Abstract
We studied the lineage distribution of JAK2 mutations in peripheral blood of 8 polycythemia vera (PV) patients with exon 12 mutations and in 21 PV patients with JAK2-V617F. Using a quantitative allele discrimination assay, we detected exon 12 mutations in purified granulocytes, monocytes, and platelets of 8 patients studied, but lymphoid cells showed variable involvement and the mutation was absent in T cells. Endogenous erythroid colonies grew in all patients analyzed. One patient displayed erythroid colonies homozygous for the exon 12 mutation with evidence for mitotic recombination on chromosome 9p. In some patients with exon 12 mutations or JAK2-V617F, a proportion of endogenous erythroid colonies were negative for both JAK2 mutations. One patient carried 2 independent clones: one with an exon 12 mutation and a second with JAK2-V617F. The finding of clonal heterogeneity is compatible with the hypothesis that additional clonal events are involved in the pathogenesis of PV.
Introduction
Mutations in exon 12 of JAK2 are detected selectively in patients with polycythemia vera (PV) that are negative for JAK2-V617F and in some patients with idiopathic erythrocytosis.1 The JAK2-V617F and exon 12 mutations represent clonal markers useful to track the hematopoietic lineages involved in myeloproliferative disorder (MPD).2-6 In patients with MPD, JAK2-V617F is present in purified hematopoietic stem cells, in myeloid lineages of the peripheral blood, and in variable proportions of lymphoid cells.7-11 Using a novel sensitive assay, we quantitated the involvement of exon 12 mutations in purified peripheral blood lineages and in erythroid progenitor assays. In addition, we addressed the question of whether JAK2-V617F is present in T cells by clonal analysis.
Methods
Patients
The screening for JAK2 exon 12 mutation in MPD patients was performed by DNA sequencing.12 All patients except p024 fulfilled the diagnostic criteria of PV according to the World Health Organization (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).13,14 Patient p024 was initially diagnosed with essential thrombocythemia, and several months later phlebotomies were started because of rising hemoglobin (175 g/L). Two patients with JAK2 exon 12 mutations (Vi064, Vi327) were from Vienna, Austria. All other patients were from Basel, Switzerland. The collection of patient samples was approved by the “Ethik Kommission Beider Basel” and the “Ethik Kommission der Universität Wien und des Allgemeinen Krankenhauses der Stadt Wien-AK.” Written consent was obtained from all patients in accordance with the Declaration of Helsinki.
Cells, DNA, and RNA analyses
Isolation of granulocytes, platelets, and peripheral blood mononuclear cells was performed as described.5,15 Sorting of peripheral blood mononuclear cells, colony assays in methylcellulose, T-cell cloning,16 and the SNaPshot assay for RNA samples are described in Document S1. The allele discrimination assay for detection and quantification of JAK2 exon 12 mutations is described in Figure S1. Allele-specific polymerase chain reaction (PCR) for the detection of JAK2-V617F and microsatellite PCR for chromosome 9p were performed as reported.5,17
Statistical analysis
We used SPSS version 15.0 (Chicago, IL) to calculate linear and ordinal regression for the correlations between disease duration and the percentage of mutant allele and between the percentage of mutant allele and the number of lineages involved.
Results and discussion
We studied the lineage distribution of JAK2 mutations in peripheral blood of 8 PV patients with mutations in exon 12 and in 21 PV patients with JAK2-V617F (Figure 1). Five different JAK2 exon 12 mutations were observed by sequencing, and all of them contained deletions of 3 or 6 bases (Figure 1A). We devised a novel assay to quantitate JAK2 exon 12 mutations with a sensitivity of 1% mutant alleles (Figure S1). In all patients analyzed, exon 12 mutations were detectable in granulocytes, platelets, and monocytes, with the highest allelic ratios in most cases present in platelets and the lowest in monocytes (Figure 1B top panel). Similarly, the JAK2-V617F mutation was present in granulocytes, platelets, and with the exception of p104, also in monocytes (Figure 1B bottom panel). Interestingly, in patient p021, we detected 2 different JAK2 mutations: N542-E543del (exon 12) and JAK2-V617F (exon 14). In granulocytes, platelets, and monocytes of patient p021, the exon 12 mutation was present in higher allelic ratios than JAK2-V617F.
Distribution of JAK2 mutations in peripheral blood lineages. (A) The location of exon 12 mutations and JAK2-V617F in the Jak2 protein is shown (top). The amino acid changes caused by the individual exon 12 mutations are shown below using the single letter code. Previously described mutations (asterisk) and newly found mutations (no asterisk) are shown with the unique patient numbers (UPN) of the patients included in this study. (B) Lineage distribution of JAK2 exon 12 mutations (top) and JAK2-V617F (bottom part). Numbers in boxes indicate the percentages of chromosomes 9 with exon 12 mutations, and the shading of boxes corresponds to the ranges shown at the bottom. UPN indicates unique patient number; F, female; M, male; GRA, granulocytes; NK cells, natural killer cells; nd, not determined. Numbers in column for T-cell cloning indicate JAK2-V617F positive clones/total clones analyzed. The phenotypes of JAK2-V617F positive clones were determined by flow cytometry. NK cell phenotype: CD3−CD56+; T-cell phenotype: CD3+CD56−. * Note that patient p021 was positive for exon 12 mutation N542-E543del and for JAK2-V617F. (C) Phenotypic analysis of JAK2-V617F positive clones. Flow cytometic analyses. One JAK2-V617F positive clone from patient p035 consisted of CD3+CD56− T cells. All other positive clones from patients p136 and p116 were CD3−CD56+ NK cells (top panel). Allele-specific PCR for JAK2-V617F showing T-cell clone from p035 was homozygous for JAK2-V617F, whereas the clones from patients p136 and p116 were heterozygous for JAK2-V617F (bottom panel).
Distribution of JAK2 mutations in peripheral blood lineages. (A) The location of exon 12 mutations and JAK2-V617F in the Jak2 protein is shown (top). The amino acid changes caused by the individual exon 12 mutations are shown below using the single letter code. Previously described mutations (asterisk) and newly found mutations (no asterisk) are shown with the unique patient numbers (UPN) of the patients included in this study. (B) Lineage distribution of JAK2 exon 12 mutations (top) and JAK2-V617F (bottom part). Numbers in boxes indicate the percentages of chromosomes 9 with exon 12 mutations, and the shading of boxes corresponds to the ranges shown at the bottom. UPN indicates unique patient number; F, female; M, male; GRA, granulocytes; NK cells, natural killer cells; nd, not determined. Numbers in column for T-cell cloning indicate JAK2-V617F positive clones/total clones analyzed. The phenotypes of JAK2-V617F positive clones were determined by flow cytometry. NK cell phenotype: CD3−CD56+; T-cell phenotype: CD3+CD56−. * Note that patient p021 was positive for exon 12 mutation N542-E543del and for JAK2-V617F. (C) Phenotypic analysis of JAK2-V617F positive clones. Flow cytometic analyses. One JAK2-V617F positive clone from patient p035 consisted of CD3+CD56− T cells. All other positive clones from patients p136 and p116 were CD3−CD56+ NK cells (top panel). Allele-specific PCR for JAK2-V617F showing T-cell clone from p035 was homozygous for JAK2-V617F, whereas the clones from patients p136 and p116 were heterozygous for JAK2-V617F (bottom panel).
Only 3 of 8 patients (38%) with exon 12 mutations displayed detectable signal in lymphoid cells. In patients p221 and Vi064, a small subset of natural killer (NK) cells carried the mutation, and only patient Vi327 showed an allelic ratio greater than 10% in NK and B cells. JAK2-V617F also showed variable engagement of lymphoid lineages, with NK cells being most frequently involved (14 of 21, 67%) and in some cases showing very high (> 70%) allelic ratios (p016, p033, p035, and p103). B cells had low JAK2-V617F allelic ratios (< 15%), except in one patient (p035). JAK2 exon 12 mutations have not been observed in T cells by DNA sequencing,1 and were also absent in our study using a more sensitive detection assay. Only 2 of 21 patients (p016 and p035) displayed JAK2-V617F in T cells. The presence of JAK2-V617F in T cells from PV patients has been described in one study10 but was rare in other reports.8,9,18-22 All these studies were performed using bulk cell populations. We established T- and NK-cell clones from peripheral blood of 10 PV patients with high JAK2-V617F allelic ratios. In 4 of 10 patients, less than 1% of the total clones were JAK2-V617F positive (Figure 1B). Surprisingly, only a single JAK2-V617F positive clone in patient p035 consisted of T cells, whereas JAK2-V617F positive clones in 2 of 3 patients examined were NK cells (Figure 1B,C). JAK2-V617F positive CD34+ progenitors from MPD patients were shown to be capable of differentiating into T cells in thymic organ cultures in vitro.9 However, in patients, maturation of JAK2-V617F positive T cells occurs very infrequently, possibly because of low frequency of de novo T-cell genesis in adults. A correlation between the disease duration and the allelic ratios of JAK2-V617F in granulocytes, platelets, monocytes, and NK cells was noted (Figure S2), but no such correlation was found for exon 12 mutations (not shown). The percentages of mutant alleles also correlated with the number of lineages involved for both the exon 12 mutations and JAK2-V617F (Figure S2).
Erythroid colonies that grew in the absence of Epo (EECs) were detected in all patients (Figure 2), although in some cases the total number of EECs was small (p041, Vi327, and p115). In 2 patients with exon 12 mutations (p002 and p041) and one patient with JAK2-V617F (p136), we found EECs with only wild-type JAK2 sequences in 2 independent experiments. The erythroid phenotype of the colonies was confirmed by the presence of glycophorin A mRNA (Figure S3). Sequencing of the entire JAK2 coding region in these colonies did not reveal any additional JAK2 mutations. Growth of EECs with only wild-type JAK2 has recently been reported in PV patients positive for JAK2-V617F.21,23,24 In contrast, no wild-type EECs in PV patients were found by others.1,3,25,26 Our results confirm the finding of EECs with only wild-type JAK2 and suggest clonal heterogeneity of erythroid progenitors, not only in patients with JAK2-V617F but also in some patients with exon 12 mutations. Interestingly, in the patient with 2 different JAK2 mutations (p021), none of the erythroid colonies carried both mutations simultaneously (Figure 2), indicating that the exon 12 mutation and JAK2-V617F represent 2 separate clones. Concurrent presence of JAK2-V617F with MPLW515 mutations was previously found in 3 patients with primary myelofibrosis and 3 patients with essential thrombocythemia.27,28 The allelic ratios for MPLW515 were higher than those for JAK2-V617F, but clonal analysis was not reported. Therefore, it remains to be determined whether these cases represent sequential somatic mutations that occurred in the same clone. Thus, our patient p021 is the first case with a documented biclonal pattern. The finding of clonal heterogeneity is compatible with a model in which a stem cell or progenitor carries a predisposing mutation that increases the likelihood for acquiring somatic mutations in JAK2 or other as yet unknown gene(s).
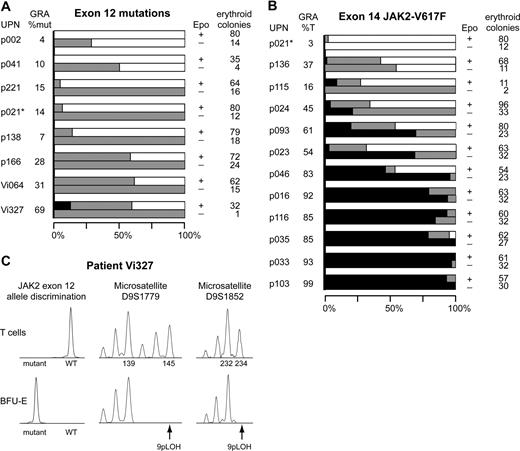
Distribution of JAK2 mutations in erythroid progenitors and loss of heterozygosity on chromosome 9p (9pLOH) analysis in patient Vi327. The total number of erythroid colonies analyzed and the percentages of colonies with homozygous or heterozygous JAK2 mutation or wild-type JAK2 are shown. Colony assays in methylcellulose were performed with peripheral blood cells of patients with JAK2 exon 12 mutations (A) or JAK2-V617F mutation (B). Single erythroid colonies were picked and analyzed individually. Horizontal bars indicate the percentages of colonies with homozygous mutation (■), heterozygous mutation ( ), or wild-type JAK2 (□). For each patient, 2 bars are shown: the upper representing colonies grown in the presence of erythropoietin (Epo+) and the lower representing colonies grown without erythropoietin (Epo−). The unique patient numbers (UPN) and the allelic ratios of the JAK2 mutations (%mut or %T) in granulocytes (GRA) are shown in the 2 left columns, and the total number of erythroid colonies analyzed is shown in the right column. *Note that in patient p021 colonies positive for exon 12 mutation and colonies with JAK2-V617F were found. None of these colonies carried both mutations simultaneously. (C) Molecular analysis of individual erythroid colonies of patient Vi327. Data from 1 of 4 BFU-E homozygous for the E543-D544del mutation is shown. T-cell DNA from patient Vi327 was used as control (top row). Allele discrimination assay shows the presence of a homozygous E543-D544del mutation (left panel). Two microsatellite markers, D9S1779 and D9S1852, demonstrate loss of heterozygosity on chromosome 9p (9pLOH) in the same colony (middle and right panels). Numbers indicate allele sizes.
), or wild-type JAK2 (□). For each patient, 2 bars are shown: the upper representing colonies grown in the presence of erythropoietin (Epo+) and the lower representing colonies grown without erythropoietin (Epo−). The unique patient numbers (UPN) and the allelic ratios of the JAK2 mutations (%mut or %T) in granulocytes (GRA) are shown in the 2 left columns, and the total number of erythroid colonies analyzed is shown in the right column. *Note that in patient p021 colonies positive for exon 12 mutation and colonies with JAK2-V617F were found. None of these colonies carried both mutations simultaneously. (C) Molecular analysis of individual erythroid colonies of patient Vi327. Data from 1 of 4 BFU-E homozygous for the E543-D544del mutation is shown. T-cell DNA from patient Vi327 was used as control (top row). Allele discrimination assay shows the presence of a homozygous E543-D544del mutation (left panel). Two microsatellite markers, D9S1779 and D9S1852, demonstrate loss of heterozygosity on chromosome 9p (9pLOH) in the same colony (middle and right panels). Numbers indicate allele sizes.
Distribution of JAK2 mutations in erythroid progenitors and loss of heterozygosity on chromosome 9p (9pLOH) analysis in patient Vi327. The total number of erythroid colonies analyzed and the percentages of colonies with homozygous or heterozygous JAK2 mutation or wild-type JAK2 are shown. Colony assays in methylcellulose were performed with peripheral blood cells of patients with JAK2 exon 12 mutations (A) or JAK2-V617F mutation (B). Single erythroid colonies were picked and analyzed individually. Horizontal bars indicate the percentages of colonies with homozygous mutation (■), heterozygous mutation ( ), or wild-type JAK2 (□). For each patient, 2 bars are shown: the upper representing colonies grown in the presence of erythropoietin (Epo+) and the lower representing colonies grown without erythropoietin (Epo−). The unique patient numbers (UPN) and the allelic ratios of the JAK2 mutations (%mut or %T) in granulocytes (GRA) are shown in the 2 left columns, and the total number of erythroid colonies analyzed is shown in the right column. *Note that in patient p021 colonies positive for exon 12 mutation and colonies with JAK2-V617F were found. None of these colonies carried both mutations simultaneously. (C) Molecular analysis of individual erythroid colonies of patient Vi327. Data from 1 of 4 BFU-E homozygous for the E543-D544del mutation is shown. T-cell DNA from patient Vi327 was used as control (top row). Allele discrimination assay shows the presence of a homozygous E543-D544del mutation (left panel). Two microsatellite markers, D9S1779 and D9S1852, demonstrate loss of heterozygosity on chromosome 9p (9pLOH) in the same colony (middle and right panels). Numbers indicate allele sizes.
), or wild-type JAK2 (□). For each patient, 2 bars are shown: the upper representing colonies grown in the presence of erythropoietin (Epo+) and the lower representing colonies grown without erythropoietin (Epo−). The unique patient numbers (UPN) and the allelic ratios of the JAK2 mutations (%mut or %T) in granulocytes (GRA) are shown in the 2 left columns, and the total number of erythroid colonies analyzed is shown in the right column. *Note that in patient p021 colonies positive for exon 12 mutation and colonies with JAK2-V617F were found. None of these colonies carried both mutations simultaneously. (C) Molecular analysis of individual erythroid colonies of patient Vi327. Data from 1 of 4 BFU-E homozygous for the E543-D544del mutation is shown. T-cell DNA from patient Vi327 was used as control (top row). Allele discrimination assay shows the presence of a homozygous E543-D544del mutation (left panel). Two microsatellite markers, D9S1779 and D9S1852, demonstrate loss of heterozygosity on chromosome 9p (9pLOH) in the same colony (middle and right panels). Numbers indicate allele sizes.
In patient Vi327, we found a homozygous exon 12 mutation in 4 of 32 colonies examined (Figure 2A). In this patient, the allelic ratio of the exon 12 mutation was more than 50% in granulocytes, platelets, and monocytes (Figure 1B). The homozygous colonies exhibited loss of heterozygosity on chromosome 9p (9pLOH; Figure 2C). Copy number analysis of JAK2 by real-time PCR excluded gene amplification or deletion as the mechanism (not shown). Two other cases of homozygous exon 12 mutations were recently found by sequencing, but the mechanism has not been studied.12,26 All PV patients with JAK2-V617F exhibited at least some homozygous colonies (Figure 2B), as reported.25 The only exception in our series was patient p021, in whom we found 2 different JAK2 mutations. The reason why exon 12 mutations are more invariably associated with increased erythropoiesis than JAK2-V617F remains to be determined.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Verena Jäggin for FACS sorting, Michael Medinger and Andre Tichelli for contributing clinical data, Martin Stern for statistical analysis, and Jürg Schwaller, Ralph Tiedt, Alois Gratwohl, and Nico Ghilardi for comments on the manuscript.
This work was supported by grant 310000-108006/1 from the Swiss National Science Foundation and the Swiss Cancer League (grant OCS-01 742-08-2005) (R.C.S.) and from the Initiative for Cancer Research of the Medical University of Vienna and the Austrian Science Fund FWF (P20033-B11; R.K.).
Authorship
Contribution: S.L. performed research, analyzed data, and wrote the paper; R.K. performed research and analyzed data; G.D., A.T., and H.G. analyzed data; R.C.S. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Radek C. Skoda, Department of Research, Experimental Hematology, University Hospital Basel, Hebelstrasse 20, 4031 Basel, Switzerland; e-mail: radek.skoda@unibas.ch.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal