In addition to classic homeostatic granulocyte production mediated by G-CSF and GM-CSF, an independent pathway stimulated by IL6 complexed with its soluble receptor (sIL6R) contributes to emergency granulopoiesis.
Granulocytes are produced in the bone marrow through a dynamic process (see figure) regulated by specific hematopoietic growth factors. Cytokines promoting the proliferation and differentiation of neutrophils include IL3, IL6, G-CSF, and GM-CSF (reviewed in Dinauer et al1 ), the last 2 of which serve as the primary regulators of granulopoiesis.
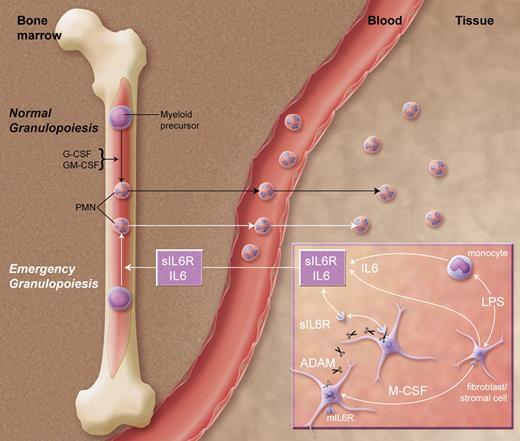
Pathways of homeostatic and emergency granulopoiesis. Top pathway: under normal conditions, G-CSF and GM-CSF regulate production of neutrophils by myeloid precursors in the bone marrow. During times of infection, the proposed emergency pathway is initiated by bacterial lipopolysaccharide (LPS) stimulation of tissue fibroblasts, stromal cells, and mononuclear phagocytes, resulting in the secretion of IL6 and M-CSF. The latter cytokine promotes cleavage of membrane IL6 receptor (mIL6R) to soluble sIL6R by ADAMs (a disintegrin and metalloproteases). The resultant soluble IL6/sIL6R complexes then circulate to the bone marrow, where they stimulate emergency granulopoiesis independent of G-CSF and GM-CSF.
Pathways of homeostatic and emergency granulopoiesis. Top pathway: under normal conditions, G-CSF and GM-CSF regulate production of neutrophils by myeloid precursors in the bone marrow. During times of infection, the proposed emergency pathway is initiated by bacterial lipopolysaccharide (LPS) stimulation of tissue fibroblasts, stromal cells, and mononuclear phagocytes, resulting in the secretion of IL6 and M-CSF. The latter cytokine promotes cleavage of membrane IL6 receptor (mIL6R) to soluble sIL6R by ADAMs (a disintegrin and metalloproteases). The resultant soluble IL6/sIL6R complexes then circulate to the bone marrow, where they stimulate emergency granulopoiesis independent of G-CSF and GM-CSF.
Accordingly, double-knockout G-CSF−/−/GM-CSF−/− mice show pro-found defects in neutrophil production and function.2 However, when challenged with microbial pathogens, these mice respond with increased production of both myeloid precursors and mature neutrophils, indi-cating an alternative pathway of “emergency” granulopoiesis.
In this issue of Blood, Walker and colleagues have identified IL6/sIL6R complexes as the mediators of this pathway in an in vitro model of emergency granulopoiesis in the absence of G-CSF and GM-CSF. In vivo experiments using triple-knockout G-CSF−/−/GM-CSF−/−/IL6−/− mice proved infeasible due to their poor viability. Therefore, the authors developed an in vitro culture system using colony formation from G-CSF−/−/GM-CSF−/− bone marrow as a bioassay for neutrophil-promoting activity in conditioned media from LPS-stimulated G-CSF−/−/GM-CSF−/− embryonic fibroblasts.
Previous studies had indicated essential roles for IL6 and, surprisingly, M-CSF for this activity, but neither was sufficient. The present work has elucidated a multicomponent pathway in which binding of IL6 to the soluble form of its receptor produces a transstimulatory IL6/sIL6R complex. The requirement for M-CSF may reflect its amplification of IL6 secretion or augmentation of sIL6R release by protease cleavage of membrane-bound IL6 receptor (mIL6R).
The primary limitation of this work, as the authors acknowledge, is the in vitro model system that proved essential for identification of the neutrophil-promoting complex, but which lacked proof of in vivo applicability. Due to the redundancy of cytokine controls for neutrophil production, future studies may more easily demonstrate that IL6/sIL6R complexes are sufficient rather than that they are necessary for emergency granulopoiesis in vivo.
In the future, the physiological role of IL6/sIL6R complexes will undoubtedly be compared with that of IL6, the only form of the cytokine heretofore associated with hematopoiesis. IL6 directly binds to mIL6R, and the resultant multimer associates with the signal transduction protein gp130 (reviewed in Scheller et al3 ). mIL6R expression is limited to hepatocytes and some hematopoietic cells, but the ubiquitous expression of gp130, which also responds to IL6/sIL6R, renders nearly all cells potentially responsive to the IL6/sIL6R complex.
mIL6R has been detected in myeloid progenitor cells in vitro, and increases with their differentiation.4 If its expression is dependent upon G-CSF or GM-CSF, then IL6/sIL6R would provide a bypass mechanism unique to double-knockout cells. However, the soluble complex could also represent an independent physiological signal to myeloid precursors from tissue stromal and immune cells encountering microbial pathogens. The current study helps to solve the mystery of the neutrophil response in double-knockout mice. Further studies must determine whether the pathway is a phenomenon of cell culture or an important emergency signal from infected or stressed tissue to the bone marrow.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal