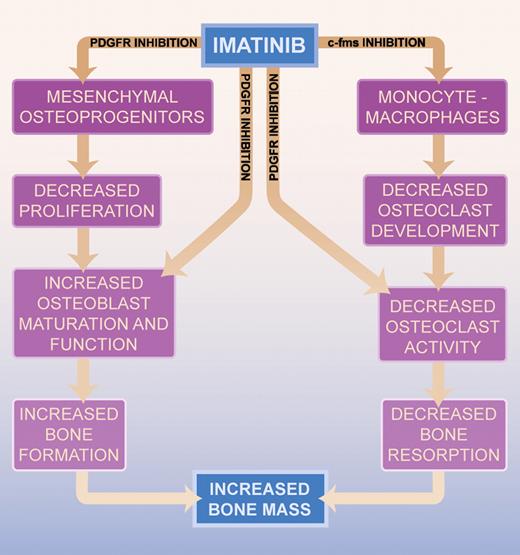
Functional studies presented in this issue of Blood, and previously by the same group1 and others,2 suggest that imatinib inhibits osteoclast maturation and function (via c-fms inhibition and decreased RANK expression) and promotes osteoblast maturation (via platelet-derived growth factor [PDGF] receptor inhibition) from mesenchymal osteoprogenitors—effectively tilting the balance in favor of bone formation. Since PDGF is mitogenic for mesenchymal osteoprogenitors, a reduction in the available pool of osteoblast precursors with imatinib therapy may diminish bone-mass formation even if differentiation is potentiated; however, preliminary findings from Fitter and colleagues suggest that this is not the case. Prospective studies to better characterize the cellular mechanisms and resultant effects of imatinib on the dynamic balance between osteoclast and osteoblast function will require detailed bone histomorphometry from undecalcified bone specimens aligned with results from body imaging, such as bone densitometry and microcomputed tomography of the spine and pelvis.
Fitter and colleagues suggest that emerging long-term safety data justify the study of imatinib for disorders associated with bone loss if bone-mass data are confirmed. Concerns raised with respect to the potential cardiotoxicity of imatinib via c-abl inhibition in cardiomyocytes3 will have to be sufficiently dispelled to justify its long-term use in treating nonneoplastic conditions. Engineered variants of imatinib that retain beneficial activity without cardiac effects4 present an interesting alternative. Further studies of imatinib or its variants alone, in combination with, or in sequence with anticatabolic agents such as raloxifene, bisphosphonates, and denusomab, or anabolic agents such as the parathormone fragment teriparetide, may then follow.
Might imatinib favorably influence neoplastic bone microenvironments that generate significant skeletal morbidity, unlike CML? There are few observations to this effect in the literature; however, in advanced castration-resistant prostate cancer with osteoblastic bone metastases, potent reduction in a marker of bone resorption (urine N-telopeptide) with a stable or increased marker of bone formation (bone-specific alkaline phosphatase) was seen when imatinib was combined with docetaxel.5 These data are in concordance with the inferences from Fitter and colleagues, although no clinical benefit to patients was identified. Tumor resistance may arise from an imatinib-driven tilt in the balance of neoplastic allegiance to osteoblast-generated growth factors rather than from osteoclasts. Studies in pure or dominant-lytic phenotypes of metastatic bone disease may thus be worth exploring; designs that incorporate pharmacodynamic monitoring, bone turnover markers, and skeleton-related endpoints may assist in defining biological subsets that benefit from the addition of imatinib to existing standard therapeutics.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal