Previously, we and others showed that mitotic Aurora-A kinase (Aur-A) was required for accurate mitotic entry and proper spindle assembly. In this study, we found that expression of Aur-A was markedly elevated in bone marrow mononuclear cells (BMMCs) obtained from a significant portion of de novo acute myeloid leukemia (AML) patients. Targeting human primary AML cells with Aur-A kinase inhibitory VX-680 led to apoptotic cell death in a dose-dependent manner. Importantly, VX-680–induced cell death was preferentially higher in Aur-A-high primary leukemic blasts compared with Aur-A-low AML (P < .001) or normal BMMCs (P < .001), suggesting the possible pharmacologic window in targeting Aurora kinase among Aur-A-high VX-680–sensitive leukemia patients. VX-680–induced cell death in AML cell lines was accompanied by formation of monopolar mitotic spindles, G2/M phase arrest, decreased phosphorylated(p)-Akt-1, and increased proteolytic cleavage of procaspase-3 and poly(ADP)ribose polymerase. Notably, VX-680 increased Bax/Bcl-2 expression ratio, a favorable proapoptotic predictor for drug response and survival in AML. Lastly, VX-680 enhanced the cytotoxic effect of the chemotherapeutic agent etoposide (VP16) on AML cells. Together, we concluded that Aurora kinases were potentially therapeutic targets for AML and that Aur-A-high expression may serve as a differential marker for selective treatment.
Introduction
Acute myeloid leukemia (AML) consists of heterogeneous subgroups of neoplastic hematopoietic precursor cells of uncontrolled clonal expansion. Current therapeutic regiments, relying on remission induction followed by postremission therapy with additional intensive chemotherapy or stem-cell transplantation, have produced limited survival benefits.1,2 Development of less toxic and specific therapies in AML is urgently needed, prompting an intensive search for appropriate target molecules. Acute promyelocytic leukemia (APL), characterized by t(15;17) translocation resulting in a fusion transcript of promyelocytic leukemia-retinoic acid receptor α (PML-RARα), became the first subtype of AML treated with an agent targeting a specific genetic mutation. All-trans retinoic acid, inducing terminal differentiation and apoptosis, has since changed the standard of care in APL for its high complete remission rate.3 Several other mutations have recently been recognized to cause hyperactivation of signal transduction pathways that render growth and/or survival advantage, including Flt3 internal tandem duplication (Flt3/ITD), Flt3 tyrosine kinase domain (Flt3/TKD), c-Kit, and Ras.4,–6 Collectively, these mutations account for 30% to 50% of AML patients, raising the opportunity for more target-directed therapies. Small molecules targeting against Ras (farnesyl transferase inhibitor), Flt3, and Kit signaling have recently been tested in clinical samples, generating promising, however limited, clinical responses.7,–9
Serine/threonine kinases Aurora family, including Aur-A, -B, and -C, are key players in ensuring accurate chromosome segregation during cell cycle, maintaining genetic integrity in cell division.10,11 Aur-C is highly expressed in sperm cells and plays a crucial role in spermatogenesis.12 Aur-B, together with survivin and inner centromere protein, forms chromosome passenger protein complex, playing a crucial role in proper microtubule attachment to chromosomes and cytokinesis.13 We and others have shown that Aur-A is essential in proper timing of mitotic entry and formation of bipolar spindles.13,14 Ectopic overexpression of Aur-A transformed NIH3T3 and immortalized Rat1 cells, and these transformed cells could form tumors when implanted in nude mice.15,16 Indeed, high Aur-A expression was found in various types of commonly occurring malignancy,16,,,–20 and in some cases, correlated with aneuploidy in tumor tissues and poor prognosis.21,22 Small-molecule Aurora kinase inhibitors have been recently developed as potential targeting therapeutics.23 Among these, VX-680 with more Aur-A selectivity (Ki = 0.6 nM; 18 nM for Aur-B and 4.6 nM for Aur-C) showed appealing preclinical evidence of anticancer activity in vivo.24 Recently, VX-680 was shown to be effective against multiple myeloma, especially in patients with RHAMM overexpression.25 More interestingly, VX-680 demonstrated potent anticancer activity in chronic myeloid leukemia (CML) harboring imatinib-resistant T351I and dasatinib-resistant V299L Bcr-Abl mutations.26,27 The potential antileukemia mechanism of VX-680 and its association with Aurora kinase expression in AML, however, have not been investigated.
Here we show that Aurora kinases were highly expressed in primary leukemia bone marrow blasts compared with that in normal donors. Importantly, we report that VX-680, an Aurora kinase inhibitor, induced apoptosis preferentially in the leukemic blasts with high Aur-A expression, but not in normal bone marrow mononuclear cells (BMMCs) or Aur-A-low AML cells, suggesting a potential pharmacologic window for VX-680 therapeutic response in Aur-A-high AMLs. Moreover, we studied the potential mechanism of apoptosis induced by VX-680, which was accompanied by destruction of normal bipolar spindles, G2/M phase arrest, reduction of phosphorylated(p)-Akt-1, and activation of cellular caspases. Bax/Bcl-2 ratio, a known favorable survival factor in AML, was increased by VX-680 in a dose-dependent manner. Lastly, we found that VX-680 synergistically enhanced the cytotoxic effect of VP16 in AML cells. Thus, we showed that Aurora kinase inhibitor VX-680 may be of therapeutic use in AML, particularly in Aur-A-high cases and in combination with VP16.
Methods
Patients, samples, and reagents
A total of 98 untreated de novo AML patients and 12 healthy volunteers were included. According to the French-American-British (FAB) classification, the patient subtypes were as follows: 3 had M0, 6 had M1, 25 had M2, 18 had M3, 8 had M4, 35 had M5, and 3 had M6. At recruitment, informed consent was obtained from each subject in accordance with the Declaration of Helsinki. This study was approved by the Institute Research Ethics Committee at Sun Yan-Sen University. Samples from patient unique number (UPN) 1 to 53, UPN 54 to 78, UPN 79 to 90 and UPN 91 to 98 (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) were collected, respectively, from patients at Departments of Hematology in First Affiliated Hospital of Sun Yat-Sen University, Guangdong Provincial People's Hospital, Cancer Center of Sun Yat-Sen University, and NanFang Hospital. Primary leukemic cells were enriched from the diagnostic bone marrow samples of patients with de novo AML by Ficoll-Hypaque (Sigma-Aldrich, St Louis, MO) density gradient centrifugation. Normal BMMCs were obtained from healthy bone marrow donors. VX-680 (Kava Tech, Santiago, CA) was stored in dimethyl sulfoxide (DMSO, 400 μM) and kept at −20°C. VP16 was purchased from Sigma-Aldrich.
Cell culture
Normal BMMCs were labeled with CD34 microbeads isolated by magnetic positive selection (MACS cell isolation kit; Miltenyi Biotec, Paris, France). Each separated aliquot was confirmed to contain more than 95% CD34+ cells by flow cytometry (not shown). Primary leukemia blasts and normal BMMCs were resuspended in RPMI 1640 (Invitrogen, Carlsbad, CA) containing appropriate antibiotics and 10% fetal bovine serum (HyClone Laboratories, Logan, UT). HL-60 and U937 cell lines obtained from American Type Culture Collection (Manassas, VA) were cultured according to supplier's instructions.
Cell growth assay
Cell proliferation was assessed by standard MTT assay (Sigma-Aldrich). The absorbance was determined at a test wavelength of 570 nm on a multiwell plate reader (Microplate Reader; Bio-Rad, Hercules, CA).
Measurement of apoptosis by annexin V analysis
The annexin V assays were performed according to the manufacturer's protocol (Annexin V-FITC Apoptosis Detection Kit, EMD Biosciences, San Diego, CA). The percentage of apoptotic cells was determined using FACS flow cytometer equipped with CellQuest software (BD Immunocytometry Systems, San Jose, CA).
Cell-cycle analysis
Cells were collected, fixed, and resuspended in cell-cycle buffer (0.38 mM sodium citrate, 0.5 mg/mL RNase A, and 0.01 mg/mL propidium iodide [PI]) at a concentration of 106 cells/mL. Cell-cycle analysis was carried out using a FACSCalibur flow cytometer equipped with Modfit LT for Mac V2.0 software (BD Biosciences, San Jose, CA).
Cell synchronization
Cells were synchronized at the G1/S boundary by standard double thymidine (Sigma-Aldrich) blocking method. Briefly, cells were first blocked for 16 hours with thymidine, released in fresh media for 8 hours, reexposed to thymidine for an additional 16 hours, followed by release in fresh media. Cells were then incubated in the presence of VX-680 or DMSO, and collected at the indicated time points.
Immunocytochemical staining of Aur-A expression
Primary leukemia blasts and normal BMMCs were washed with phosphate-buffered saline and smeared glass slides by cytospin (Hettich, Kirchlengern, Germany). The slides were fixed and incubated in H2O2 for 10 minutes. After washes, slides were blocked with 1% bovine serum albumin (BSA, Sigma-Aldrich) followed by anti–Aur-A antibody (Upstate Biotechnology, Charlottesville, VA). Slides were then incubated with the secondary antibody and finally with H2O2-diaminobenzidine until the desired stain intensity developed. Slides were all counterstained with hematoxylin, dehydrated, and mounted. In the negative control, primary antibody was replaced by the nonimmune mouse IgG of the same isotype. Moderate or strong cytoplasm staining was considered as positive reaction. The degree of immunocytostaining of specimens was evaluated by 3 independent pathologists.
Short interfering RNA transfection
HL-60 and primary leukemic cells were seeded onto 6-well plates for 16 hours before transfection. siRNA (50 nM) Aur-A:5′-AUGCCCUCUCUUACUGUCA-3′,28 siRNA Aur-B: 5′-AACGCGGCACUUCACAAUUGA-3′29 or control scramble sequences and 10 μL lipofectamine 2000 (Invitrogen) were added to Opti-MEM (Invitrogen) and mixed. After incubation, the siRNA and lipofectamine 2000 solutions were mixed gently and added to the plates until ready for further assay.
Immunofluorescence staining
Cultured cells were smeared glass slides by cytospin before fixation in 4% paraformaldehyde–phosphate-buffered saline. The fixed cells were then permeabilized and then covered with 1% BSA for 30 minutes. Slides were incubated with mouse anti–p-histone H3 (Ser10) antibody (Cell Signaling Technology, Danvers, MA), rabbit anti–Aur-A antibody (Upstate Biotechnology), and monoclonal anti–α-tubulin antibody (Sigma-Aldrich) for 60 minutes. The immune complexes were stained with a goat antimouse secondary antibody conjugated to Alexa-488 or a goat antirabbit secondary antibody conjugated to Alexa-680 (Invitrogen). Nuclei were stained with DAPI and viewed with a laser confocal microscope (LSM 510, Zeiss MicroImaging, Thornwood, NY).
Cell lysis and Western blot analysis
Cells were lysed in RIPA buffer. The protein concentration was determined by Bradford method with BSA (Sigma-Aldrich) as the standard (Bio-Rad). Equal amounts of cell extract (50 μg) were subjected to electrophoresis in SDS-polyacrylamide gel and transferred to nitrocellulose membrane (Bio-Rad). The membrane was blocked and then incubated with glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Ambion), Aur-A (Upstate), cyclinB1, Bax, and Flt3 antibodies (all from Santa Cruz Biotechnology, Santa Cruz, CA), p-Aur-A/AIK (Thr288), Aur-B, cleaved poly(ADP)ribose polymerase (PARP) (Asp214), p-histone H3 (Ser10), p-Akt-1 (Ser473), cleaved caspase-3 (Asp175), and Bcl-2 antibodies (all from Cell Signaling). Quantification of band intensities was done using TINA software, version 2.10e (Raytest Isotopenmessgeraete, Straubenhardt, Germany).
Screening mutation of Flt3 gene
Genomic DNA was extracted from AMLs with Trizol (Invitrogen). The presence of Flt3/ITD was identified by amplifying a region spanning exons 14 and 15 with primers 14F (TGTAAAACGACGGCCAGTCAATTTAGGTATGAAAGCC) and 15R (GAGGAAACAGCTATGACCCTTTCAGCATTTTGACGGAACC),30 followed by electrophoresis on 2.5% agarose gel. To detect Flt3/TKD mutation, genomic DNA was used to amplify Flt3 exon 20 using primers 20F (TGTAAAACGACGGCCAGTC CGCCAGGAACGTGCTTG) and 20R (CAGGAAACAGCTATGACCGCAGCCTC ACATTGCCCC).30 The amplified products were then digested with EcoRV and subjected to electrophoresis on agarose gels. An EcoRV restriction site exists within the wild-type Flt3 sequence in codon 835/836. Any mutation that alters the coding region of this region results in the elimination of this restriction site. All undigested bands seen after digestion were cut from the gel, purified with QIAquick gel extraction kit (QIAGEN, Hilden, Germany), and directly sequenced.
Evaluation of drug interactions
The interaction between VX-680 and VP16 was analyzed using the CalcuSyn software program (Biosoft, Cambridge, United Kingdom) to determine whether the combination was additive or synergistic. Data from annexin V assays were expressed as the proportion of apoptotic cells induced by the individual drugs or the combination in drug-treated compared with untreated cells. This program is based on the Jin's method,31 which is performed based on the following equation: q = D1 + 2/(D1 + D2 − D1 × D2), where D1 + 2 indicates the effect when cells were used in combination with drug 1 and 2, and D1 and D2 indicate the effect when used alone. The value of q indicates synergism when greater than 1.15, antagonism when smaller than 0.85, and additivity when located between 0.85 and 1.15.
Statistical analysis
The patients were divided into 2 groups (Aur-A-low and Aur-A-high) based on the expression level of Aur-A protein in AML cells by Western blot analysis and confirmed by immunocytostaining. High expression of target protein (Aur-A, Aur-B, and Flt3) was defined as the ratio of (target protein absorbance − background absorbance) versus (GAPDH absorbance − background absorbance) ≥ 0.50. The Aur-A-high cases were also defined arbitrarily as specimens showing strong staining for Aur-A, with more than 30% cells displayed visible brown granules in the cytoplasm. The comparisons among characteristics of the groups were made using a χ2 test for the binary variables and a Mann-Whitney test for the continuous variables. Statistics were calculated by GraphPad Prism software (GraphPad Software, San Diego, CA) and SPSS software, version 11.5 (SPSS). Analysis of variance (paired analysis of variance) and the protective least significant difference Fisher test were performed. A probability of less than 0.05 was considered significant.
Results
Aur-A expression in primary leukemia cells and AML cell lines
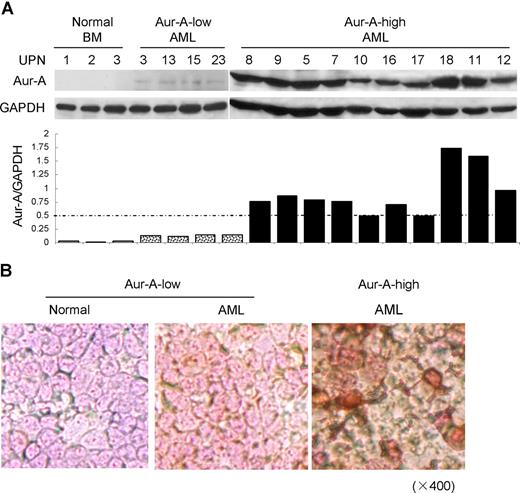
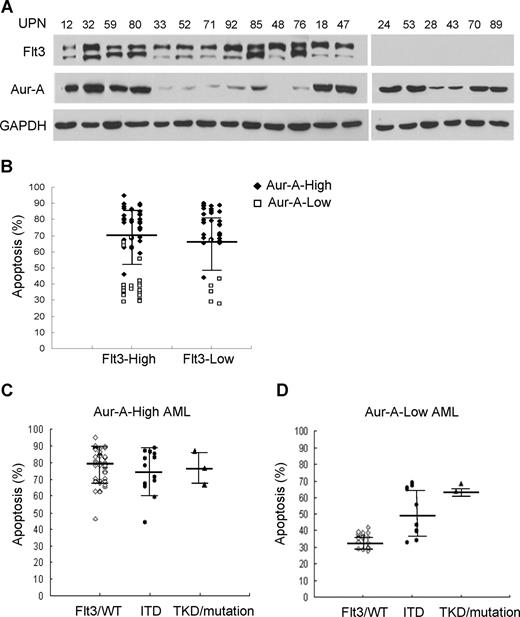
We first asked if the level of Aur-A protein was elevated in BMMCs of AML patients. Ninety-eight bone marrows aspirated from pathologically confirmed de novo AML patients and 12 normal donors were included in the study (Table S1). Western blot analysis showed that the expression of Aur-A protein was highly elevated in a significant proportion of AML cases compared with normal BMMCs. Because of the apparent variation of Aur-A expression among the AML samples, we further divided AML cases into 2 groups: Aur-A-high (65 of 98, 66.3%) versus Aur-A-low (33 of 98, 33.7%), as defined in “Methods.” In contrast, the normal bone marrow specimens revealed negligible level of Aur-A protein expression (12 of 12). Representative Western blot analyses were shown (Figure 1A). The association of Aur-A expression with clinical variables was also evaluated. As shown in Table 1, there was no significant difference between the patient age, sex, white blood cell count, or FAB classification subtype in relation to Aur-A expression (P > .05). Similarly, Flt3 and Aur-B protein expression levels were also evaluated by Western blot analysis and grouped in Table 1. The detailed expression status of these 3 proteins in all cases is listed in Table S1.
Aur-A is highly expressed in de novo primary AML bone marrow cells. (A) Endogenous expression of Aur-A in the representative AML samples and normal BMMCs (top). Primary leukemic blasts obtained from patients with AML were lysed and proteins were analyzed by Western blot analysis. GAPDH served as a loading control. The ratio of the intensity of the bands corresponded to Aur-A and GAPDH (bottom). AML samples were arbitrary divided into Aur-A-low and Aur-A-high cases, as described in “Statistical analysis.” UPN indicates unique patient number. (B) Bone marrow (BM) samples were collected from AML patients or normal donors and subjected to immunocytochemical staining with antibody against Aur-A. The Aur-A-high group exhibited strong staining of Aur-A (right), whereas the normal (left) and Aur-A-low group (middle) of AML samples showed weak staining (original magnification, ×400).
Aur-A is highly expressed in de novo primary AML bone marrow cells. (A) Endogenous expression of Aur-A in the representative AML samples and normal BMMCs (top). Primary leukemic blasts obtained from patients with AML were lysed and proteins were analyzed by Western blot analysis. GAPDH served as a loading control. The ratio of the intensity of the bands corresponded to Aur-A and GAPDH (bottom). AML samples were arbitrary divided into Aur-A-low and Aur-A-high cases, as described in “Statistical analysis.” UPN indicates unique patient number. (B) Bone marrow (BM) samples were collected from AML patients or normal donors and subjected to immunocytochemical staining with antibody against Aur-A. The Aur-A-high group exhibited strong staining of Aur-A (right), whereas the normal (left) and Aur-A-low group (middle) of AML samples showed weak staining (original magnification, ×400).
Clinical characteristics according to Aur-A expression in leukemia blasts
| . | Total (N = 98) . | Aur-A expression . | |
|---|---|---|---|
| Low (N = 33) . | High (N = 65) . | ||
| Median age, y (range) | 40.1 (12-73) | 41.4 (12-73) | 39.4 (12-72) |
| Sex, male/female | 59/39 | 22/11 | 37/28 |
| WBC, median no. ×103/μL (range) | 43.45 (0.25-212.4) | 29.38 (0.44-196.2) | 50.59 (0.25-212.4) |
| FAB classification | |||
| M0 | 3 | 0 | 3 |
| M1 | 6 | 1 | 5 |
| M2 | 25 | 10 | 15 |
| M3 | 18 | 8 | 10 |
| M4 | 8 | 2 | 6 |
| M5 | 35 | 10 | 25 |
| M6 | 3 | 2 | 1 |
| Flt3 genotype | |||
| WT | 67 | 20 | 47 |
| ITD | 27 | 11 | 16 |
| TKD mutations | 7 | 4 | 3 |
| ITD/TKD mutations* | 3 | 2 | 1 |
| Flt3 expression | |||
| High | 63 | 27 | 36 |
| Low | 35 | 6 | 29 |
| Aur-B expression | |||
| High | 40 | 7 | 33 |
| Low | 58 | 26 | 32 |
| . | Total (N = 98) . | Aur-A expression . | |
|---|---|---|---|
| Low (N = 33) . | High (N = 65) . | ||
| Median age, y (range) | 40.1 (12-73) | 41.4 (12-73) | 39.4 (12-72) |
| Sex, male/female | 59/39 | 22/11 | 37/28 |
| WBC, median no. ×103/μL (range) | 43.45 (0.25-212.4) | 29.38 (0.44-196.2) | 50.59 (0.25-212.4) |
| FAB classification | |||
| M0 | 3 | 0 | 3 |
| M1 | 6 | 1 | 5 |
| M2 | 25 | 10 | 15 |
| M3 | 18 | 8 | 10 |
| M4 | 8 | 2 | 6 |
| M5 | 35 | 10 | 25 |
| M6 | 3 | 2 | 1 |
| Flt3 genotype | |||
| WT | 67 | 20 | 47 |
| ITD | 27 | 11 | 16 |
| TKD mutations | 7 | 4 | 3 |
| ITD/TKD mutations* | 3 | 2 | 1 |
| Flt3 expression | |||
| High | 63 | 27 | 36 |
| Low | 35 | 6 | 29 |
| Aur-B expression | |||
| High | 40 | 7 | 33 |
| Low | 58 | 26 | 32 |
FAB indicates French-American-British; WT, wild-type Flt3; ITD, Flt3 internal tandem duplication; and TKD, the activation loop of the tyrosine kinase domain.
ITD/TKD mutations indicate that AML patients had both ITD and TKD mutations and were included in both ITD and TKD mutations groups.
We also verified expression of Aur-A in AML bone marrow specimens by immunocytochemical staining. Closely correlated with the findings from Western blot analysis, the Aur-A-high cases displayed strong immunocytochemical staining, defined as more than 30% cells showed visible brown granules in the cytoplasm. Typical immunocytochemical stainings of Aur-A-high or Aur-A-low expression subgroups were shown (Figure 1B).
VX-680 induces apoptosis in AML but not in normal primary bone marrow cells
We studied the inhibition of Aurora kinases in AML cells using a small molecule inhibitor VX-680.24 Aur-A is autophosphorylated in its activation loop on Thr288 on activation.32 Histone H3 is a direct downstream target of the Aurora kinase.33 Incubation of HL-60 cells with VX-680 for 24 hours led to a dose-dependent decrease in Aur-A phosphorylation at Thr288 (Figure S1A). Phosphorylation inhibition was also observed in histone H3 at Ser10 by VX-680 at much higher doses (Figure S1B,C).
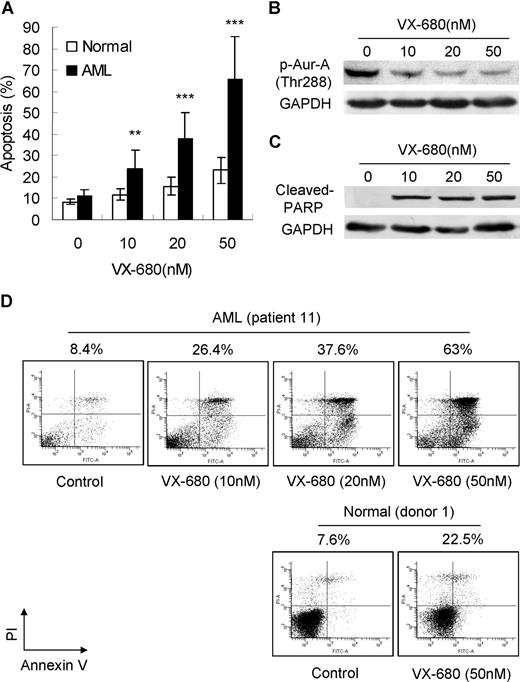
We then asked if Aurora kinase inhibitor VX-680 could induce apoptosis in primary leukemia blasts. Apoptotic cell death was assessed by annexin V-FITC staining and Western blot analysis with a specific antibody to cleaved PARP. Exposure of primary leukemia blasts to VX-680 for 72 hours induced marked apoptosis in a dose-dependent manner, compared with normal BMMCs (Figure 2A). A detailed analysis performed on patient 11 (M2, > 90% blasts) was also displayed. With increasing doses of VX-680, a decrease of Aur-A phosphorylation at Thr288 (Figure 2B) was accompanied by elevated levels of cleaved PARP (Figure 2C). Incubation with VX-680 of 10, 20, and 50 nM for 72 hours led to an increased apoptotic response with 26.4%, 37.6%, and 63% of annexin V-positive cells in primary blasts from patient 11. In contrast, VX-680 (50 nM) induced less cell death in normal bone marrow blasts (healthy donor 1, 22.5%; Figure 2D).
VX-680 inhibits activation of Aur-A and causes apoptotic cell death in AML blasts but not in normal BMMCs. (A) VX-680 induces primary leukemic blast apoptosis in a dose-dependent manner. Blasts from 98 AML patients and 12 normal donors were incubated with VX-680 as indicated doses for 72 hours. The apoptosis was assessed by annexin V assays. Mean percentage of apoptosis was shown (P < .001). (B) VX-680 inhibits autophosphorylation of Aur-A at Thr288 in primary leukemia cells. Cells from patient 11 were incubated with increasing amounts of VX-680 or DMSO for 24 hours. Cell lysates were subject to Western blot analysis with phospho-Aur-A (Thr288) antibody. (C) VX-680 induces cleavage of PARP. Blasts from patient 11 (> 90% blasts) were treated with VX-680 with indicated doses for 72 hours before analysis of PARP cleavage by Western blot. GAPDH levels represent loading controls. (D) Cells from patient 11 (top panel) and normal donor 1 (bottom panel) were incubated for 72 hours with indicated doses of VX-680 before staining with annexin V-FITC and PI. The percentages of apoptotic cells were displayed.
VX-680 inhibits activation of Aur-A and causes apoptotic cell death in AML blasts but not in normal BMMCs. (A) VX-680 induces primary leukemic blast apoptosis in a dose-dependent manner. Blasts from 98 AML patients and 12 normal donors were incubated with VX-680 as indicated doses for 72 hours. The apoptosis was assessed by annexin V assays. Mean percentage of apoptosis was shown (P < .001). (B) VX-680 inhibits autophosphorylation of Aur-A at Thr288 in primary leukemia cells. Cells from patient 11 were incubated with increasing amounts of VX-680 or DMSO for 24 hours. Cell lysates were subject to Western blot analysis with phospho-Aur-A (Thr288) antibody. (C) VX-680 induces cleavage of PARP. Blasts from patient 11 (> 90% blasts) were treated with VX-680 with indicated doses for 72 hours before analysis of PARP cleavage by Western blot. GAPDH levels represent loading controls. (D) Cells from patient 11 (top panel) and normal donor 1 (bottom panel) were incubated for 72 hours with indicated doses of VX-680 before staining with annexin V-FITC and PI. The percentages of apoptotic cells were displayed.
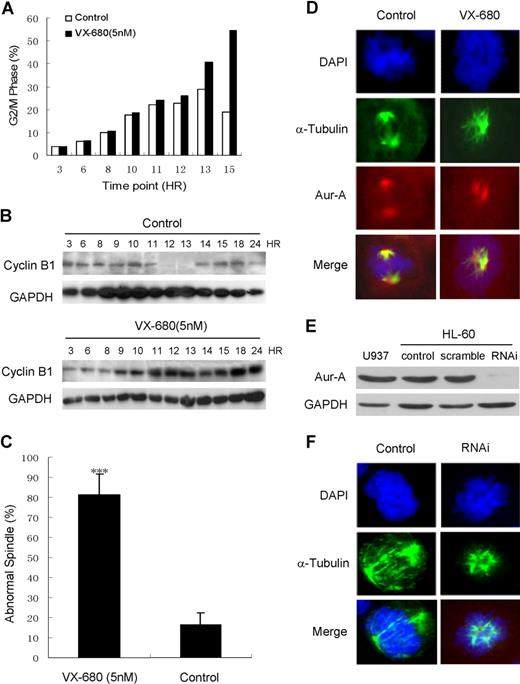
We next suppressed the expression of Aur-A or Aur-B by siRNA in both HL-60 and primary AML cells, as detected by Western blot analysis (Figure S2A,B). Aur-A siRNA-induced apoptosis (45.1% ± 5.4%, 48 hours, Figure S2C) was similar to the degree of cell death seen in VX-680 (5 nM) treated HL-60 cells (52.2% ± 8.6%, Figure 3B). In contrast, a significantly less amount of apoptosis was observed after Aur-B siRNA (23.5% ± 5%, 48 hours, Figure S2C). A detailed apoptosis annexin V analysis performed in primary AML blasts was also shown (Figure S2D). Suppression of Aur-A generated a significant amount of apoptosis (54.7%, 72 hours), similar to that in VX-680-treated AMLs (65.5% ± 19.9%, 50 nM, Figure 2A). Aur-B siRNA-transfected AML cells, however, produced less apoptosis (19.8%, 72 hours). These results indicated that suppression of Aur-A was indeed critical for VX-680-induced apoptosis in AML cells.
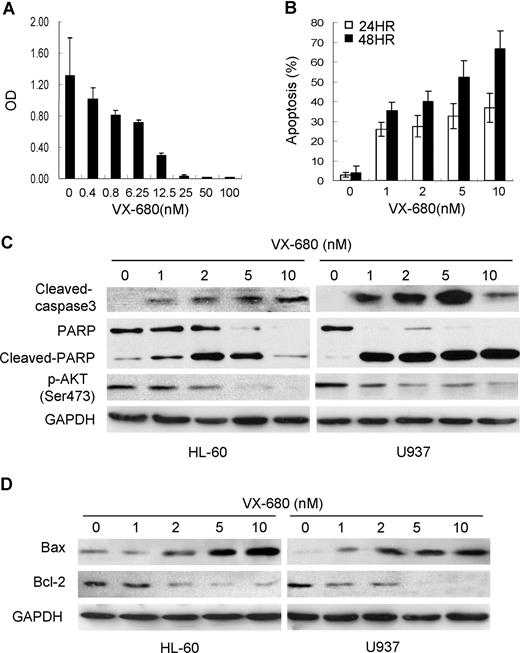
VX-680 induces cellular caspase activation and increases Bax/Bcl-2 ratio in AML cells. (A)VX-680 inhibits cell proliferation in a dose-dependent manner. HL-60 cells were incubated with increasing doses of VX-680 or DMSO for 72 hours. Cell survival rate was measured by MTT assay. (B) VX-680 induces apoptosis in HL-60 cells. Cells were incubated with VX-680 and collected at time points as indicated. The apoptosis was assessed as sub-G1 population by flow cytometry. VX-680 induces HL-60 cells apoptosis in both dose-dependent and time-dependent manners. (C-D) AML HL-60 and U937 cells were treated with increasing concentrations of VX-680. Cells were collected, lysed, and subjected to Western blot analysis with cleaved-caspase3, cleaved-PARP, p-Akt-1(Ser473), Bax, Bcl-2 specific antibodies. GAPDH was used as a loading control. Data shown are representative of 3 independent experiments.
VX-680 induces cellular caspase activation and increases Bax/Bcl-2 ratio in AML cells. (A)VX-680 inhibits cell proliferation in a dose-dependent manner. HL-60 cells were incubated with increasing doses of VX-680 or DMSO for 72 hours. Cell survival rate was measured by MTT assay. (B) VX-680 induces apoptosis in HL-60 cells. Cells were incubated with VX-680 and collected at time points as indicated. The apoptosis was assessed as sub-G1 population by flow cytometry. VX-680 induces HL-60 cells apoptosis in both dose-dependent and time-dependent manners. (C-D) AML HL-60 and U937 cells were treated with increasing concentrations of VX-680. Cells were collected, lysed, and subjected to Western blot analysis with cleaved-caspase3, cleaved-PARP, p-Akt-1(Ser473), Bax, Bcl-2 specific antibodies. GAPDH was used as a loading control. Data shown are representative of 3 independent experiments.
VX-680 preferentially induces apoptosis in Aur-A-high leukemia blasts
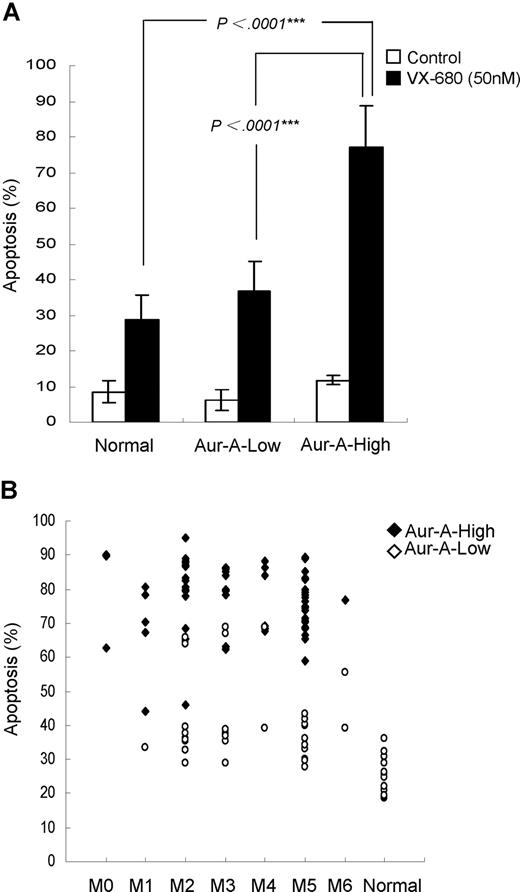
We noticed that a majority of AML blasts were sensitive to VX-680, whereas a certain proportion was not, as the variable drug responses were evident. We next sought to address whether the differential effect of VX-680 on induction of apoptosis in these leukemic blasts was related to Aur-A expression. VX-680 induced a significant high amount of apoptosis (77.1% ± 11.7%, P < .001) in Aur-A-high leukemic blasts (Figure 4A). In contrast, a remarkably smaller amount of apoptosis (36.9% ± 8.3%) was observed in Aur-A-low AMLs, similar to the degree of cell death (28.9% ± 6.7%) in normal BMMCs, where Aur-A levels were also low. However, we did not find a correlation between Aur-B expression and the response to VX-680–induced apoptosis (Figure S3). Thus, VX-680 preferentially targeted AML cells with Aur-A-high expression.
VX-680 preferentially induces apoptosis in Aur-A-high leukemia blasts. (A) Primary leukemic blasts of AML patients were treated with VX-680 (50 nM) for 72 hours. The apoptotic cells were measured by annexin V-FITC staining. The VX-680–induced apoptosis was significantly increased in the Aur-A-high AML cells compared with the normal BMMCs (P < .001) or Aur-A-low AML cells (P < .001). (B) Distribution of the apoptosis in the leukemic blasts induced by VX-680 was indicated according to the FAB type. Normal BMMCs included 12 healthy donors.
VX-680 preferentially induces apoptosis in Aur-A-high leukemia blasts. (A) Primary leukemic blasts of AML patients were treated with VX-680 (50 nM) for 72 hours. The apoptotic cells were measured by annexin V-FITC staining. The VX-680–induced apoptosis was significantly increased in the Aur-A-high AML cells compared with the normal BMMCs (P < .001) or Aur-A-low AML cells (P < .001). (B) Distribution of the apoptosis in the leukemic blasts induced by VX-680 was indicated according to the FAB type. Normal BMMCs included 12 healthy donors.
We then distributed the apoptosis levels of all 98 cases according to FAB type. There was a significant different degree of apoptosis between AML and normal hematopoietic cells (P < .001), but not among the each FAB types of AML (Figure 4B, P > .05). Further analysis revealed that apoptosis was significantly different between Aur-A-high and Aur-A-low expression in the M2 (79.9% ± 12.1% vs 44% ± 14.1%, P < .001), M3 (78.4% ± 8.9% vs 47.8% ± 17.1%, P < .001), and M5 (76.4% ± 8.3% vs 35.6% ± 5.5%, P < .001) but not in M4 (77.4% ± 10% vs 54 ± 21%, P = .061) FAB types. Because of the small number of M0, M1, and M6 cases, we were unable to get the value P by statistics analysis.
Flt3 mutations, but not expression level, in primary AML blasts indicates sensitive to VX-680
Careful analysis of the VX-680 responders detected a few cases of the relatively high degree of apoptosis in low Aur-A expressers. VX-680, a pan-aurora kinase inhibitor, also possesses off-targets, including Flt3, which is highly expressed in 70% to 100% of cases of AML.34 Flt3 expression in AML samples was closely correlated with neither Aur-A (Figure 5A; Table S1) nor with VX-680 responsiveness (Figure 5B). Given that Flt3 activating mutations (ITD and TKD) are also found at a relatively high portion of AMLs,34 we further determined whether these Flt3 mutant subgroups would show preferential sensitive to VX-680. Thirty-one primary AML cells with activating mutations of Flt3 were detected in total 98 cases, including 7 with Flt3/TKD mutations and 27 with Flt3/ITD (3 with both of the 2 mutations; Table S1). In Aur-A-high AML cells, both wild-type and mutant forms of Flt3 were sensitive to VX-680 with no significant difference among groups (P = .600, Figure 5C). Interestingly, among samples with low Aur-A expression, more pronounced apoptosis was observed in cells with Flt3/ITD (52.8% ± 14.8%, P < .01) and Flt3/TKD mutations (66.25% ± 3.5%), compared with Flt3/WT (35% ± 4.1%; Figure 5D). Because of a few cases of Flt3/TKD mutation in Aur-A-low AMLs (4 of 98), we were unable to show the statistical difference. Thus, high-Aur-A expression or low-Aur-A expression with Flt3/ITD mutation was associated with increased sensitivity to VX-680.
Flt3 mutations, but not expression level, in primary AML blasts indicate sensitivity to VX-680. (A) Expression of Aur-A and Flt3 in the representative AML samples. Primary leukemic blasts obtained from AML patients were lysed, and proteins were analyzed by Western blot analysis with antibodies as indicated. GAPDH served as a loading control. (B) Expression of Flt3 does not predict apoptotic response to VX-680 (P > .05). Dot plot of apoptotic response in individual AML samples to VX-680 (50 nM) was grouped by level of Flt3 expression. (C,D) AML cases were further stratified by Flt3 mutation status in the Aur-A-high or Aur-A-low group. Flt3/WT indicates wild-type Flt3; ITD, Flt3 internal tandem duplication; TKD, the activation loop of the tyrosine kinase domain mutations. Error bars represent SEM.
Flt3 mutations, but not expression level, in primary AML blasts indicate sensitivity to VX-680. (A) Expression of Aur-A and Flt3 in the representative AML samples. Primary leukemic blasts obtained from AML patients were lysed, and proteins were analyzed by Western blot analysis with antibodies as indicated. GAPDH served as a loading control. (B) Expression of Flt3 does not predict apoptotic response to VX-680 (P > .05). Dot plot of apoptotic response in individual AML samples to VX-680 (50 nM) was grouped by level of Flt3 expression. (C,D) AML cases were further stratified by Flt3 mutation status in the Aur-A-high or Aur-A-low group. Flt3/WT indicates wild-type Flt3; ITD, Flt3 internal tandem duplication; TKD, the activation loop of the tyrosine kinase domain mutations. Error bars represent SEM.
VX-680 induces cellular caspase activation and increases Bax/Bcl-2 ratio in AML cells
To study the molecular events triggered by VX-680 inhibition, we chose 2 AML cell lines, HL-60 and U937, both expressing high levels of Aur-A (Figure 6E). VX-680 suppressed growth of HL-60 cells in a dose-dependent manner, as assessed by MTT assay (Figure 3A). Similar results were also generated in U937 cells (not shown). Flow cytometric analysis showed that VX-680 led to apoptotic cell death in both dose-dependent and time-dependent manners (Figure 3B). Western blot analysis showed that inhibition of Aurora kinase with VX-680 for 48 hours in both cell lines induced the increasing amounts of cleaved caspase-3 in a dose-dependent manner (Figure 3C). The cleavage of the PARP, a major target for caspases, was also detected in VX-680 treatment. Interestingly, VX-680–induced activation of caspase was correlated with down-regulation of Akt-1 phosphorylation at its activation site Ser473. Moreover, in a dose-dependent manner, treatment of AML cells with VX-680 was associated with increasing amounts of the proapoptotic factor Bax and decreasing levels of the antiapoptotic protein Bcl-2, both regulating apoptotic cell death by mitochondrial-mediated pathway (Figure 3D), indicating that VX-680 may trigger AML cells apoptosis by impairing the functional balance of Bcl-2 proteins in caspase activation pathways.
VX-680 induces G2/M arrest and disturbs mitotic spindle assembly. HL-60 cells were released from double thymidine-induced G1/S block in the presence of 5 nM VX-680 or DMSO. (A) DNA content of cells collected at the indicated time points was assessed by flow cytometric analysis of cells labeled with PI. Percentage of the cell population in G2/M phase was shown. (B) Cyclin B1 expression at these time points was assessed by Western blot analysis. GAPDH was used as a loading control. A representative of 3 independent experiments was shown. (C) Samples were taken during mitosis and stained with anti-α-tubulin antibody. The percentage of distinct spindle structures in mitosis was averaged from 3 independent experiments. At each time point, more than 100 spindle structures were counted (P < .001). (D) The morphology of mitotic spindles and chromosomes was shown by immunofluorescence staining with anti–α-tubulin antibody and anti–Aur-A antibody. Microtubules are stained as green, Aur-A protein as red, and chromosomes as blue. Bars represent 10 μm. (E) Aur-A expression was suppressed by RNAi in HL-60 cells. (F) The morphology of mitotic spindles and chromosomes in siRNA-transfected HL-60 cells was similar to that in VX-680–treated cells. Microtubules are stained as green and chromatosomes as blue. Bars represent 10 μm.
VX-680 induces G2/M arrest and disturbs mitotic spindle assembly. HL-60 cells were released from double thymidine-induced G1/S block in the presence of 5 nM VX-680 or DMSO. (A) DNA content of cells collected at the indicated time points was assessed by flow cytometric analysis of cells labeled with PI. Percentage of the cell population in G2/M phase was shown. (B) Cyclin B1 expression at these time points was assessed by Western blot analysis. GAPDH was used as a loading control. A representative of 3 independent experiments was shown. (C) Samples were taken during mitosis and stained with anti-α-tubulin antibody. The percentage of distinct spindle structures in mitosis was averaged from 3 independent experiments. At each time point, more than 100 spindle structures were counted (P < .001). (D) The morphology of mitotic spindles and chromosomes was shown by immunofluorescence staining with anti–α-tubulin antibody and anti–Aur-A antibody. Microtubules are stained as green, Aur-A protein as red, and chromosomes as blue. Bars represent 10 μm. (E) Aur-A expression was suppressed by RNAi in HL-60 cells. (F) The morphology of mitotic spindles and chromosomes in siRNA-transfected HL-60 cells was similar to that in VX-680–treated cells. Microtubules are stained as green and chromatosomes as blue. Bars represent 10 μm.
VX-680 induces G2/M phase mitotic arrest in AML cells
We then examined the effects of VX-680 on cell-cycle progression in double-thymidine synchronized cells by measuring DNA contents and cyclin B1 expression, which peaks and then drops at metaphase-anaphase transition in mitosis.35 VX-680 treatment resulted in HL-60 cells with higher levels of 4N DNA content 10 to 15 hours after release from a G1/S block compared with control cultures. The percentage of 4N cells were 25.9%, 40.9%, and 54.5%, respectively, in VX-680–treated cells, compared with 22.8%, 28.9%, and 19% in control cells at 12, 13, and 15 hours after G1/S releasing (Figure 6A). Additionally, cyclin B1 was continuing to accumulate to a much late time point in VX-680–treated cells compared with control culture (Figure 6B), suggesting a delay in cell-cycle progression. These data indicated that VX-680 induced G2/M arrest in AML cells, in accordance with recent reports of Aurora inhibition on cell-cycle progression.24,36,–38
The morphology of mitotic spindles and chromosome alignment were examined in HL-60 cells treated with VX-680. The percentage of abnormal spindle was more significant in VX-680 treated cells (81.3% ± 10.2%) than control (18.6% ± 11.1%, P < .001; Figure 6C). The DMSO-treated control cells displayed normal bipolar mitotic spindles with chromosomes properly aligned along the metaphase plate. VX-680 (5 nM) induced the formation of abnormal mitotic spindles, with various chromosome alignment defects (Figure 6D). Similar spindle defects were generated in HL-60 with Aur-A suppressed by RNAi (Figure 6E,F). Formation of abnormal mitotic spindles is consistent with a known Aur-A inhibition phenotype.39,,–42 Therefore, inhibition of Aur-A activity by VX-680 inhibited AML cells from progressing through mitosis properly and results in aberrant mitotic spindle formation.
Effects of combinations of VP16 and VX-680 on induction of apoptosis
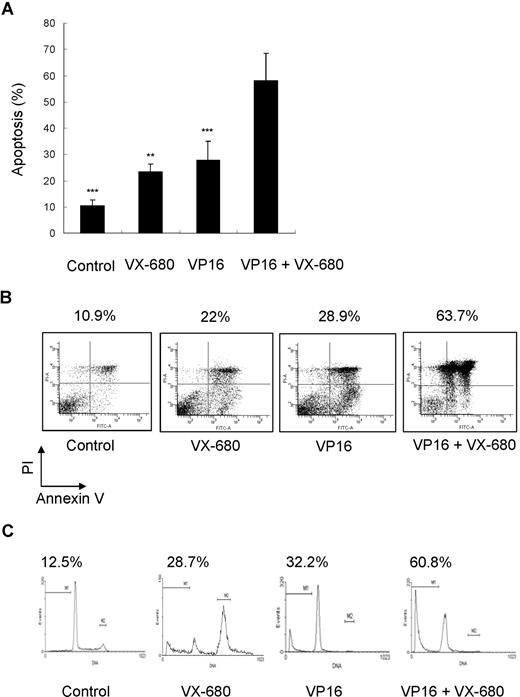
We have demonstrated that VX-680 inhibited Aurora kinase activity in AML cells and preferentially suppressed proliferation and induced apoptosis in primary blasts with high Aur-A expression. VP16 is one of the most active chemotherapeutic agents for the treatment of AML and remains the backbone of induction and consolidation regimens.43 We sought to determine whether the combination of VX-680 with antileukemia chemotherapeutic drugs may potentate single-agent therapy of VP16. Primary blasts were treated with single-agent VX-680 (10 nM), VP16 (10 μM), or combination of VX-680 and VP16. VX-680 and VP16 were capable of inducing apoptosis (23.42% ± 2.92% and 27.78% ± 7.20%, respectively; Figure 7A; Table 2). Combination therapy produced a higher apoptosis (58.10% ± 10.51%) and suggested a synergistic effect of these 2 agents (mean q = 1.299; Figure 7A; Tables 2,3). IC50 for VX-680 ranged from 8.6 nM (patient 18) to 54 nM (patients 3). Five patients (nos. 2, 3, 13, 15, and 23) were low responders for VX-680 (IC50 > 50 nM). The combination of VX-680 with VP16 also produced higher apoptosis in these patients than single-agent treatment (not shown). In addition, experiments were performed to determine any sequence-dependent effect of VP16 followed by VX-680 and did not find a superior synergism between combination and sequential therapy (Figure S4).
Effect of combination of VX-680 and VP16 on apoptosis of AML blasts. (A) Cells from AML blasts were incubated with indicated doses of VX-680, VP16, or a combination of both for 72 hours before staining with annexin V-FITC and PI. The apoptosis was assessed by flow cytometry; Columns, mean; bars, SD. **P < .01; ***P < .001. (B) Cells from patient 12 (71% blasts) were incubated for 72 hours with indicated doses of VX-680 and VP16 before staining with annexin V-FITC. The percentages of apoptotic cells are displayed. (C) Sub-G1 analysis of apoptotic blasts. Blasts from patient 12 were stained with PI. The percentage of apoptotic blasts in sub-G1 (gate M1) is displayed.
Effect of combination of VX-680 and VP16 on apoptosis of AML blasts. (A) Cells from AML blasts were incubated with indicated doses of VX-680, VP16, or a combination of both for 72 hours before staining with annexin V-FITC and PI. The apoptosis was assessed by flow cytometry; Columns, mean; bars, SD. **P < .01; ***P < .001. (B) Cells from patient 12 (71% blasts) were incubated for 72 hours with indicated doses of VX-680 and VP16 before staining with annexin V-FITC. The percentages of apoptotic cells are displayed. (C) Sub-G1 analysis of apoptotic blasts. Blasts from patient 12 were stained with PI. The percentage of apoptotic blasts in sub-G1 (gate M1) is displayed.
Comparison of VX-680 and VP16 apoptosis responses
| Treatment . | Apoptotic responses . |
|---|---|
| Control | 10.52 ± 2.03 |
| VX-680 (10 nM) | 23.42 ± 2.92 |
| VP16 (10 μM) | 27.78 ± 7.20 |
| VP16 + VX-680 | 58.10 ± 10.51 |
| Treatment . | Apoptotic responses . |
|---|---|
| Control | 10.52 ± 2.03 |
| VX-680 (10 nM) | 23.42 ± 2.92 |
| VP16 (10 μM) | 27.78 ± 7.20 |
| VP16 + VX-680 | 58.10 ± 10.51 |
Values are fluorescence intensities (mean ± SD).
Statistical analysis of apoptosis responses to VX-680 and VP16
| Statistical analysis . | P . |
|---|---|
| VP16 more efficient than VX-680 | .598 |
| VP16 + VX-680 more efficient than VX-680 | .000 |
| VP16 + VX-680 more efficient than VP16 | .003 |
| Statistical analysis . | P . |
|---|---|
| VP16 more efficient than VX-680 | .598 |
| VP16 + VX-680 more efficient than VX-680 | .000 |
| VP16 + VX-680 more efficient than VP16 | .003 |
A detailed analysis performed on patient 12 (M5, Aur-A-high expression, Figure 7B). These cells displayed a low spontaneous apoptosis in culture (10.9%). Incubation with VX-680 led to an increased apoptotic response (22%). Similar response was observed in cells treated with VP16 (28.9%). Combination of VP16 with VX-680 had a synergistic effect (63.7%; q = 1.43). Cell-cycle analysis revealed that addition of VX-680 for 72 hours led to 28.7% of cell death (sub-G1 population), similar to 32.2% induced by VP16 (Figure 7C). A combination of these compounds had synergistic effects (60.8%; q = 1.18).
Discussion
In this study, we found that mitotic Aur-A kinase was overexpressed in BMMCs of a significant proportion of de novo AML patients. More importantly, overexpression of Aur-A indicated the sensitivity of AML cells to VX-680–induced apoptosis, thus raising the possibility of stratifying a selected subgroup of AML patients for this novel target-guided treatment. Abnormally high levels of Aurora kinase protein have been observed in various human cancers.16,,,–20 Overexpression of Aur-A not only promotes tumor cell proliferation but also reflects the pathobiologic characteristics of the tumors. Elevated levels of Aur-A are highly correlated with genetic instability, undifferentiated histologic grade, and poor clinical outcome, further reinforcing its tumorigenic potential and role as an oncogene.44,–46 Although overexpression of Aur-A has been shown in many types of solid tumors, we were among the first reporting Aur-A expression and its potential therapeutic implication in human de novo AMLs.
Western blot analysis revealed that Aur-A was overexpressed in a substantial proportion of AML cases (65 of 98, 66.3%), further supported by immunocytochemical staining (Figure 1). Consistently, one recent study also found the aberrant expression of Aurora kinases in several leukemia cell lines as well as primary leukemia cells.47 Aur-A interacts with several key cell- cycle regulators, including p5328,48,49 and BRCA-1.50 Ectopic transfection of Aur-A impairs the regulatory function of these tumor suppressor proteins. Intact p53 serves also an essential role in DNA damage checkpoint, preventing genetic instability. These findings support a potential role of abnormal Aur-A function in overriding the DNA damage checkpoint,38,51 thus resulting in mutational change and chromosomal abnormalities, commonly seen in AML.52
Abnormal expression of several protein markers has been recently emerging as a useful tool directing treatment selection in tumors. For example, expression of Bcr-Abl fusion protein in CML indicates the use of its selective inhibitors, imatinib and dasatinib.48,53 Amplification of ErbB2/Her2/neu points Herceptin/Tratuzumab as the first line regimen against metastatic breast cancer in combination with chemotherapy.54 In this study, we observed that VX-680, a selective Aurora kinase inhibitor, was effective at nanomolar concentrations, inducing apoptosis in both primary leukemia blasts and AML cell lines in a dose-dependent manner (Figures 2,3). Interestingly, we found that the extent of VX-680–induced cell death differed significantly depending on the levels of Aur-A expression (Figure 4); Overexpression of Aur-A was not closely correlated with Aur-B level (Table S1), although Aur-B was also highly expressed in a proportion of AML cells (40 of 98, 40.8%). Accordingly, cells treated with VX-680 showed monopolar spindle defects (Figure 6), typically found in cells with impaired Aur-A activity. In addition, siRNA studies revealed that suppression of Aur-A led to apoptosis in primary AML cells (Figure S2), suggesting that the cytotoxic effect of VX-680 in AMLs was largely the result of inhibition of Aur-A activation.
Apoptosis was markedly increased by VX-680 treatment in leukemia blasts from Aur-A-high AML cases. In contrast, VX-680–induced apoptosis was negligible in either normal or leukemia blasts that express low levels of Aur-A. Thus, VX-680 preferentially induces apoptosis in Aur-A-high AML cells, whereas normal hematopoietic bone marrow cells escape apoptosis, reducing possible drug-related general myelosuppression. VX-680 has been shown to have an inhibitory effect on several other important cellular kinases besides Aurora family members, including mainly Flt3 (Ki = 30 nM), Abl (Ki = 68 nM), and Lck (Ki = 80 nM),24,55 albeit at higher doses. Indeed, VX-680 has significant inhibitory activity against Bcr-Abl bearing the T315I and V299L mutations in CML and Philadelphia-positive ALL cells,26,27,56 which is resistant to current Abl inhibitors, including imatinib and dual Abl/Src inhibitor dasatinib. Flt3 activating ITD and TKD mutations are also frequently found in AMLs (20%-30%) and predict poor prognosis.57,58 We found that expression of Flt3 was not closely associated with Aur-A (Table S1; Figure 4A) and failed to direct VX-680 responsiveness (Figure 5B). Interestingly, although Flt3 mutation status did not predict drug sensitivity in AML cells with high Aur-A expression, cells with Flt3/ITD mutation, however, showed preferential apoptotic response to VX-680 in Aur-A-low group (Figure 5D). These results indicated that a combination analysis of both Aur-A expression and Flt3 activating mutations may distinguish a sensitive subgroup of patients who may benefit from potential VX-680 therapy. Thus, activating Flt3/ITD mutation offered as an additional target for VX-680 in AMLs, particularly in cases of low Aur-A expression.
It is noteworthy to point out that VX-680 appears to have similar cytotoxic action on primary leukemia blasts as topoisomerase poison VP16, currently used as a first line agent for patients with AML. Importantly and in most cases, stronger and synergistic apoptotic responses were obtained when VP16 was combined with the Aurora kinase inhibitor (Figure 7). However, we did not observe a sequential treatment advantage to synergism over combination therapy (Figure S4), suggesting that they may target distinct cytotoxic pathways. Interestingly, new evidence showed sequential Abl kinase inhibitor therapy in CML selected for compound drug-resistant Bcr-Abl mutations, demonstrating potential hazards of sequential kinase inhibitor therapy and suggesting a beneficial effect of combination therapy.27 A similar synergistic effect was also observed recently when short hairpin RNA suppression of Aur-A expression increased chemosensitivity to docetaxel in both in vitro and in vivo models of esophageal squamous cell carcinoma.59 Our results suggested that combining VX-680 with classic chemotherapy could not only improve the therapeutic actions but also lower the toxic effect of AML treatment.
Resistance to chemotherapy-induced apoptosis is a crucial mechanism of drug resistance and disease relapse in AML.60 Analysis of key protein regulators in apoptosis pathway may thus predict the potential outcome of drug treatment. Bcl-2 inhibits apoptosis by forming inactivating heterodimers with Bax, the dominant proapoptotic protein that functions to release cytochrome c from mitochondria.61,62 Overexpression of Bcl-2 has been reported to confer drug resistance in leukemia, whereas high Bax expression is associated with a favorable prognosis. Indeed, the Bax/Bcl-2 ratio is positively correlated with clinical outcome in AMLs.63 Here, we found that VX-680 down-regulated Bcl-2 and up-regulated Bax in a dose-dependent fashion (Figure 3D). The ratio of Bax/Bcl-2, which favors the disease prognosis, was increased by VX-680, thus rendering cells more prone to induction of apoptosis. Recently, Aur-A was found to activate nuclear factor-κB (NF-κB) via mediating phosphorylation of its inhibitory sequester IκB.64 Either VX-680 inhibition or siRNA suppression of Aur-A led to decreased IκB phosphorylation and degradation, thus down-regulating NF-κB. Previous studies showed that NF-κB promoted Bcl-2 expression through the cyclic AMP response element and Sp1 binding sites.65 Thus, it is conceivable that Aur-A may activate Bcl-2 expression via up-regulating NF-κB. On the other hand, Aur-A physically binds and phosphorylates p53 at Ser315, targeting it for murine double minute-2-mediated degradation.49 We recently showed that VX-680 inhibition of Aur-A led to increased expression of p53, rendering cancer cells more sensitive to radiotherapy.66 Bax, one of the major downstream target genes of p53, therefore may be up-regulated in VX-680–treated AMLs via suppression of Aur-A–mediated p53 phosphorylation and degradation. However, p53 may not be functional in some leukemic cell lines. Future study will be needed to address whether these or other hypothesis account for the increased Bax/Bcl-2 ratio in VX-680–induced apoptosis.
Taken together, our results suggested a therapeutic potential of Aurora kinase inhibitor VX-680 in de novo AML cases, either as a single agent or in combination with other first line antitumor drugs, such as VP16. More importantly, leukemia cells with high-Aur-A expression showed greater sensitivity to VX-680, indicating Aur-A as a therapeutic target and surrogate marker to guide the selection of AML patients susceptible to VX-680 treatment.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Xiao-Feng Zhu for critical reading of the manuscript and helpful comments and the other members of the Liu laboratory (Jun-xia Cao, Zi-jie Long, Jine Yao, and Yan Zhao) for invaluable advice and discussion.
This work was supported by grants from Sun Yat-Sen University 985 Fund (84 000-3222101) and Chinese NSF (30772476; Q.L.).
Authorship
Contribution: X.-F.H. designed and performed the research, analyzed data, and wrote the paper; S.-K.L. designed the research; J.X. performed research and analyzed data; J.L. and D.-R.X. analyzed data; L.-H.W. and M.Y. performed research; X.-R.W., X.-B.W, F.-M.Z., and Y.-X.Z. analyzed data; Q.L. designed the research, analyzed data, and wrote the paper; all authors checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Quentin Liu, State Key Laboratory of Oncology in South China, Cancer Center, Sun Yat-Sen University, 651 Dongfeng Road, Guangzhou 510060, People's Republic of China; e-mail: liuq9@mail.sysu.edu.cn and Shao-Kai Luo, Department of Hematology, First Affiliated Hospital, Sun Yat-Sen University, 58 Zhongshan II Road, Guangzhou 510080, People's Republic of China; e-mail: luoshaokai@163.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal