Abstract
Nucleophosmin (NPM1) gene has been heavily implicated in cancer pathogenesis both as a putative proto-oncogene and tumor suppressor gene. NPM1 is the most frequently mutated gene in acute myeloid leukemia (AML), while deletion of 5q, where NPM1 maps, is frequent in patients with myelodysplastic syndromes (MDS). We have previously shown that mice heterozygous for Npm1 (Npm1+/−) develop a hematologic syndrome with features of human MDS. Here we analyzed Npm1+/− mutants to determine their susceptibility to cancer. Npm1+/− mice displayed a greater propensity to develop malignancies compared with Npm1+/+ mice. The Npm1+/− cohort frequently developed hematologic malignancies of both myeloid and lymphoid origin with myeloid malignancies displaying the highest incidence. Malignant cells retained the wild-type allele with normal localization and expression of Npm1 at the protein level, suggesting that complete Npm1 loss is not a prerequisite for tumorigenesis. Our results conclusively demonstrate that Npm1 acts as a haploinsufficient tumor suppressor in the hematopoietic compartment.
Introduction
Nucleophosmin (NPM1) is a nucleolar phosphoprotein involved in critical cellular processes including ribosome biogenesis, maintenance of genomic stability, regulation of p53 and p19Arf tumor suppressor stability, modulation of DNA transcription, and response to stress stimuli.1 The NPM1 gene has been implicated in human cancer as it is frequently a target of genetic alterations primarily in hematopoietic tumors. It is found translocated with distinct partner genes in several diseases such as acute promyelocytic leukemia (APL), anaplastic large cell lymphoma (ALCL), acute myeloid leukemia (AML), and myelodysplasia.2-4 Moreover, it is mapped to a region of chromosome 5 that is frequently deleted in therapy-related MDS and in nonhematopoietic tumors, such as non–small cell lung carcinoma.5,6 More recently, NPM1 has been found mutated and aberrantly localized in the cytoplasm of the leukemic blasts in a high proportion of AML patients (approximately 35%), rendering NPM1 the most frequently mutated gene in AML.7
Characterization of primary cells from an Npm1 hypomorphic series in vitro indicated that Npm1 could exert tumor suppressive functions.8 Npm1 loss significantly affected genomic stability and Npm1 was shown to be haploinsufficient for this function. Npm1+/− mouse embryonic fibroblasts (MEFs) displayed genomic instability, which resulted in increased susceptibility to oncogenic transformation in vitro.8 Moreover, Npm1+/− mice displayed hematologic features similar to those observed in patients with MDS, a preleukemic condition in humans.8 However, whether Npm1 functional loss may lead to cancer susceptibility per se remained unclear. In this report, we demonstrate that Npm1 acts indeed as a haploinsufficient tumor suppressor gene in vivo.
Methods
The Research Animal Resources Center at Memorial Sloan Kettering Cancer Center and Harvard Medical School Animal Facility approved and monitored all protocols that used animals in this study.
Mouse line
The generation of Npm1 heterozygous mice was previously described.8
Histology and immunohistochemistry
Mouse tissues sections were stained with hematoxylin and eosin (H&E) for histopathological examination. Antibodies for immunohistochemical staining were CD45R/B220 for B-cell lineage, CD3 for T-cell lineage, polyclonal prediluted anti-lysozyme muramidase (Ventana, Tucson, AZ) for myeloid cells, and mouse antinucleophosmin/B23 (Zymed, South San Francisco, CA), used according to the manufacturer's instructions. Pictures of the stained tissue sections were obtained using an Olympus BX41 microscope and a DP20 camera (Olympus, Center Valley, PA). All image acquisition and processing was carried out with Adobe Photoshop 7.0 (Adobe Systems, Mountain View, CA).
Immunofluorescence
Immunofluorescence was performed as previously described8 using anti–mouse γ-tubulin antibody (Sigma-Aldrich, St Louis, MO).
CGH array analysis
Genomic DNA from tumors of 6 Npm1 heterozygous mice was subjected to comparative genomic hybridization (CGH) array analysis at the MSKCC Genomic Core Lab Microarray Facility.
Flow cytometry
Bone marrow cells were stained with phycoerythrin (PE)-conjugated anti-Mac1 and allophycocyanin (APC)-conjugated anti-Gr1 (BD Pharmingen, San Diego, CA) and analyzed using FACSCalibur (BD Biosciences).
Southern blot analysis
Southern blot analysis was performed using the 5′ probe used to identify the Npm1-null allele as previously described.8
Results and discussion
To determine the susceptibility of Npm1+/− mice to tumorigenesis, we conducted a 2-year follow-up on a colony of 102 mice (47 wild-type and 55 heterozygotes mice), and have evaluated incidence, latency, and pathological features of neoplasias occurring in these mice compared with wild-type littermates.
Strikingly, Npm1+/− mice displayed higher susceptibility to develop malignancies than their wild-type counterparts. Of the 55 heterozygous mice under observation, 16 (29%) developed malignancies (Table 1). By comparison, only 3 cases (6.3%) were identified in the wild-type population (P < .01). Tumors were observed after a long latency that ranged from 10 to 24 months.
Tumor spectra and incidences in Npm1+/− and Npm1+/+ mice
| Genotype/ID . | Age, mo . | Type of solid tumor . | Hematologic malignancies . |
|---|---|---|---|
| Npm1+/−, n = 16/55 (29%) | |||
| 70 | 20 | — | Myeloid leukemia |
| 36 | 24 | — | Myeloid leukemia |
| 80 | 23 | — | Myeloproliferative disease–like myeloid leukemia |
| 32D | 20 | — | Myeloid leukemia |
| 90D | 18 | Lung carcinoma | — |
| 75A | 24 | Salivary adenocarcinoma | — |
| 72D | 23 | — | T cell lymphoma/leukemia |
| B24 | 10 | — | B cell lymphoma |
| B3 | 23 | — | Myeloproliferative disease–like myeloid leukemia |
| A73 | 24 | — | Myeloid leukemia |
| A66 | 23 | Hepatocellular carcinoma | — |
| B20 | 23 | — | B cell lymphoma |
| B47 | 24 | — | Myeloproliferative disease–like myeloid leukemia |
| B22 | 24 | Lung adenocarcinoma | — |
| 3B | 24 | — | Myeloproliferative disease–like myeloid leukemia |
| 75 | 19 | — | Myeloproliferative disease–like myeloid leukemia |
| Npm1+/+, n = 3/47 (6.3%) | |||
| 85 | 21 | Cutaneous squamous cell carcinoma | — |
| A76 | 24 | Hepatocellular adenoma | — |
| 83 | 18 | Hepatocellular carcinoma | — |
| Genotype/ID . | Age, mo . | Type of solid tumor . | Hematologic malignancies . |
|---|---|---|---|
| Npm1+/−, n = 16/55 (29%) | |||
| 70 | 20 | — | Myeloid leukemia |
| 36 | 24 | — | Myeloid leukemia |
| 80 | 23 | — | Myeloproliferative disease–like myeloid leukemia |
| 32D | 20 | — | Myeloid leukemia |
| 90D | 18 | Lung carcinoma | — |
| 75A | 24 | Salivary adenocarcinoma | — |
| 72D | 23 | — | T cell lymphoma/leukemia |
| B24 | 10 | — | B cell lymphoma |
| B3 | 23 | — | Myeloproliferative disease–like myeloid leukemia |
| A73 | 24 | — | Myeloid leukemia |
| A66 | 23 | Hepatocellular carcinoma | — |
| B20 | 23 | — | B cell lymphoma |
| B47 | 24 | — | Myeloproliferative disease–like myeloid leukemia |
| B22 | 24 | Lung adenocarcinoma | — |
| 3B | 24 | — | Myeloproliferative disease–like myeloid leukemia |
| 75 | 19 | — | Myeloproliferative disease–like myeloid leukemia |
| Npm1+/+, n = 3/47 (6.3%) | |||
| 85 | 21 | Cutaneous squamous cell carcinoma | — |
| A76 | 24 | Hepatocellular adenoma | — |
| 83 | 18 | Hepatocellular carcinoma | — |
Npm1+/− versus Npm1+/+ P less than .01. The P value was calculated by chi-square test.
— indicates not applicable.
Pathological analysis revealed that 75% of the diseased Npm1+/− mice were affected by hematologic malignancies (Figure 1A), while no abnormalities were detected in the hematopoietic organs of wild-type animals analyzed. Interestingly, among the solid tumors developed by the Npm1+/− mice, 2 were cancers of the lung.
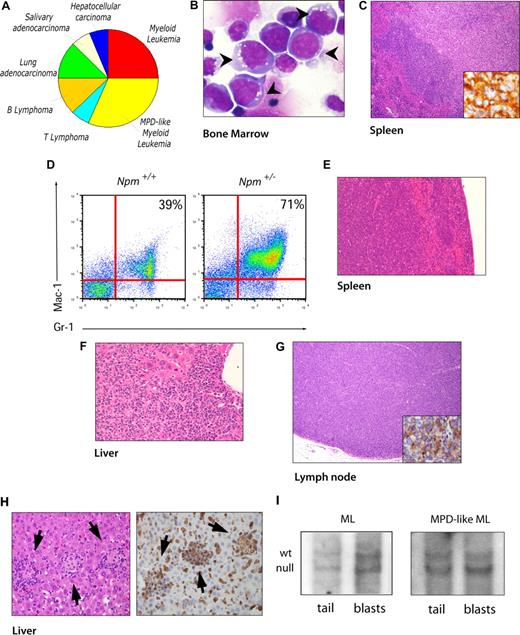
Features of Npm1+/− mice hematologic malignancies. (A) Pie chart showing tumor spectrum in Npm1+/− mice. (B) Bone marrow cytospin (60×/0.80 NA oil objective magnification) from an ML Npm1+/− mouse showing blasts with intracytoplasmic vacuoles, high nuclear/cytoplasmic ratio, basophilic cytoplasm, and multiple nucleoli (arrowheads). (C) Spleen section (10×/0.30 NA oil objective magnification) from the same mouse showing mass involving the red pulp composed of atypical mononuclear cells. The inset is a 40×/0.75 NA oil objective magnification of the immunohistochemical staining for muramidase indicating blasts originating from the myeloid lineage. (D) Flow cytometry analysis of bone marrow from an MPD-like ML Npm1+/− mouse demonstrates an expansion of Gr-1/Mac-1 mature cells. Numbers on graphs are percentages of cells in total bone marrow. (E) Spleen section (10×/0.30 NA oil objective magnification) from the same mouse in panel D showing both red and white pulp replaced by atypical mononuclear infiltrate. (F) Liver section (40×/0.75 NA oil objective magnification) from the same mouse in panel D showing infiltration of periportal area extended into the parenchyma as well. (G) Lymph node (10×/0.30 NA oil objective magnification) replacement by atypical lymphoid infiltrate of CD45R+ cells (inset 40×/0.75 NA oil objective) supporting the involvement of a B-cell lymphoma. (H) Hematoxylin and eosin staining of liver lobular infiltrates (40×/0.75 NA oil objective magnification, arrows in left panel). Immunohistochemistry shows lobular lymphoid infiltrates in liver positive for CD3 (arrows in right panel). (I) Southern blot analysis on tail and blast DNA from representative ML (left panel) and MPD-like ML (right panel) affected Npm1+/− mice.
Features of Npm1+/− mice hematologic malignancies. (A) Pie chart showing tumor spectrum in Npm1+/− mice. (B) Bone marrow cytospin (60×/0.80 NA oil objective magnification) from an ML Npm1+/− mouse showing blasts with intracytoplasmic vacuoles, high nuclear/cytoplasmic ratio, basophilic cytoplasm, and multiple nucleoli (arrowheads). (C) Spleen section (10×/0.30 NA oil objective magnification) from the same mouse showing mass involving the red pulp composed of atypical mononuclear cells. The inset is a 40×/0.75 NA oil objective magnification of the immunohistochemical staining for muramidase indicating blasts originating from the myeloid lineage. (D) Flow cytometry analysis of bone marrow from an MPD-like ML Npm1+/− mouse demonstrates an expansion of Gr-1/Mac-1 mature cells. Numbers on graphs are percentages of cells in total bone marrow. (E) Spleen section (10×/0.30 NA oil objective magnification) from the same mouse in panel D showing both red and white pulp replaced by atypical mononuclear infiltrate. (F) Liver section (40×/0.75 NA oil objective magnification) from the same mouse in panel D showing infiltration of periportal area extended into the parenchyma as well. (G) Lymph node (10×/0.30 NA oil objective magnification) replacement by atypical lymphoid infiltrate of CD45R+ cells (inset 40×/0.75 NA oil objective) supporting the involvement of a B-cell lymphoma. (H) Hematoxylin and eosin staining of liver lobular infiltrates (40×/0.75 NA oil objective magnification, arrows in left panel). Immunohistochemistry shows lobular lymphoid infiltrates in liver positive for CD3 (arrows in right panel). (I) Southern blot analysis on tail and blast DNA from representative ML (left panel) and MPD-like ML (right panel) affected Npm1+/− mice.
Interestingly, three quarters of the hematologic neoplasms developed by Npm1+/− mice were myeloid malignancies, with a myeloproliferative disease–like myeloid leukemia (MPD-like ML) having the highest incidence (41.6% of all hematologic malignancies). MPD-like ML was characterized by leukocytosis (white blood cell [WBC] count = 18.1 ± 0.7 × 109/L) and extreme expansion of well-differentiated myeloid cells in bone marrow (Figure 1D), spleen (Figure 1E), and also in other nonhematopoietic tissues, such as liver (Figure 1F), and kidney and intestine (Figure S1C and S1D, respectively, available on the Blood website; see the Supplemental Materials link at the top of the online article).
The remaining mice affected by myeloid malignancies presented with myeloid leukemia (ML). ML was characterized by severe anemia (hemoglobin [HGB] level = 69 ± 8 g/L), mild leukocytosis (WBC count = 17.4 ± 0.5 ×109/L), and thrombocytopenia (platelet [PLT] count = 290 ± 60 × 109/L). Both peripheral blood and bone marrow contained a significant percentage of leukemic blasts (20% ± 4% and 36% ± 2%, respectively). Morphologically, blasts presented like immature myeloid cells with increased nucleus-cytoplasm ratio and highly vacuolated cytoplasm (Figure 1B arrowheads). Hepatomegaly and splenomegaly were invariably detected in these leukemic mice (Figure S1A). Pathologically, spleen and liver were filled with a large amount of leukemic blasts that compromised the architecture of the tissues (Figures 1C, S1B). Immunohistologic analysis of spleen and liver sections identified cells whose cytoplasm was positive for muramidase (lysozyme) staining, and positivity is usually associated with monocytic differentiation in human and mouse leukemias9 (Figure 1C inset). These are all features distinctive of myeloid leukemia in the mouse.10
In addition to an increased rate of myeloid malignancies, Npm1+/− mice also developed B- and T-cell lymphomas (one quarter of all hematologic malignancies observed). These mice presented with enlarged lymph nodes with atypical lymphoid infiltrates affecting the normal architecture (Figure 1G). Lymphadenopathy was accompanied by hepatosplenomegaly with both spleen pulps and liver parenchyma replaced by nodules of atypical lymphoid cells (Figure 1H left panel; Figure S1F,G). In two thirds of lymphomas, the atypical lymphoid infiltrates were positive for CD45R staining (Figure 1G inset), supporting the diagnosis of B-cell lymphoma. In the other cases, neoplastic lymphoid infiltrates displayed strong positivity for CD3 staining (Figure 1H right panel), suggestive of T-cell lymphomagenesis.
We next analyzed the status of the Npm1 gene in genomic DNA extracted from blasts of focal homogeneous infiltrates localized in the spleen of ML and MPD-like ML affected mice (6 mice in total), using the corresponding tail DNA as reference. Loss of the wild-type Npm1 allele was never observed (Figure 1I). The nuclear expression of Npm1 in these tumors was confirmed by immunohistochemistry (Figure S2A).
As Npm1 function is required for maintaining genomic stability, we have analyzed centrosome number and the presence of chromosomal abnormalities in Npm1+/− mice tumors. Bone marrow cytospins from MPD-like ML affected mice were immunostained for the centrosome marker γ-tubulin. Two independent cytospins clearly showed an increased number of cells displaying multiple centrosomes (Figure S2B). On average, 70% of cells displayed amplified centrosomes in these MPD-like ML, compared with 6% of bone marrow cells from nonleukemic Npm1+/− mice. These data demonstrate that centrosome amplification is associated with Npm1 loss and may result in leukemia pathogenesis in Npm1+/− mice.
Genomic DNA analysis of 6 hematologic malignancies performed by comparative genomic hybridization invariably revealed the presence of numeric and structural chromosomal abnormalities (Figure S2C).
Although Npm1+/− mice display features resembling some of those identified in human MDS, it was not clear whether this represented a genuine preleukemic situation as is the case for the human disease. Here, we addressed this question by following a large cohort of Npm1+/− mice to establish whether Npm1 heterozygosity results in an increased susceptibility to leukemogenesis. Indeed, a number of these mice were found to develop a variety of hematologic neoplasms, with myeloid malignancies having the highest incidence. These data confirm our hypothesis that the Npm1 phenotype originally characterized has the potential to progress to overt leukemia.
The absence of loss of heterozygosity (LOH) indicates that Npm1 haploinsufficiency, rather than biallelic inactivation, is promoting the development of tumors in these mice. This suggests that while Npm1 heterozygosity may render the cells more susceptible to oncogenic transformation, the complete somatic loss of the Npm1 gene is neither required for the transformation nor selected for by the transformed cell itself.
In conclusion, we demonstrate that Npm1 is a haploinsufficient suppressor of myeloid and lymphoid malignancies in the mouse. Npm1+/− mice are prone to spontaneous tumor formation and in particular to the development of disease of myeloid origin.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Linda Disantis and Owen Milbury for editing the paper, Manoj Kumar of BIDMC Genomics Center for help with CGH analysis, and members of the Pandolfi laboratory for helpful discussions.
This work was supported by National Institutes of Health grant (CA-71692) to P.P.P. P.S. was supported in part by the Associazione Umbra Leucemie e Linfomi (AULL).
National Institutes of Health
Authorship
Contribution: P.S. and S.G. designed and performed experiments, analyzed data, and wrote the paper; S.M.M. performed experiments; K.C. and J.G.C. performed experiments and edited the paper; A.V. performed the CGH analysis; J.T.-F. performed the pathological analysis of tumor samples; P.P.P. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pier Paolo Pandolfi, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, NRB 1038A, Boston, MA 02215; e-mail: ppandolf@bidmc.harvard.edu.
References
Author notes
P.S. and S.G. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal