific associations with other and nonlocalized lymphoma subtypes is less clear.
In rheumatoid arthritis (RA)10,11 and systemic lupus erythematosus (SLE),12 an increased risk of malignant lymphomas has been described repeatedly, whereas the evidence is less consistent for other inflammatory disorders that display autoimmune phenomena, such as psoriasis,13 inflammatory bowel disorders,14 and sarcoidosis.15 Discrepant results between studies may be the result of differences in study size and design and of differences in composition of lymphoma subtypes studied and failure to disentangle subtype-specific associations. Indeed, large studies are needed to investigate associations between autoimmune disorders and NHL subtypes, as both disease groups are rare in the population.
Further elucidation of the nature of associations between autoimmune disorders and NHL subtypes may give clues to the underlying biology of autoimmune-related lymphomagenesis, and may also have implications for NHL development in general. The aims of this large pooled study, encompassing almost 30 000 subjects in 12 countries in Europe and North America and Australia, were to investigate associations between a range of autoimmune disorders and risk of NHL, to explore potential variation in associations among NHL subtypes by histology and anatomic site and to identify possible reasons for heterogeneity between studies.
Methods
Study population
We performed a pooled analysis of individual data from 12 case-control studies identified through the InterLymph consortium (www.epi.grants.cancer.gov/InterLymph). Studies that met the following criteria were eligible to contribute to the pooled analysis: case patients diagnosed with incident NHL as adults (17-89 years of age); collection of personal history of one or more autoimmune conditions; population- or hospital-based design; study completion between 1992 and 2005; and electronic datasets available in August 2005. In Table 1, we present selected characteristics of the participating studies.
Characteristics of case-control studies participating in the pooled analysis of history of autoimmune conditions and risk of non-Hodgkin lymphoma (NHL)
| Study acronym . | Location . | Years . | Age, y . | Matching variables . | Cases . | Controls . | |||
|---|---|---|---|---|---|---|---|---|---|
| N . | Rate,* % . | N . | Rate,* % . | Source . | |||||
| Avi-N | Aviano; Napoli, Italy | 1999-2002 | 18-84 | None | 225 | 97 | 492 | 91 | Patients admitted to hospital for non-neoplastic, nonimmunologic conditions |
| BC | Vancouver, Victoria, BC | 2000-2004 | 20-80 | Age, sex, region | 828 | 79 | 848 | 46 | Random selection from client registry of Ministry of Health |
| Connecticut | Connecticut | 1995-2001 | 21-84 | Age | 598 | 72 | 716 | 47-69 | <65 y: RDD; ≥65 y: random selection from CMMS |
| EpiLymph | Spain | 1998-2003 | 17-96 | Age, sex, region | 435 | 82 | 630 | 96 | Patients admitted to hospital for infectious, parasitic, mental, nervous, circulatory, digestive, endocrine, metabolic, or respiratory conditions |
| Germany | 1999-2002 | 18-82 | Age, sex, region | 518 | 87 | 710 | 44 | Random selection from population registries | |
| Ireland | 1998-2004 | 19-85 | Age, sex, center | 144 | 90 | 208 | 75 | Patients admitted to hospital for infectious, parasitic, mental, nervous, circulatory, digestive, endocrine, metabolic, or respiratory conditions | |
| Czech Republic | 2001-2003 | 19-82 | Age, sex, region | 199 | 90 | 304 | 60 | Patients admitted to hospital for infectious, parasitic, mental, nervous, circulatory, digestive, endocrine, metabolic, and respiratory conditions | |
| France | 2000-2003 | 18-82 | Age, sex, region | 217 | 91 | 260 | 74 | Patients admitted to hospital for infectious, parasitic, mental, nervous, circulatory, digestive, endocrine, metabolic, and respiratory conditions | |
| Italy (Sardinia) | 1998-2004 | 25-81 | Age, sex, region | 219 | 93 | 336 | 66 | Random selection from population registries | |
| Italy | Turin, Novara, Forli, Vercelli, Varese, Verona, Florence, Siena, Latina, Ragusa, Imperia | 1990-1993 | 20-74 | Age, sex, region | 1426 | 82 | 1771 | 74 | Random selection from demographic or National Health Service files |
| NCI-SEER | Detroit, MI; Iowa; Los Angeles, CA; Seattle, WA | 1998-2001 | 20-74 | Age, sex, region, race | 1316 | 76 | 1055 | 52 | <65 y: RDD; ≥65 y: random selection from CMMS |
| Nebraska | Nebraska | 1999-2002 | 20-75 | Age, sex | 387 | 74 | 535 | 78 | RDD |
| Northern Italy | Aviano; Milan, Italy | 1983-1992 | 17-85 | None | 429 | >97 | 1155 | >97 | Patients admitted for non-neoplastic, nonimmunologic conditions in hospitals where cases diagnosed |
| NSW | New South Wales, Australian Capital Territory, Australia | 2000-2001 | 20-74 | Age, sex, region | 694 | 85 | 694 | 61 | Random selection from electoral rolls |
| SCALE | Denmark; Sweden | 1999-2002 | 18-74 | Age, sex, country | 3055 | 81 | 3187 | 71 | Random selection from population registries |
| UCSF | San Francisco, CA | 1988-1993 | 21-74 | Age, sex, region | 1305 | 72 | 2404 | 78 | <65 y: RDD; ≥65 y: random selection from CMMS |
| UK | Parts of north and southwest England | 1998-2003 | 16-69 | Age, sex, region | 828 | 75 | 1139 | 71 | Random selection from general practice lists |
| Study acronym . | Location . | Years . | Age, y . | Matching variables . | Cases . | Controls . | |||
|---|---|---|---|---|---|---|---|---|---|
| N . | Rate,* % . | N . | Rate,* % . | Source . | |||||
| Avi-N | Aviano; Napoli, Italy | 1999-2002 | 18-84 | None | 225 | 97 | 492 | 91 | Patients admitted to hospital for non-neoplastic, nonimmunologic conditions |
| BC | Vancouver, Victoria, BC | 2000-2004 | 20-80 | Age, sex, region | 828 | 79 | 848 | 46 | Random selection from client registry of Ministry of Health |
| Connecticut | Connecticut | 1995-2001 | 21-84 | Age | 598 | 72 | 716 | 47-69 | <65 y: RDD; ≥65 y: random selection from CMMS |
| EpiLymph | Spain | 1998-2003 | 17-96 | Age, sex, region | 435 | 82 | 630 | 96 | Patients admitted to hospital for infectious, parasitic, mental, nervous, circulatory, digestive, endocrine, metabolic, or respiratory conditions |
| Germany | 1999-2002 | 18-82 | Age, sex, region | 518 | 87 | 710 | 44 | Random selection from population registries | |
| Ireland | 1998-2004 | 19-85 | Age, sex, center | 144 | 90 | 208 | 75 | Patients admitted to hospital for infectious, parasitic, mental, nervous, circulatory, digestive, endocrine, metabolic, or respiratory conditions | |
| Czech Republic | 2001-2003 | 19-82 | Age, sex, region | 199 | 90 | 304 | 60 | Patients admitted to hospital for infectious, parasitic, mental, nervous, circulatory, digestive, endocrine, metabolic, and respiratory conditions | |
| France | 2000-2003 | 18-82 | Age, sex, region | 217 | 91 | 260 | 74 | Patients admitted to hospital for infectious, parasitic, mental, nervous, circulatory, digestive, endocrine, metabolic, and respiratory conditions | |
| Italy (Sardinia) | 1998-2004 | 25-81 | Age, sex, region | 219 | 93 | 336 | 66 | Random selection from population registries | |
| Italy | Turin, Novara, Forli, Vercelli, Varese, Verona, Florence, Siena, Latina, Ragusa, Imperia | 1990-1993 | 20-74 | Age, sex, region | 1426 | 82 | 1771 | 74 | Random selection from demographic or National Health Service files |
| NCI-SEER | Detroit, MI; Iowa; Los Angeles, CA; Seattle, WA | 1998-2001 | 20-74 | Age, sex, region, race | 1316 | 76 | 1055 | 52 | <65 y: RDD; ≥65 y: random selection from CMMS |
| Nebraska | Nebraska | 1999-2002 | 20-75 | Age, sex | 387 | 74 | 535 | 78 | RDD |
| Northern Italy | Aviano; Milan, Italy | 1983-1992 | 17-85 | None | 429 | >97 | 1155 | >97 | Patients admitted for non-neoplastic, nonimmunologic conditions in hospitals where cases diagnosed |
| NSW | New South Wales, Australian Capital Territory, Australia | 2000-2001 | 20-74 | Age, sex, region | 694 | 85 | 694 | 61 | Random selection from electoral rolls |
| SCALE | Denmark; Sweden | 1999-2002 | 18-74 | Age, sex, country | 3055 | 81 | 3187 | 71 | Random selection from population registries |
| UCSF | San Francisco, CA | 1988-1993 | 21-74 | Age, sex, region | 1305 | 72 | 2404 | 78 | <65 y: RDD; ≥65 y: random selection from CMMS |
| UK | Parts of north and southwest England | 1998-2003 | 16-69 | Age, sex, region | 828 | 75 | 1139 | 71 | Random selection from general practice lists |
RDD indicates random digit dialing; CMMS Centers for Medicare and Medicaid Services.
Participation rate.
Eight of the studies have previously reported data on some autoimmune conditions and risk of NHL.16-24 However, analyses of risk by NHL subtypes according to the World Health Organization (WHO) 2001 classification of lymphoid neoplasms1 were not performed in 5 of these studies17-19,21,24 and subtype risk estimation was hampered by low statistical power in the remaining 3 studies.20,22,23 Case patients were identified through rapid case ascertainment systems in direct collaboration with treating physicians, nurses, and/or pathologists (8 studies) and through regional population-based cancer registries (4 studies), of which one used a rapid clinical reporting system. All but one study included both men and women; the Connecticut study was restricted to women. Individuals with HIV infection were either excluded from the original studies based on reports from the treating physician (5 studies) and/or self-reports (1 study) or excluded from the pooled analysis based on self-reports (4 studies) and/or blood test results (2 studies). Individuals with a history of organ transplantation were also excluded from the pooled analysis.
All studies used a frequency-matched case-control design, except the United Kingdom study and 2 EpiLymph centers (Germany and Czech Republic) where cases and controls were matched individually, and the Aviano-Napoli and Northern Italy studies where no matching criteria were applied. For hospital-based controls (19% of all controls), the disease at admission was examined, and subjects admitted for autoimmune conditions were excluded (27 subjects, 0.9% of all hospital-based controls).
Exposure assessment
The participating studies used either telephone or in-person interviews with trained interviewers and standardized structured questionnaires: one study used a self-completed questionnaire. Included were questions about one or more autoimmune disorders and a range of potential confounding factors. All autoimmune disorders were self-reported, none was confirmed by medical record review, but most studies (70%) asked for personal history of physician-diagnosed autoimmune conditions.
The autoimmune disorders assessed by more than one study, and thus eligible for pooling, included Sjögren syndrome, SLE, RA, systemic sclerosis or scleroderma, poly- or dermatomyositis, immune thrombocytopenic purpura, type 1 diabetes, pernicious anemia, multiple sclerosis, myasthenia gravis, celiac disease, psoriasis, sarcoidosis, and inflammatory bowel disorders (Crohn disease and ulcerative colitis). Autoimmune hemolytic anemia was assessed in 2 studies, and 3 additional studies assessed the broader term hemolytic anemia. These reports were grouped together as hemolytic anemia under the assumption that the majority of subjects reporting hemolytic anemia probably have the autoimmune form of disease. We further classified Sjögren syndrome into primary (report of Sjögren syndrome only) or secondary disease (concomitant report of RA, SLE, or poly- or dermatomyositis) in 8 studies. Participants who reported a diagnosis of diabetes at age 30 or less were classified as having type 1 diabetes.23 We assessed duration of the autoimmune disorder as years between autoimmune disease onset and NHL diagnosis (cases) or interview (controls). For all conditions, reports of autoimmune disease with a duration of less than 2 years were not counted. If 2 incompatible autoimmune disorders were reported by the same subject (eg, RA and SLE), the most recently diagnosed disorder was retained. Information on treatment with systemic corticosteroids was collected in 7 studies, and on use of immunosuppressants (including methotrexate, cyclophosphamide, cyclosporine, azathioprine, and unspecified immunosuppressive drugs) in 5 studies. Information on treatment with tumor necrosis factor blocking agents was not collected because these agents were introduced after initiation of almost all participating studies.
The pooled analysis was approved by the University of New South Wales Human research Ethics Committee. Each participating study obtained local ethical approval and informed consent from participants and provided a de-identified dataset with individual information on history of assessed autoimmune conditions, matching variables and demographic and potentially confounding variables for their study participants.
Tumor classification
All studies verified NHL diagnoses by histopathology review (report review in 4, slide review in 2, and both in 6 studies). All NHL subtypes according to the WHO classification,1 except multiple myeloma, were included in this analysis. In most studies, patients with chronic lymphocytic leukemia, which constitutes an NHL subtype according to the WHO classification, were recruited as cases, whereas such patients were excluded in a few studies. Case patients classified by the WHO/ICD-O-3 classification (6 studies) were placed directly into the hierarchical groupings of a proposed nested classification based on the WHO classification,1 developed by a group of expert hematopathologists for epidemiologic research.25 Subtype diagnoses using earlier classifications (REAL, 1 study; ICD-O-2 1994, 2 studies; Working Formulation, 3 studies) were converted to hierarchical groups and where possible to WHO subtypes using algorithms from the nested classification.25 The overall distribution of WHO NHL subtypes in the pooled data and according to the proposed hierarchical groups is given in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Results are presented in tables for the most frequent NHL subtypes or hierarchical groupings, for which the statistical power was the greatest, whereas results for rare subtypes are presented in the text where relevant. Site of lymphoma, for cases with extranodal NHL, was recorded in 6 studies for a total of 7829 cases. True extranodal sites were defined as extranodal and extralymphatic (excluding spleen, Waldeyer ring, and thymus). Patients with unspecified lymphoma (ie, neither non-Hodgkin nor Hodgkin lymphoma) were not included in most participating studies and thus not in this pooled analysis. In a few of the participating studies, unspecified lymphomas diagnosed in the same populations were however recorded in parallel, and the numbers were low (maximum 3% of all lymphomas diagnosed during the same time periods).
Statistical analysis
Odds ratios (OR) and 95% confidence intervals (CI) were calculated from unconditional logistic regression models. We first assessed study-specific risk estimates, and where available, the data were checked against previously published results.17-24 Because of the variation in control ascertainment between the 6 member study centers in EpiLymph (Table 1), this study was treated statistically as 6 separate studies, to allow for assessment of center-specific effects and heterogeneity by center. For studies with individually matched cases and controls, we compared study-specific results using conditional and unconditional regression. Because there were only marginal differences, we used unconditional regression that led to inclusion of a larger number of controls and, thus, increased statistical power. All hypotheses tested were stated a priori in a predefined analysis plan.
Given the rarity of the assessed disorders, with some studies having no exposed cases and/or no exposed controls for one or several autoimmune disorders, we used a joint fixed-effects model for the pooled analysis26 to allow for inclusion of all studies. For studies with at least one exposed case and control, between-study heterogeneity was assessed using a likelihood-ratio test, and where numbers allowed, further explored with the Cochran Q statistic and the I2 statistic27 in a 2-stage random-effects model.26 Because of the potential limitations of the joint fixed-effects model, including underestimation of the standard error and overweighting of larger studies,26 we compared pooled risk estimates computed using the joint fixed-effects model and the 2-stage random-effects model, and the estimates differed only marginally for all disorders. Statistically significant between-study heterogeneity was found for one disorder, RA, for which pooled estimates using the 2-stage random-effects model are presented. Possible causes of between-study heterogeneity were investigated in relation to differences in study design (such as questionnaire and data collection format, population vs hospital controls, response rates, and control exposure prevalence rates) as an aid in the identification of outlying studies. For all disorders, we also compared risk estimates in logistic regression models stratified by design-related variables, and we did not observe any general differences in effect by these factors.
All models were adjusted for the matching variables age, sex, region/study center and race. We considered additional potential confounders if they were collected across the majority of studies (socioeconomic status, family history of lymphoma) or if the variable was shown to be an important confounder in a participating study. In individual studies, the inclusion of a range of exposures including tobacco smoking, body mass index, sun exposure, and occupational exposure to pesticides and solvents were not found to change study-specific estimates by more than 10%.17,19-23 Family history of lymphoma and socioeconomic status (assessed as educational level in 10 studies, and from census data in 2 studies) did not change pooled estimates significantly. However, socioeconomic status was considered to be a potentially important study-specific confounder, especially in the United States where socioeconomic status is related to healthcare access, and was thus retained in the pooled model.
We compared risk estimates by sex, autoimmune disease duration, and treatment with systemic corticosteroids and/or immunosuppressants, and age of autoimmune and NHL onset, in stratified logistic regression models. Because there was no statistically significant heterogeneity of results by sex (although few men reported some disorders) and to maximize statistical power, women and men were analyzed together. Median age of autoimmune disease or NHL onset were used as cutoff for computing younger- and older-onset groups. Because of the large predominance of study participants with white origin, stratification by race was not meaningful. All statistical tests were 2-sided, and the level of statistical significance was set to α = 0.05. Analyses were performed using the STATA software version 8.2 (Stata, College Station, TX).
Results
Characteristics of the participants are shown in Table 2. Personal history of the assessed autoimmune conditions and pooled risks of NHL overall are shown in Table 3. Results by study are shown in Figure 1 for RA, and in Figures S1 to S13 for the other disorders assessed.
Demographic factors of the pooled study participants
| Demographic factor . | NHL cases, no. (%) . | Controls, no. (%) . |
|---|---|---|
| Pooled total | 12 982 | 16 441 |
| Sex | ||
| Men | 7029 (54.1) | 8895 (54.1) |
| Women | 5953 (45.9) | 7546 (45.9) |
| Age, y | ||
| <20 | 25 (0.2) | 69 (0.4) |
| 20-29* | 357 (2.7) | 946 (5.8) |
| 30-39* | 898 (6.9) | 1699 (10.3) |
| 40-49 | 1740 (13.4) | 2415 (14.7) |
| 50-59 | 3362 (25.9) | 3725 (22.7) |
| 60-69 | 4144 (31.9) | 4670 (28.4) |
| 70-79 | 2328 (17.9) | 2765 (16.8) |
| 80+ | 126 (1.0) | 152 (0.9) |
| Median (range) | 60 (17-89) | 58 (16-96) |
| Education/SES† | ||
| Low | 5038 (38.8) | 5823 (35.4) |
| Medium | 4483 (34.5) | 5974 (36.3) |
| High | 3382 (26.1) | 4562 (27.7) |
| Not recorded | 78 (0.6) | 82 (0.5) |
| Race/ethnicity‡ | ||
| White | 5172 (39.8) | 6507 (39.6) |
| Black | 201 (1.5) | 324 (2) |
| Other/unknown | 528 (4.1) | 556 (3.4) |
| Missing§ | 7081 (54.5) | 9054 (55.1) |
| Demographic factor . | NHL cases, no. (%) . | Controls, no. (%) . |
|---|---|---|
| Pooled total | 12 982 | 16 441 |
| Sex | ||
| Men | 7029 (54.1) | 8895 (54.1) |
| Women | 5953 (45.9) | 7546 (45.9) |
| Age, y | ||
| <20 | 25 (0.2) | 69 (0.4) |
| 20-29* | 357 (2.7) | 946 (5.8) |
| 30-39* | 898 (6.9) | 1699 (10.3) |
| 40-49 | 1740 (13.4) | 2415 (14.7) |
| 50-59 | 3362 (25.9) | 3725 (22.7) |
| 60-69 | 4144 (31.9) | 4670 (28.4) |
| 70-79 | 2328 (17.9) | 2765 (16.8) |
| 80+ | 126 (1.0) | 152 (0.9) |
| Median (range) | 60 (17-89) | 58 (16-96) |
| Education/SES† | ||
| Low | 5038 (38.8) | 5823 (35.4) |
| Medium | 4483 (34.5) | 5974 (36.3) |
| High | 3382 (26.1) | 4562 (27.7) |
| Not recorded | 78 (0.6) | 82 (0.5) |
| Race/ethnicity‡ | ||
| White | 5172 (39.8) | 6507 (39.6) |
| Black | 201 (1.5) | 324 (2) |
| Other/unknown | 528 (4.1) | 556 (3.4) |
| Missing§ | 7081 (54.5) | 9054 (55.1) |
The inclusion of Hodgkin lymphoma cases in several studies led to an imbalance in the age distribution of controls compared with the NHL cases only.
Education/socioeconomic status (SES) groups in each study were based on the tertile distribution of years of education (10 studies) or SES levels obtained from census data (2 studies) in controls.
Participants with Hispanic origin were mainly categorized as “white” (n = 307), but a few individuals were also categorized as “black” (n = 4) or with “other/unknown” ethnicity (n = 24), based on the original categorization in the participating studies.
Ethnicity was generally not recorded in the European studies because of the large predominance of individuals with white origin.
Personal history of selected autoimmune disorders and pooled relative risk of non-Hodgkin lymphoma (NHL)
| Disorder* . | No. of studies . | Prevalence† . | Pooled relative risk . | ||
|---|---|---|---|---|---|
| All NHL: ever/never (%) . | Controls: ever/never (%) . | OR (95% CI)‡ . | Study heterogeneity P§ . | ||
| Rheumatoid arthritis | 12 | 504/11735 (4.3) | 556/15222 (3.7) | 1.06 (0.87-1.29)‖ | <.01 |
| Psoriasis | 7 | 278/7460 (3.7) | 279/10122 (2.8) | 1.16 (0.98-1.38) | .82 |
| Ulcerative colitis | 9 | 125/9775 (1.3) | 138/12148 (1.1) | 1.02 (0.79-1.31) | .84 |
| Type 1 diabetes | 9 | 32/10289 (0.3) | 58/13613 (0.4) | 0.76 (0.49-1.19) | .79 |
| Sarcoidosis | 6 | 19/7607 (0.2) | 27/8856 (0.3) | 0.73 (0.40-1.32) | .70 |
| Pernicious anemia | 5 | 13/3410 (0.4) | 16/5865 (0.3) | 1.08 (0.51-2.30) | .25 |
| Crohn disease | 7 | 23/9230 (0.2) | 27/10614 (0.3) | 0.89 (0.50-1.56) | .74 |
| Celiac disease | 7 | 33/9343 (0.4) | 25/10424 (0.2) | 1.50 (0.89-2.54) | .72 |
| Hemolytic anemia | 5 | 21/3242 (0.6) | 13/5585 (0.2) | 2.57 (1.27-5.21) | .57 |
| Systemic lupus erythematosus | 11 | 57/12034 (0.5) | 26/15237 (0.2) | 2.69 (1.68-4.30) | .39 |
| Multiple sclerosis | 10 | 15/9666 (0.2) | 18/12341 (0.1) | 0.96 (0.48-1.92) | .89 |
| Sjögren syndrome | 8 | 52/8178 (0.6) | 8/10543 (0.1) | 6.56 (3.10-13.9) | .72 |
| Primary Sjögren syndrome | 8 | 23/8176 (0.3) | 5/10543 (0.0) | 4.75 (1.79-12.6) | .93 |
| Secondary Sjögren syndrome | 8 | 29/8178 (0.4) | 3/10542 (0.0) | 9.57 (2.90-31.6) | ND |
| Scleroderma | 7 | 4/7616 (0.1) | 7/10093 (0.1) | 0.69 (0.20-2.40) | .81 |
| Immune thrombocytopenia | 5 | 4/4095 (0.1) | 3/6529 (0.0) | 2.13 (0.47-9.73) | .54 |
| Myasthenia gravis | 6 | 4/6385 (0.1) | 3/7413 (0.0) | 1.45 (0.31-6.82) | .53 |
| Polymyositis/dermatomyositis | 5 | 6/6662 (0.1) | 0/7947 (0.0) | ND | ND |
| Disorder* . | No. of studies . | Prevalence† . | Pooled relative risk . | ||
|---|---|---|---|---|---|
| All NHL: ever/never (%) . | Controls: ever/never (%) . | OR (95% CI)‡ . | Study heterogeneity P§ . | ||
| Rheumatoid arthritis | 12 | 504/11735 (4.3) | 556/15222 (3.7) | 1.06 (0.87-1.29)‖ | <.01 |
| Psoriasis | 7 | 278/7460 (3.7) | 279/10122 (2.8) | 1.16 (0.98-1.38) | .82 |
| Ulcerative colitis | 9 | 125/9775 (1.3) | 138/12148 (1.1) | 1.02 (0.79-1.31) | .84 |
| Type 1 diabetes | 9 | 32/10289 (0.3) | 58/13613 (0.4) | 0.76 (0.49-1.19) | .79 |
| Sarcoidosis | 6 | 19/7607 (0.2) | 27/8856 (0.3) | 0.73 (0.40-1.32) | .70 |
| Pernicious anemia | 5 | 13/3410 (0.4) | 16/5865 (0.3) | 1.08 (0.51-2.30) | .25 |
| Crohn disease | 7 | 23/9230 (0.2) | 27/10614 (0.3) | 0.89 (0.50-1.56) | .74 |
| Celiac disease | 7 | 33/9343 (0.4) | 25/10424 (0.2) | 1.50 (0.89-2.54) | .72 |
| Hemolytic anemia | 5 | 21/3242 (0.6) | 13/5585 (0.2) | 2.57 (1.27-5.21) | .57 |
| Systemic lupus erythematosus | 11 | 57/12034 (0.5) | 26/15237 (0.2) | 2.69 (1.68-4.30) | .39 |
| Multiple sclerosis | 10 | 15/9666 (0.2) | 18/12341 (0.1) | 0.96 (0.48-1.92) | .89 |
| Sjögren syndrome | 8 | 52/8178 (0.6) | 8/10543 (0.1) | 6.56 (3.10-13.9) | .72 |
| Primary Sjögren syndrome | 8 | 23/8176 (0.3) | 5/10543 (0.0) | 4.75 (1.79-12.6) | .93 |
| Secondary Sjögren syndrome | 8 | 29/8178 (0.4) | 3/10542 (0.0) | 9.57 (2.90-31.6) | ND |
| Scleroderma | 7 | 4/7616 (0.1) | 7/10093 (0.1) | 0.69 (0.20-2.40) | .81 |
| Immune thrombocytopenia | 5 | 4/4095 (0.1) | 3/6529 (0.0) | 2.13 (0.47-9.73) | .54 |
| Myasthenia gravis | 6 | 4/6385 (0.1) | 3/7413 (0.0) | 1.45 (0.31-6.82) | .53 |
| Polymyositis/dermatomyositis | 5 | 6/6662 (0.1) | 0/7947 (0.0) | ND | ND |
ND indicates not determined.
Restricted to a history of the autoimmune disorder diagnosed at least 2 years before interview/NHL diagnosis.
Please note that the denominator differs for each AI condition because not all participating studies ascertained history of each AI condition. The exact number of exposed cases and controls contributing from each study are shown in Figures S1 to S13 (including all but the last four disorders listed).
Odds ratio (OR) and 95% confidence interval (CI) computed using a joint fixed-effects model adjusted for age (in 5-year categories), sex, race/ethnicity, education/SES, and study center, with no history of the autoimmune disease in question as reference category.
P value for heterogeneity using a likelihood-ratio test, between studies with at least one exposed case and one exposed control (as shown in Figures S1 to S13).
Results computed using the two-stage random effects model due to statistically significant between-study heterogeneity (P < .01).
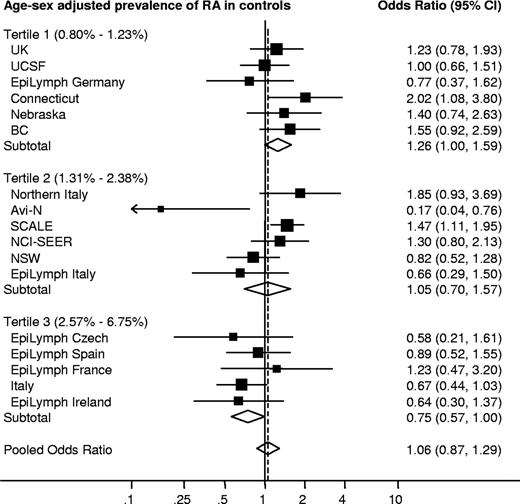
Personal history of rheumatoid arthritis (RA) and risk of non-Hodgkin lymphoma (NHL) by study. Individual study results are sorted and stratified by the age- and sex-adjusted prevalence of RA in controls.
Personal history of rheumatoid arthritis (RA) and risk of non-Hodgkin lymphoma (NHL) by study. Individual study results are sorted and stratified by the age- and sex-adjusted prevalence of RA in controls.
Sjögren syndrome
Sjögren syndrome was associated with a 6.6-fold increased risk of NHL, and secondary Sjögren syndrome yielded a higher risk than the primary form (Table 3). Women with Sjögren (92% of all affected participants) experienced a 6-fold risk increase, whereas a lack of exposed controls precluded risk estimation among men (data not shown). In all Sjögren syndrome and in primary disease, a 5-fold increased risk of NHL persisted with more than 10 years of disease (Table 4). Statistically significant associations were confined to ages 60 years or older at NHL diagnosis. Neither age at Sjögren syndrome onset nor corticosteroid/immunosuppressive drug use modified the results (data not shown).
Personal history of selected autoimmune disorders and pooled relative risk of non-Hodgkin lymphoma (NHL) by autoimmune disease duration
| Disorder* . | Never . | 2-5 years . | 6-10 years . | More than 10 years . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases . | Controls . | OR (95% CI)† . | Cases . | Controls . | OR (95% CI)† . | Cases . | Controls . | OR (95% CI)† . | Cases . | Controls . | OR (95% CI)† . | |
| Psoriasis | 7460 | 10 122 | 1.00 (referent) | 41 | 40 | 1.35 (0.86-2.10) | 31 | 47 | 0.81 (0.51-1.28) | 200 | 188 | 1.19 (0.97-1.47) |
| Ulcerative colitis | 9775 | 12 148 | 1.00 (referent) | 12 | 19 | 0.83 (0.39-1.75) | 9 | 13 | 0.73 (0.30-0.75) | 86 | 90 | 1.08 (0.80-1.47) |
| Type 1 diabetes | 10 289 | 13 613 | 1.00 (referent) | 1 | 3 | 1.08 (0.11-10.4) | 1 | 3 | 0.66 (0.07-6.55) | 30 | 52 | 0.76 (0.48-1.20) |
| Sarcoidosis | 7607 | 8856 | 1.00 (referent) | 1 | 3 | 0.43 (0.04-4.19) | 1 | 3 | 0.34 (0.03-3.28) | 11 | 15 | 0.78 (0.35-1.71) |
| Pernicious anemia | 3410 | 5865 | 1.00 (referent) | 2 | 2 | 2.10 (0.28-15.6) | 2 | 0 | ND | 4 | 6 | 1.13 (0.31-4.08) |
| Crohn disease | 9230 | 10 614 | 1.00 (referent) | 3 | 4 | 0.92 (0.20-4.25) | 4 | 3 | 1.91 (0.41-8.85) | 12 | 16 | 0.73 (0.34-1.57) |
| Celiac disease | 9343 | 10 424 | 1.00 (referent) | 6 | 5 | 1.30 (0.39-4.28) | 3 | 5 | 0.69 (0.16-2.93) | 20 | 13 | 1.82 (0.89-3.69) |
| Hemolytic anemia | 3242 | 5585 | 1.00 (referent) | 3 | 2 | 2.37 (0.39-14.4) | 2 | 2 | 2.13 (0.29-15.8) | 15 | 9 | 2.50 (1.08-5.83) |
| Systemic lupus erythematosus | 12 034 | 15 237 | 1.00 (referent) | 10 | 1 | 14.6 (1.85-115) | 11 | 6 | 2.46 (0.90-6.75) | 26 | 16 | 1.89 (1.00-3.55) |
| Multiple sclerosis | 9666 | 12 341 | 1.00 (referent) | 1 | 3 | 0.55 (0.06-5.37) | 3 | 0 | ND | 6 | 11 | 0.61 (0.22-1.68) |
| Sjögren syndrome | 8178 | 10 543 | 1.00 (referent) | 15 | 3 | 4.54 (1.31-15.8) | 13 | 1 | 15.7 (2.04-121) | 20 | 4 | 5.07 (1.72-14.9) |
| Primary Sjögren syndrome | 8176 | 10 543 | 1.00 (referent) | 6 | 2 | 2.84 (0.57-14.2) | 2 | 1 | 2.64 (0.23-30.3) | 12 | 2 | 6.37 (1.42-28.6) |
| Secondary Sjögren syndrome | 8178 | 10 542 | 1.00 (referent) | 9 | 1 | 7.92 (1.00-62.8) | 11 | 0 | ND | 8 | 2 | 3.78 (0.79-18.1) |
| Disorder* . | Never . | 2-5 years . | 6-10 years . | More than 10 years . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases . | Controls . | OR (95% CI)† . | Cases . | Controls . | OR (95% CI)† . | Cases . | Controls . | OR (95% CI)† . | Cases . | Controls . | OR (95% CI)† . | |
| Psoriasis | 7460 | 10 122 | 1.00 (referent) | 41 | 40 | 1.35 (0.86-2.10) | 31 | 47 | 0.81 (0.51-1.28) | 200 | 188 | 1.19 (0.97-1.47) |
| Ulcerative colitis | 9775 | 12 148 | 1.00 (referent) | 12 | 19 | 0.83 (0.39-1.75) | 9 | 13 | 0.73 (0.30-0.75) | 86 | 90 | 1.08 (0.80-1.47) |
| Type 1 diabetes | 10 289 | 13 613 | 1.00 (referent) | 1 | 3 | 1.08 (0.11-10.4) | 1 | 3 | 0.66 (0.07-6.55) | 30 | 52 | 0.76 (0.48-1.20) |
| Sarcoidosis | 7607 | 8856 | 1.00 (referent) | 1 | 3 | 0.43 (0.04-4.19) | 1 | 3 | 0.34 (0.03-3.28) | 11 | 15 | 0.78 (0.35-1.71) |
| Pernicious anemia | 3410 | 5865 | 1.00 (referent) | 2 | 2 | 2.10 (0.28-15.6) | 2 | 0 | ND | 4 | 6 | 1.13 (0.31-4.08) |
| Crohn disease | 9230 | 10 614 | 1.00 (referent) | 3 | 4 | 0.92 (0.20-4.25) | 4 | 3 | 1.91 (0.41-8.85) | 12 | 16 | 0.73 (0.34-1.57) |
| Celiac disease | 9343 | 10 424 | 1.00 (referent) | 6 | 5 | 1.30 (0.39-4.28) | 3 | 5 | 0.69 (0.16-2.93) | 20 | 13 | 1.82 (0.89-3.69) |
| Hemolytic anemia | 3242 | 5585 | 1.00 (referent) | 3 | 2 | 2.37 (0.39-14.4) | 2 | 2 | 2.13 (0.29-15.8) | 15 | 9 | 2.50 (1.08-5.83) |
| Systemic lupus erythematosus | 12 034 | 15 237 | 1.00 (referent) | 10 | 1 | 14.6 (1.85-115) | 11 | 6 | 2.46 (0.90-6.75) | 26 | 16 | 1.89 (1.00-3.55) |
| Multiple sclerosis | 9666 | 12 341 | 1.00 (referent) | 1 | 3 | 0.55 (0.06-5.37) | 3 | 0 | ND | 6 | 11 | 0.61 (0.22-1.68) |
| Sjögren syndrome | 8178 | 10 543 | 1.00 (referent) | 15 | 3 | 4.54 (1.31-15.8) | 13 | 1 | 15.7 (2.04-121) | 20 | 4 | 5.07 (1.72-14.9) |
| Primary Sjögren syndrome | 8176 | 10 543 | 1.00 (referent) | 6 | 2 | 2.84 (0.57-14.2) | 2 | 1 | 2.64 (0.23-30.3) | 12 | 2 | 6.37 (1.42-28.6) |
| Secondary Sjögren syndrome | 8178 | 10 542 | 1.00 (referent) | 9 | 1 | 7.92 (1.00-62.8) | 11 | 0 | ND | 8 | 2 | 3.78 (0.79-18.1) |
ND indicates not determined.
Restricted to a history of the autoimmune disorder with onset at least 2 years before interview/NHL diagnosis. Numbers of participants reporting a particular disease may not add up to the number reporting that disease according to Table 3 because of missing data on disease duration in a few individuals.
Odds ratios (OR) and 95% confidence intervals (CI) computed in a joint-fixed effects model adjusted for age (in 5-year categories), sex, race/ethnicity, education/SES, and study center.
Sjögren syndrome was linked with an increased risk of B-cell NHL and NHL of unknown lineage (consisting mainly of B-cell NHL in predominantly white populations) (Table 5). There was a 30-fold increase in risk of the marginal zone lymphoma group (Table 6), based on 11 patients with extranodal, 3 with nodal, and one with undetermined (nonsplenic) marginal zone lymphoma. Risk of diffuse large B-cell lymphoma was increased 9-fold (exposed cases, n = 18), and risk of follicular lymphoma 4-fold (n = 7) (Table 6). Neither corticosteroid use nor immunosuppressive treatment affected the relationship between Sjögren syndrome and risk of NHL subtypes (data not shown). Among the 40 Sjögren's patients with NHL of known anatomic site, 10 were extranodal marginal zone (ie, mucosa-associated lymphoid tissue, MALT) lymphomas situated in the parotid gland, corresponding to a 260-fold risk of parotid gland NHL (OR = 258; 95% CI, 78-854), and a 1000-fold risk of parotid gland MALT lymphoma (OR = 996; 95% CI, 216-4596). Risk was also increased for nodal NHL (OR 5.42; 95% CI, 2.20-13.7, n = 21) and for true extranodal nonparotid gland NHL (OR = 5.07; 95% CI, 1.53-16.8, n = 6).
Personal history of selected autoimmune disorders and pooled relative risk of non-Hodgkin lymphoma (NHL) by cell lineage (B-cell, T-cell, unknown cell lineage)
| Disorder* . | Controls: Ever/never . | Cases . | |||||
|---|---|---|---|---|---|---|---|
| B cell† (n = 10,723) . | T cell‡ (n = 745) . | Unknown cell lineage (n = 1514) . | |||||
| Ever/never . | OR (95% CI)§ . | Ever/never . | OR (95% CI)§ . | Ever/never . | OR (95% CI)† . | ||
| Psoriasis | 279/10 122 | 231/5971 | 1.11 (0.92-1.33) | 22/351 | 1.63 (1.03-2.57) | 25/1138 | 1.30 (0.83-2.03) |
| Ulcerative colitis | 138/12 148 | 96/7996 | 1.04 (0.79-1.37) | 8/504 | 1.53 (0.73-3.21) | 21/1275 | 0.96 (0.59-1.56) |
| Type 1 diabetes | 58/13 613 | 29/8430 | 0.85 (0.53-1.35) | 0/564 | ND | 3/1295 | 0.56 (0.17-1.86) |
| Sarcoidosis | 27/8856 | 14/6627 | 0.61 (0.32-1.18) | 4/465 | 2.49 (0.85-0.27) | 1/515 | 0.66 (0.08-0.17) |
| Pernicious anemia | 16/5865 | 13/2719 | 1.32 (0.61-2.84) | 0/153 | ND | 0/538 | ND |
| Crohn disease | 27/10 614 | 17/7726 | 0.79 (0.421-.47) | 2/494 | 1.47 (0.34-6.35) | 4/1010 | 1.58 (0.52-0.78) |
| Celiac disease | 25/10 424 | 22/7916 | 1.16 (0.652-.08) | 9/554 | 6.21 (2.82-13.6) | 2/873 | 1.66 (0.36-7.62) |
| Hemolytic anemia | 13/5585 | 17/2466 | 2.62 (1.25-5.52) | 1/176 | 2.08 (0.24-17.7) | 3/600 | 2.32 (0.63-8.53) |
| Systemic lupus erythematosus | 26/15 237 | 45/9886 | 2.44 (1.49-3.99) | 3/669 | 2.43 (0.72-8.24) | 9/1479 | 4.53 (2.00-10.3) |
| Multiple sclerosis | 18/12 341 | 14/8172 | 1.06 (0.52-2.16) | 0/594 | ND | 1/900 | 0.80 (0.10-6.33) |
| Sjögren syndrome | 8/10 543 | 45/6800 | 6.52 (3.061-3.93) | 1/505 | 2.03 (0.25-16.6) | 6/873 | 16.3 (4.70-56.4) |
| Primary Sjögren syndrome | 5/10 543 | 21/6798 | 4.97 (1.86-13.29) | 0/505 | ND | 2/873 | 5.54 (0.87-35.5) |
| Secondary Sjögren syndrome | 3/10 542 | 24/6800 | 9.11 (2.72-30.46) | 1/505 | 5.13 (0.51-51.8) | 4/873 | 37.7 (7.38-192) |
| Disorder* . | Controls: Ever/never . | Cases . | |||||
|---|---|---|---|---|---|---|---|
| B cell† (n = 10,723) . | T cell‡ (n = 745) . | Unknown cell lineage (n = 1514) . | |||||
| Ever/never . | OR (95% CI)§ . | Ever/never . | OR (95% CI)§ . | Ever/never . | OR (95% CI)† . | ||
| Psoriasis | 279/10 122 | 231/5971 | 1.11 (0.92-1.33) | 22/351 | 1.63 (1.03-2.57) | 25/1138 | 1.30 (0.83-2.03) |
| Ulcerative colitis | 138/12 148 | 96/7996 | 1.04 (0.79-1.37) | 8/504 | 1.53 (0.73-3.21) | 21/1275 | 0.96 (0.59-1.56) |
| Type 1 diabetes | 58/13 613 | 29/8430 | 0.85 (0.53-1.35) | 0/564 | ND | 3/1295 | 0.56 (0.17-1.86) |
| Sarcoidosis | 27/8856 | 14/6627 | 0.61 (0.32-1.18) | 4/465 | 2.49 (0.85-0.27) | 1/515 | 0.66 (0.08-0.17) |
| Pernicious anemia | 16/5865 | 13/2719 | 1.32 (0.61-2.84) | 0/153 | ND | 0/538 | ND |
| Crohn disease | 27/10 614 | 17/7726 | 0.79 (0.421-.47) | 2/494 | 1.47 (0.34-6.35) | 4/1010 | 1.58 (0.52-0.78) |
| Celiac disease | 25/10 424 | 22/7916 | 1.16 (0.652-.08) | 9/554 | 6.21 (2.82-13.6) | 2/873 | 1.66 (0.36-7.62) |
| Hemolytic anemia | 13/5585 | 17/2466 | 2.62 (1.25-5.52) | 1/176 | 2.08 (0.24-17.7) | 3/600 | 2.32 (0.63-8.53) |
| Systemic lupus erythematosus | 26/15 237 | 45/9886 | 2.44 (1.49-3.99) | 3/669 | 2.43 (0.72-8.24) | 9/1479 | 4.53 (2.00-10.3) |
| Multiple sclerosis | 18/12 341 | 14/8172 | 1.06 (0.52-2.16) | 0/594 | ND | 1/900 | 0.80 (0.10-6.33) |
| Sjögren syndrome | 8/10 543 | 45/6800 | 6.52 (3.061-3.93) | 1/505 | 2.03 (0.25-16.6) | 6/873 | 16.3 (4.70-56.4) |
| Primary Sjögren syndrome | 5/10 543 | 21/6798 | 4.97 (1.86-13.29) | 0/505 | ND | 2/873 | 5.54 (0.87-35.5) |
| Secondary Sjögren syndrome | 3/10 542 | 24/6800 | 9.11 (2.72-30.46) | 1/505 | 5.13 (0.51-51.8) | 4/873 | 37.7 (7.38-192) |
ND indicates not determined.
Restricted to a history of the autoimmune disorder diagnosed at least 2 years before interview/NHL diagnosis.
Apart from subgroups of patients displayed in Table 6, patients with B-cell NHL also included 289 patients with lymphoplasmacytic lymphoma, 116 with hairy-cell leukemia, 106 with Burkitt lymphoma, 24 with precursor B-cell lymphoma, and 927 with unspecified B-cell NHL (see also Table S1).
The T-cell NHL group consisted of 389 patients with peripheral T-cell NHL (127 with anaplastic large cell lymphoma, 20 with cutaneous T-cell lymphoma not otherwise specified, 14 with primary cutaneous anaplastic large cell lymphoma, 53 with angioimmunoblastic, 19 with enteropathy-type, 4 with subcutaneous panniculitis-like, 2 with hepatosplenic T-cell lymphoma, and 150 with other peripheral T-cell NHLs), 29 with precursor T-cell NHL, 19 with NK/T-cell lymphoma nasal type or aggressive NK-cell leukemia, 7 with T-cell large granular lymphocytic leukemia, 7 with T-cell prolymphocytic leukemia and 44 with unspecified T-cell NHL (see also Table S1).
Odds ratios (OR) and 95% confidence intervals (CI) computed using a joint-fixed effects model adjusted for age (in 5-year categories), sex, race/ethnicity, education/SES, and study center, with no history of the autoimmune disease in question as reference category.
Personal history of selected autoimmune disorders and pooled relative risk of major B-cell non-Hodgkin lymphoma (NHL) subtypes or subtype groups*
| Disorder† . | Diffuse large B-cell lymphoma (n = 3709) . | Follicular lymphoma (n = 2712) . | CLL/SLL/PLL/MCL‡ (n = 2096) . | Marginal-zone lymphoma group§ (n = 744) . | ||||
|---|---|---|---|---|---|---|---|---|
| Ever/ never . | OR (95% CI)‖ . | Ever/never . | OR (95% CI)‖ . | Ever/never . | OR (95% CI)‖ . | Ever/never . | OR (95% CI)‖ . | |
| Psoriasis | 70/1974 | 1.11 (0.85-1.46) | 43/1381 | 0.95 (0.68-1.32) | 69/1260 | 1.18 (0.89-1.57) | 10/200 | 1.08 (0.56-2.08) |
| Ulcerative colitis | 38/2664 | 1.23 (0.85-1.78) | 20/2040 | 0.89 (0.55-1.45) | 16/1579 | 1.02 (0.59-1.79) | 6/417 | 1.28 (0.54-3.04) |
| Type 1 diabetes | 7/2875 | 0.59 (0.27-1.31) | 4/1963 | 0.53 (0.19-1.49) | 13/1800 | 1.97 (1.00-3.88) | 2/506 | 1.17 (0.27-5.00) |
| Sarcoidosis | 5/2217 | 0.69 (0.26-1.80) | 4/1810 | 0.63 (0.22-1.82) | 2/1363 | 0.39 (0.09-1.66) | 1/410 | 0.64 (0.08-4.80) |
| Pernicious anemia | 8/1138 | 2.12 (0.87-5.16) | 0/917 | ND | 0/110 | ND | 3/204 | 2.29 (0.58-8.94) |
| Crohn disease | 10/2523 | 1.49 (0.71-3.13) | 1/1970 | 0.16 (0.02-1.23) | 2/1574 | 0.49 (0.11-2.16) | 3/406 | 2.41 (0.69-8.46) |
| Celiac disease | 11/2574 | 1.83 (0.89-3.74) | 6/1795 | 1.13 (0.45-2.85) | 3/1937 | 0.73 (0.21-2.52) | 0/518 | ND |
| Hemolytic anemia | 8/951 | 3.22 (1.31-7.89) | 3/756 | 1.46 (0.40-5.37) | 1/245 | 2.90 (0.31-27.2) | 1/143 | 2.23 (0.24-21.0) |
| Systemic lupus erythematosus | 17/3347 | 2.74 (1.47-5.11) | 9/2455 | 1.70 (0.77-3.74) | 6/2045 | 2.10 (0.825-.42) | 10/583 | 7.52 (3.39-16.7) |
| Multiple sclerosis | 5/2897 | 1.03 (0.38-2.80) | 5/2267 | 1.08 (0.39-2.98) | 2/1483 | 1.07 (0.23-4.96) | 2/603 | 1.37 (0.30-6.20) |
| Sjögren syndrome | 18/2350 | 8.92 (3.83-20.7) | 7/1794 | 3.91 (1.39-11.0) | 1/1397 | 0.62 (0.07-5.07) | 15/396 | 30.6 (12.3-76.1) |
| Primary Sjögren syndrome | 8/2348 | 6.57 (2.12-20.3) | 2/1794 | 1.78 (0.34-9.37) | 1/1397 | 1.01 (0.11-9.15) | 8/396 | 23.1 (7.16-74.6) |
| Secondary Sjögren syndrome | 10/2350 | 12.8 (3.49-47.3) | 5/1794 | 7.55 (1.75-32.7) | 0/1397 | ND | 7/396 | 44.6 (10.6-187) |
| Disorder† . | Diffuse large B-cell lymphoma (n = 3709) . | Follicular lymphoma (n = 2712) . | CLL/SLL/PLL/MCL‡ (n = 2096) . | Marginal-zone lymphoma group§ (n = 744) . | ||||
|---|---|---|---|---|---|---|---|---|
| Ever/ never . | OR (95% CI)‖ . | Ever/never . | OR (95% CI)‖ . | Ever/never . | OR (95% CI)‖ . | Ever/never . | OR (95% CI)‖ . | |
| Psoriasis | 70/1974 | 1.11 (0.85-1.46) | 43/1381 | 0.95 (0.68-1.32) | 69/1260 | 1.18 (0.89-1.57) | 10/200 | 1.08 (0.56-2.08) |
| Ulcerative colitis | 38/2664 | 1.23 (0.85-1.78) | 20/2040 | 0.89 (0.55-1.45) | 16/1579 | 1.02 (0.59-1.79) | 6/417 | 1.28 (0.54-3.04) |
| Type 1 diabetes | 7/2875 | 0.59 (0.27-1.31) | 4/1963 | 0.53 (0.19-1.49) | 13/1800 | 1.97 (1.00-3.88) | 2/506 | 1.17 (0.27-5.00) |
| Sarcoidosis | 5/2217 | 0.69 (0.26-1.80) | 4/1810 | 0.63 (0.22-1.82) | 2/1363 | 0.39 (0.09-1.66) | 1/410 | 0.64 (0.08-4.80) |
| Pernicious anemia | 8/1138 | 2.12 (0.87-5.16) | 0/917 | ND | 0/110 | ND | 3/204 | 2.29 (0.58-8.94) |
| Crohn disease | 10/2523 | 1.49 (0.71-3.13) | 1/1970 | 0.16 (0.02-1.23) | 2/1574 | 0.49 (0.11-2.16) | 3/406 | 2.41 (0.69-8.46) |
| Celiac disease | 11/2574 | 1.83 (0.89-3.74) | 6/1795 | 1.13 (0.45-2.85) | 3/1937 | 0.73 (0.21-2.52) | 0/518 | ND |
| Hemolytic anemia | 8/951 | 3.22 (1.31-7.89) | 3/756 | 1.46 (0.40-5.37) | 1/245 | 2.90 (0.31-27.2) | 1/143 | 2.23 (0.24-21.0) |
| Systemic lupus erythematosus | 17/3347 | 2.74 (1.47-5.11) | 9/2455 | 1.70 (0.77-3.74) | 6/2045 | 2.10 (0.825-.42) | 10/583 | 7.52 (3.39-16.7) |
| Multiple sclerosis | 5/2897 | 1.03 (0.38-2.80) | 5/2267 | 1.08 (0.39-2.98) | 2/1483 | 1.07 (0.23-4.96) | 2/603 | 1.37 (0.30-6.20) |
| Sjögren syndrome | 18/2350 | 8.92 (3.83-20.7) | 7/1794 | 3.91 (1.39-11.0) | 1/1397 | 0.62 (0.07-5.07) | 15/396 | 30.6 (12.3-76.1) |
| Primary Sjögren syndrome | 8/2348 | 6.57 (2.12-20.3) | 2/1794 | 1.78 (0.34-9.37) | 1/1397 | 1.01 (0.11-9.15) | 8/396 | 23.1 (7.16-74.6) |
| Secondary Sjögren syndrome | 10/2350 | 12.8 (3.49-47.3) | 5/1794 | 7.55 (1.75-32.7) | 0/1397 | ND | 7/396 | 44.6 (10.6-187) |
ND indicates not determined.
In analyses of B-cell NHL subtypes, all controls (see Table 5) were used for modeling of risk of diffuse large B-cell lymphoma, follicular lymphoma, and CLL/SLL/PLL/MCL. However, in modeling of risk of the marginal-zone lymphoma group, studies using the older working classification of lymphomas were excluded (Italy, Northern Italy, University of California–San Francisco, representing about 30% of all participants).
Restricted to a history of the autoimmune disorder diagnosed at least 2 years before interview/NHL diagnosis.
Includes patients with chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), prolymphocytic leukemia (PLL), and mantle cell lymphoma (MCL) (25).
Includes patients with extranodal (MALT), nodal, splenic, and undetermined marginal-zone lymphoma (25).
Odds ratios (OR) and 95% confidence intervals (CI) computed using a joint-fixed effects model adjusted for age (in 5-year categories), sex, race/ethnicity, education/SES, and study center, with no history of the autoimmune disease in question as reference category.
Systemic lupus erythematosus
SLE was associated with a 2.7-fold increase in risk of NHL overall (Table 3). The risk was of similar magnitude in both sexes but statistically significant only among women (data not shown). NHL risk was highest among patients with SLE of short duration (2-5 years), but a near 2-fold increase was also observed with more than 10 years of disease (Table 4). The association was confined to patients with older-onset SLE (≥ 39 years), whereas age at NHL diagnosis or corticosteroid/immunosuppressive drug use did not modify the results (data not shown). Risk was increased for B-cell NHL and NHL of unknown lineage (Table 5), and among the B-cell subtypes, for the marginal zone lymphoma group (OR = 7.5; n = 10) and diffuse large B-cell NHL (OR = 2.7; n = 17; Table 6). In the marginal zone lymphoma group, 7 patients had MALT lymphoma (OR = 12.9; 95% CI, 4.91-33.8). The exclusion of patients with secondary Sjögren syndrome did not alter these associations.
Hemolytic anemia
Hemolytic anemia was associated with a 2.6-fold increase in NHL risk (Table 3), which was slightly more pronounced among men than women (data not shown). The association was most evident among participants reporting long disease duration (> 10 years, Table 4). There was a statistically significant positive association with B-cell NHL (Table 5), and a 3-fold increased risk of diffuse large B-cell lymphoma (Table 6).
Celiac disease
Celiac disease was not associated with risk of NHL overall (Table 3). However, those diagnosed with celiac disease at an older age (≥ 33 years) had an increased risk (OR = 2.34; 95% CI, 1.06-5.21, n = 19). There were no clear differences in risk by sex, or celiac disease duration (Table 4). Risk of T-cell NHL was increased 6-fold (n = 9, Table 5) because of a substantially increased risk of enteropathy-type T-cell NHL (ETTL; OR = 255; 95% CI, 30-2199, n = 3), and of anaplastic large cell lymphoma (OR = 24; 95% CI, 8.8-65; 2 nodal, 4 true extranodal, including 1 small intestine). The risk of small intestinal NHL was increased 30-fold (OR = 31; 95% CI, 10-96, n = 5).
Psoriasis
Psoriasis was not associated with risk of NHL overall (Table 3), and there was no large variation in risk by sex or psoriasis duration (Table 4). However, a weak positive association was observed among psoriatic patients with a late NHL diagnosis (> 60 years, OR = 1.28; 95% CI, 1.01-1.63, n = 159). There was a statistically significant 1.6-fold increase in risk of T-cell NHL (Table 5) and a doubled risk of anaplastic large cell lymphoma (OR = 2.25; 95% CI, 1.00-5.06; 3 nodal, 1 parotid gland, 1 bone, 2 unknown site). There were no statistically significant associations with the cutaneous T-cell NHL subtypes mycosis fungoides and Sezary syndrome (OR = 1.38; 95% CI, 0.50-3.83, n = 4) or cutaneous NHL of any type (OR = 1.61; 95% CI, 0.86-3.03, n = 11).
Rheumatoid arthritis
RA was not associated with risk of NHL overall, but there was statistically significant heterogeneity of the results among studies (Table 3; Figure 1). This heterogeneity could not be fully explained by any single study center but was reduced among studies that used physician-diagnosed RA as the definition of the exposure (7 studies: OR = 1.15; 95% CI, 0.94-1.41, P for heterogeneity = .15; I2 = 30.2%). The heterogeneity also appeared to be related to the prevalence of reported RA among controls in different studies. For the 6 studies within the lowest tertile of age- and sex-adjusted RA control prevalence, the pooled NHL risk was borderline significantly increased, whereas it was decreased for the 5 studies in the highest tertile (Figure 1). RA and concomitant use of corticosteroids or immunosuppressants was positively associated with risk of NHL (OR = 1.57; 95% CI, 1.12-2.21), whereas RA without such use was not (OR = 1.04; 95% CI, 0.77-1.41). We did not assess risk by RA duration or NHL subtype because of the between-study heterogeneity and the lower statistical power in multiple stratified models.
Other disorders
Overall risk of NHL was not linked to a history of inflammatory bowel disorders (ulcerative colitis or Crohn disease), type 1 diabetes, sarcoidosis, pernicious anemia, or multiple sclerosis (Table 3). Examination by autoimmune disease duration (Table 4), sex or age at autoimmune disease, or NHL diagnosis (data not shown) did not affect these results. Type 1 diabetes was borderline significantly associated with the nested group of chronic lymphocytic leukemia/small lymphocytic lymphoma/prolymphocytic leukemia/mantle cell lymphoma (Table 6), but there was no statistically significant association with any of these 4 subtypes in separate analyses (data not shown). Scleroderma, immune thrombocytopenia, myasthenia gravis, or poly- or dermatomyositis were all rarely reported (Table 3) and subanalyses were therefore not pursued.
Discussion
In these large pooled analyses, we found evidence that an increased risk of NHL is associated with only a few autoimmune disorders and that these associations are stronger for some NHL subtypes than others. Besides confirming the known link between all NHL combined and Sjögren syndrome and SLE, we demonstrated an increased risk of marginal zone and diffuse large B-cell lymphomas in both of these disorders, and an increased risk of specific T-cell NHL subtypes in celiac disease and psoriasis. Our results further indicate an association between hemolytic anemia and risk of NHL, and diffuse large B-cell lymphoma.
The risk of NHL was increased almost 7-fold in patients with Sjögren syndrome, which is in the lower range compared with other reports,8,10 but not unexpected in comparison with cohorts of hospitalized Sjögren patients with presumably more severe disease.10 Relative risks of NHL subtypes in Sjögren syndrome have seldom been quantified.21 We observed a 250-fold increase in risk of parotid gland NHL and a dramatic 1000-fold increase in risk of parotid gland MALT lymphoma. This finding is consistent with prior Sjögren lymphoma case series,28 and with biologic evidence of antigen-driven clonal expansions in affected salivary glands.6 However, we also noted positive associations with other subtypes, most notably diffuse large B-cell lymphoma, and with nodal lymphomas regardless of WHO subtype in Sjögren syndrome, associations that were much less dramatic in relative terms but more important in terms of patient numbers. Interestingly, in a recent registry-based study of incident Sjögren patients who developed NHL, the majority of the lymphomas were of the diffuse large B-cell type.8
In SLE, we observed a close to 3-fold increased risk of NHL, consistent with the hitherto largest cohort studies showing a 3- to 4-fold increase.12 The patients with SLE were at an increased risk of marginal zone lymphoma, predominantly of the MALT type, and of diffuse large B-cell lymphoma, which is in accordance with recent descriptive data.29 We also observed a positive association with risk of NHL in hemolytic anemia, in line with a previous study based on patients hospitalized with autoimmune hemolytic anemia,30 and we provide the first report of a possible association between hemolytic anemia and diffuse large B-cell lymphoma. However, these results should be interpreted with caution because hemolytic anemia could represent secondary phenomena31 and was not characterized in detail in all participating studies. Of note, the increased risks of NHL in Sjögren syndrome, SLE, and hemolytic anemia persisted with more than 10 years of autoimmune disease duration, making misclassification of incipient lymphomas an improbable explanation for our findings.
In celiac disease, we did not observe an association with risk of NHL overall, but the risk was doubled among patients diagnosed at age more than or equal to 33 years. Previous population-based studies showed a 2- to 6-fold increase in risk of NHL in celiac disease.9,32 However, in the largest cohort study,32 the increase was confined to celiac disease patients diagnosed as adults, and there was a trend of decreasing NHL risk over successive calendar periods. If true, such a trend may partly explain our results for NHL overall. We confirmed previous reports of a strong association with ETTL and of NHL located in the small intestine.33,34 In addition, we observed a marked association with anaplastic large cell lymphoma, which has not been described previously but is consistent with prior observations of nonintestinal T-cell NHL in celiac disease.35 Because only 1 of 6 cases of anaplastic large cell lymphoma was situated in the small intestine, misdiagnosis of ETTL with numerous CD30 positive cells is an improbable explanation for the association. The indication that only those diagnosed with celiac disease later in life may be at risk of NHL could imply that early diagnosis and early start of diet therapy reduce the risk of lymphoma. This hypothesis is not new but has not been adequately tested.23
In psoriasis, previously reported results of risk of NHL overall are mixed.36,37 Whereas some have noted an increase in risk of T-cell NHL,20,36,37 little is known about T-cell subtype-specific risks. A possible misclassification of early cutaneous T-cell NHL (ie, mycosis fungoides) as psoriasis could have resulted in false positive associations. Therefore, our finding of an increased risk of T-cell NHL of the anaplastic large cell type and not the cutaneous T-cell NHL forms is especially informative and supports a true risk increase of T-cell lymphoma in psoriasis. However, in 2 of 7 cases of anaplastic large cell lymphoma with no site information, misdiagnosis of transformed mycosis fungoides with numerous CD30+ cells cannot be excluded.
In RA, we did not find an increased risk of NHL overall, unlike most previous reports.10,11,38 However, a moderately increased NHL risk was observed among RA patients treated with corticosteroids or immunosuppressants, supporting a gradient in risk by treatment or disease severity.38 In most participating studies, the prevalence of RA reported by the controls was higher than expected,39 which could reflect a degree of misclassification with other joint or connective tissue disorders. The indication of a positive association between RA and NHL in studies with a low RA control prevalence as opposed to an inverse association among studies with a high RA control prevalence lends some support for this interpretation. An alternative explanation for the overall null result is a possible underrepresentation of RA patients with severe disease and presumably higher risk of lymphoma38 through self-selection in case-control studies as opposed to in registry-based cohort studies.10
Inflammatory bowel disorders, type 1 diabetes, sarcoidosis, pernicious anemia, and multiple sclerosis were not associated with risk of NHL overall, consistent with the majority of previous reports,14,30,40-42 and there was no strong evidence for subtype-specific associations. In Crohn's disease, a recent large meta-analysis43 reported a moderately increased risk of lymphoma overall, but latency was not evaluated; therefore a possible risk inflation by initial misdiagnoses of lymphomas cannot be excluded.
It is thus noteworthy that Sjögren syndrome, SLE, and perhaps hemolytic anemia were all associated with risk of diffuse large B-cell NHL, and Sjögren syndrome and SLE also with marginal zone lymphoma. Celiac disease and psoriasis were both associated with T-cell NHL of the anaplastic large cell type in addition to the well-known close link between celiac disease and ETTL. Biologically, the importance of antigen-driven immune stimulation for the development of MALT lymphoma in the parotid gland in Sjögren syndrome,44 and ETTL in celiac disease4 is well documented. In Sjögren patients, there is also evidence that oligoclonal expansions of B cells, arising in the parotid gland, may give rise to MALT lymphomas at distant sites.45 Whether local antigen-driven immune responses or inflammatory processes are relevant for the development of distant NHL, specifically diffuse large B-cell lymphoma, is not known. However, in Sjögren syndrome and RA, recent evidence favors aspects of disease severity and inflammatory load as the strongest determinants of NHL risk.8,38 These same studies also noted an increased occurrence of diffuse large B-cell lymphoma in particular.8,38 Thus, it could be hypothesized that disease severity, chronic B-cell activation, and/or inflammation harbor determinants of risk of diffuse large B-cell lymphoma common to a group of autoimmune disorders, including Sjögren syndrome, RA, SLE, and autoimmune hemolytic anemia. Such determinants may include, apart from B-cell stimulation, factors related to cytokine profiles, T-cell subset balance, and apoptotic resistance,8,46 any of which could be relevant also for lymphomagenesis in general.47 In celiac disease and psoriasis, disorders characterized by an increase in T-cell activity,37,48 determinants of T-cell NHL risk may be similar, but acting to enhance oncogenic events in proliferating T cells rather than B cells.
The strengths of this pooled analysis include the large sample size, the ability to adjust for confounding factors, and the exclusion of subjects with recent onset autoimmune disorders, which could represent autoimmune phenomena triggered by yet undiagnosed lymphomas. Because of the large size of the pooled dataset, we were able to explore differences in risk by sex and autoimmune disease duration. Unique aspects of our analysis were the ability to assess risk estimates for NHL subtypes according to an epidemiologically oriented classification system25 and by location, and to compare patterns of subtype associations between different autoimmune disorders. However, despite the large study size, low numbers of exposed subjects was still a limitation in some analyses.
A limitation inherent to the design of the participating case-control studies is the use of self-reported autoimmune disease history, which has the potential for exposure misclassification, already discussed in relation to RA and hemolytic anemia. A comparison of our control prevalences with population prevalence data from different countries showed good concordance,49-52 except for ulcerative colitis, where the study control prevalences were higher than published prevalence estimates,53 and in hemolytic anemia and sarcoidosis, where no published rates could be identified. Based on these comparisons, we think that misclassification of self-reports may have been less frequent for most of the autoimmune disorders studied than for RA. In future studies of autoimmune disorders based on self-reports, we recommend a careful assessment of disease aspects reflecting established diagnostic criteria. A detailed assessment of disease severity and treatment may not only help to sort out possible diagnostic misclassification but could also shed more light on the specific determinants of lymphoma risk.
In conclusion, we observed that associations with NHL risk in Sjögren syndrome and celiac disease are less subtype-specific than previously described, and are not confined to associations with rare NHL subtypes developing in the affected organs. Our results further suggest new patterns of associations with some NHL subtypes in specified autoimmune disorders. These patterns may be based on common mechanisms of lymphomagenesis, which could be relevant for the development of the indicated NHL subtypes in a group of autoimmune disorders as well as beyond the setting of overt autoimmune disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the Leukemia Foundation of Australia (LFA GIA 24). Individual studies were supported by the Italian Association for Cancer Research and the Italian League Against Cancer (Avi-N, Northern Italy); the Canadian Cancer Society through the National Cancer Institute of Canada, the Canadian Institutes for Health Research and the Chan Sisters Foundation (British Columbia); National Cancer Institute (CA62006; Connecticut); European Commission (QLK4-CT-2000-00 422; EpiLymph); Association pour la Recherche contre le Cancer (5111) and Fondation de France (1999 008471; EpiLymph-France); Compagnia di San Paolo di Torino, Programma Oncologia 2001 (EpiLymph-Italy); Health Research Board (EpiLymph-Ireland); Spanish Ministry of Health FISS (PI040091) and CIBERESP (06/06/0073; EpiLymph-Spain); German Federal Office for Radiation Protection (StSch4261 and StSch4420; EpiLymph-Germany); National Institutes of Health (CA51086), the European Community and the Italian League against Cancer (Italy); National Cancer Institute (PC65064, PC67008, PC67009, PC67010, PC71105; NCI-SEER); American Institute for Cancer Research (99B083; Nebraska); National Health and Medical Research Council of Australia (990920; NSW); National Institutes of Health (CA69269-02) and the Swedish Cancer Society (04 0458; SCALE); National Institutes of Health (CA45614, CA89745, CA87014, CA104682; UCSF); and the Leukemia Research Fund of Great Britain. The funders did not participate in the design, data collection, or analyses of the individual studies, or in the interpretation and writing of manuscripts.
National Institutes of Health
Authorship
Contribution: K.E.S., C.M.V., E.A.E., J.T., H.H., P.V., A.S.C., P.M.B., E.A.H., E.W., J.J.S., C.L.V., T.Z., N.B., S.D.S., B.C.-H.C., L.D.M., P.C., M.M., L.F., A.S., P.B., S.D., R.S., J.R.C., A.E.G., and W.C. designed and performed individual studies and collected data; K.E.S., C.M.V., M.F., E.A.E., O.M.-M., J.T., H.H., P.V., A.S.C., P.M.B., E.A.H., E.W., J.J.S., C.L.V., T.Z., N.B., S.D.S., B.C.-H.C., L.D.M., P.C., M.M., L.F., A.S., P.B., S.D., R.S., J.R.C., E.C.B., B.B., A.E.G., and W.C. interpreted data; M.F. performed statistical analysis; K.E.S., C.M.V., and M.F. drafted the manuscript; K.E.S., C.M.V., M.F., E.A.E., O.M.-M., J.T., H.H., P.V., A.S.C., P.M.B., E.A.H., E.W., J.J.S., C.L.V., T.Z., N.B., S.D.S., B.C.-H.C., L.D.M., P.C., M.M., L.F., A.S., P.B., S.D., R.S., J.R.C., E.C.B., B.B., A.E.G., and W.C. contributed with manuscript revisions leading to a final manuscript version.
The InterLymph Immunology working group is composed of those listed in the byline.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karin Ekström Smedby, Department of Medicine, Clinical Epidemiology Unit, Karolinska University Hospital, SE-171 76 Stockholm, Sweden; e-mail: karin.ekstrom.smedby@ki.se.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal