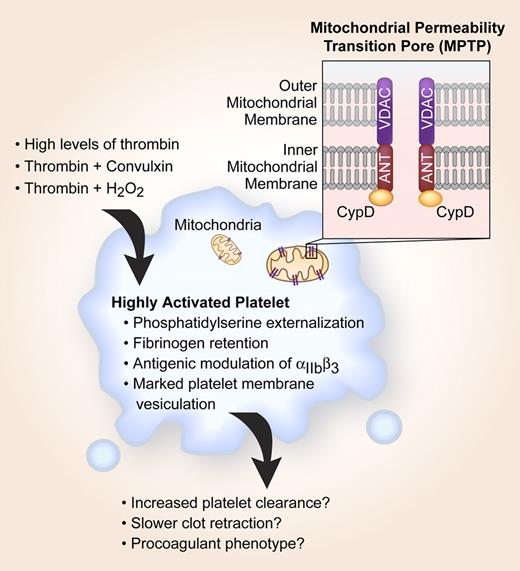
Historical roles for platelet mitochondria have been limited to supplying energy for aggregation and secretion. This view, however, continues to be refined and advanced as new functions for this old organelle emerge. It is now well accepted that mitochondria contribute to apoptosis, a process associated with cell death in nucleated cells and recently ascribed to platelets. Mitochondrial membrane potential (ΔΨm) is reduced in platelets that display apoptotic features,1 and perturbations in the ΔΨm have been observed in subsets of activated platelets that have high procoagulant activity.2 Although a reproducible phenomenon, it is generally surmised that compromises in mitochondrial integrity are the consequence of irreversible platelet activation that culminates in cell death. In the February 1 issue of Blood, Jobe and colleagues shed new light on these preconceived notions by demonstrating that formation of the mitochondrial permeability transition pore (MPTP) regulates early platelet activation events such as phosphatidylserine externalization, fibrinogen retention, and membrane vesiculation (see figure).
CypD-dependent MPTP formation plays a critical role in pushing a subset of moderately activated platelets into a highly activated state. Highly activated platelets are more likely to be cleared from the circulation, retract clots at a slower rate, and possess procoagulant activity.
CypD-dependent MPTP formation plays a critical role in pushing a subset of moderately activated platelets into a highly activated state. Highly activated platelets are more likely to be cleared from the circulation, retract clots at a slower rate, and possess procoagulant activity.
MPTP-dependent platelet activation requires cyclophilin D (CypD), a mitochondrial matrix protein that works together with the voltage-dependent anion channel (VDAC) and the adenine nucleotide translocator (ANT) to modulate ΔΨm (see figure). Although the functional significance of MPTP-dependent platelet activation is not yet known, it appears that MPTP formation pushes a subset of activated platelets past their prime and into a highly activated state. This CypD-dependent transition is regulated by both the quantity and type of agonist(s) used to activate platelets.1,2 In other words, high concentrations of thrombin or combinations of agonists such as thrombin and H2O2 induce the highly activated phenotype.
The fate of highly activated platelets remains undefined, but excessive activation may trigger clearance and thereby contribute to thrombocytopenia, which often occurs in clinical disorders such as sepsis and disseminated intravascular coagulation. High concentrations of thrombin, which are generated in severe sepsis, induce de novo expression of proapoptotic proteins including Bak and Bax,1 and Mason et al3 recently demonstrated that an intrinsic program for apoptosis controls platelet survival and clearance. Thus, pharmacological agents that affect MPTP formation may prove useful in the treatment of diseases that are associated with increased peripheral destruction of apoptotic platelets. Similar interventions may also have value in preventing defects in banked platelets that progressively lose their ΔΨm and become apoptotic during storage.4
The surprising findings of Jobe and colleagues continue to highlight the resourcefulness of platelets and raise several new possibilities regarding the function of mitochondria in these anucleate cytoplasts. As an example, it is not a foregone conclusion that highly activated platelets are immediately destined for cell death. Indeed, the authors demonstrate that CypD-dependent responses in platelets can regulate clot retraction, a prolonged process that may contribute to vessel recanalization in vivo. Mitochondrial-dependent signals may also affect thrombus growth, as suggested by the authors, and variations in the potential to form highly activated platelets may identify subjects at increased thrombotic risk. Other questions remain, including identification of the signaling pathways that link MPTP formation with early platelet activation responses. Regardless of the outcome, it is clear that we are merely on the cusp of discovering new functions for mitochondria in platelets.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal