Chronic inflammation, as seen in conditions such as rheumatoid arthritis and Crohn disease, is in part driven by discordant production of inflammatory cytokines, such as tumor necrosis factor-α and interleukin-6 (IL-6). Tyrosine kinase activity is essential to lipopolysaccharide-induced cytokine production in monocytes, and previous studies by us and others have implicated a role for the Tec kinase Bruton's tyrosine kinase (Btk) in inflammatory cytokine production. Here we show that knockdown of Btk using RNA interference results in decreased tumor necrosis factor-α, but not IL-6 production. Further investigations into the signaling mechanisms regulating IL-6 production led to the discovery that the Tec kinase bone marrow tyrosine kinase gene in chromosome X (Bmx) regulates Toll-like receptor-induced IL-6 production. Our data further showed that Bmx-dependent super-induction of IL-6 does not involve nuclear factor–κB activity. More detailed investigations of pathways downstream of Bmx signaling revealed that Bmx targets the IL-6 3′ untranslated region to increase mRNA stabilization via a novel, thus far undefined, p38 mitogen activated protein kinase-independent pathway. These data have important implications for the design of therapeutics targeted against specific cytokines and their regulators in inflammatory disease.
Introduction
The discovery of Toll-like receptors (TLR) was a major advance in our understanding of host responses to infection.1 Detection of pathogen-associated molecular patterns, such as lipopolysaccharide (LPS) by TLRs, leads to the activation of immune cells, particularly macrophages and other cells of the myeloid lineage.2 One of the most potent effects of the TLR activation is the induction of inflammatory cytokines, such as tumor necrosis factorα (TNF), interleukin-1 (IL-1), and IL-6.3 The realization that these cytokines play a key role in chronic inflammatory diseases, such as rheumatoid arthritis (RA) and Crohn disease, has focused interest onto the molecular mechanisms that regulate the production of inflammatory cytokines. This has gained particular impetus from the discovery that TLRs may also be involved in regulating/perpetuating chronic inflammatory conditions through the recognition of endogenous ligands produced as a result of tissue injury.4 TLR engagement leads to the activation of numerous signaling pathways regulating cytokine production, including the nuclear factor (NF)–κB family of transcription factors and the mitogen activated protein kinase (MAPK) p38.5,6
Tyrosine phosphorylation is detectable almost immediately after LPS stimulation in murine macrophages,7 and studies using broad range tyrosine kinase inhibitors in human blood monocytes demonstrated that tyrosine phosphorylation is required for TNF, IL-6, and IL-1β production in response to LPS.8,9 Despite this, the involvement of tyrosine kinases in TLR signaling remains unclear. Several protein tyrosine kinases are activated by LPS, including Src family kinases, the focal adhesion kinase Pyk2, and Syk kinase. Previous investigations into the Src kinases led to conflicting conclusions,10,11 whereas others ruled out a role for Pyk2 and Syk in LPS-induced cytokine production.12,13 Recently, studies from our laboratory and others have shown that the Tec kinase Bruton's tyrosine kinase (Btk) is involved in TLR signaling.14,,–17 The Tec family tyrosine kinases are structurally similar to the Src kinases, and 3 of its members are expressed in human monocytes and macrophages: tyrosine kinase expressed in hepatocellular carcinoma (Tec), bone marrow tyrosine kinase gene in chromosome X (Bmx), and Btk. Btk is associated with the human immunodeficiency X-linked agammaglobulinemia (XLA),18,19 and using XLA monocytes, we showed that Btk deficiency leads to reduced TLR-induced TNF and IL-1 production, whereas the production of IL-6 was unaffected.14,17 This was unexpected because IL-6 production is also blocked by tyrosine kinase inhibitors.8,9 Additional studies using overexpression of Btk in wild-type human macrophages showed that Btk regulated TNF mRNA stability through a pathway involving p38 MAPK, rather than transcription.14 In contrast, investigations in transformed cell lines using a dominant negative construct and Btk-deficient murine macrophages suggested that Btk signaled upstream of NF-κB, and presumably TNF transcription rather than p38 MAPK signaling.15,16 Studies by other groups have since shown no change in TNF expression in XLA monocytes,20 defects in XLA dendritic cell signaling via TLR8,21 and impairment of IL-10 expression in murine cells.22 Thus, the involvement of Btk in TLR-induced cytokine production in human and murine cells remains controversial.
To address these discordant findings, we generated Btk-depleted human macrophages using RNA interference (RNAi) knockdown, thereby reducing the chance of compensating changes in other genes, effects of transformation, or variations in the nature of Btk mutations. These studies confirmed our previous findings that Btk contributes to the regulation of TNF production after TLR stimulation, whereas IL-6 production remained unchanged.17 These data further substantiated the differential regulation of TNF and IL-6 after TLR stimulation, which seems to be in stark contrast to early studies using broad-spectrum tyrosine kinase inhibitors in human monocytes showing clearly that LPS-induced IL-6 production depends on tyrosine kinase activity.9 This led us to investigate whether the closely related Tec kinase Bmx might be involved in regulating IL-6 production after stimulation with LPS. Here we show that, unlike its family members Btk and Tec, Bmx is expressed in undifferentiated XLA and control peripheral blood mononuclear cells (PBMCs) and monocytes. These cells produce normal levels of IL-6, and we therefore went on to investigate a possible role for Bmx in TLR-induced cytokine production. This study shows, for the first time, that Bmx is activated in human macrophages stimulated with LPS and that Bmx shows partial overlap with Btk in the regulation of TNF, and a parallel but distinct mechanism regulating IL-6 independently of both p38 MAPK and NF-κB activity in primary human macrophages.
Methods
Reagents
LPS, Pam3Cys-SerLys4 (Pam3C-SK4), Malp-2, and Flagellin were obtained from Alexis Biochemicals (Nottingham, United Kingdom). SB203580 and proteasome inhibitor I (PSI) were obtained from Calbiochem (Nottingham, United Kingdom). All reagents (other than LPS) were tested for the presence of endotoxin using the Limulus amoebocyte assay (BioWhittaker UK, Wokingham, United Kingdom). Optimal dose ranges for the use of TLR ligands in primary macrophages have been assessed previously.14,17
Isolation of monocytes by elutriation and culture of macrophage colony-stimulating factor–derived macrophages
PBMCs were prepared from single donor blood buffy coat fractions using Ficoll-Hypaque centrifugation, and monocytes were then isolated by centrifugal elutriation, as previously described.23 Monocyte fractions of 80% purity or higher were routinely collected and cultured in RPMI containing 10% heat inactivated fetal calf serum at 37°C in a humidified atmosphere containing 5% CO2. For adenoviral infection, monocytes were treated with 100 ng/mL macrophage colony-stimulating factor (M-CSF; Genetics Institute, Cambridge, MA) for 96 hours before infection.
Isolation and culture of PBMCs from XLA patients and control donors
Human blood samples were collected into lithium heparin vacutainers and PBMCs isolated as previously described.14 PBMCs were cultured at a concentration of 106 cells/mL in RPMI containing 100 units/mL penicillin/streptomycin and 10% heat-inactivated fetal calf serum at 37°C in a humidified atmosphere containing 5% CO2. Ethical permission for the study was obtained from the Royal Free Hospital and Medical School research ethics committee, and written informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Generation of adenoviral vectors and cell infection
Recombinant, replication-deficient adenoviral constructs encoding wild-type human Bmx and HA-tagged Bmx (AdBmx and AdBmxHA) were prepared using the AdEasy system as previously described.14,24 pAdTrack-IL-65′ promoter-Luc-3′untranslated region (UTR; AdIL-6 5′/3′) and pAdTrack-IL-6 5′ promoter-Luc (AdIL-6 5′only) were generated as previously described.14 The NF-κBluciferase adenovirus (AdNFκB-luc) contains 4 tandem copies of the κ enhancer element located upstream of the firefly luciferase gene.6 This adenovirus was provided by P. B. McCray Jr (University of Iowa, Iowa City, IA) and is a modification of the pNFκB reporter vector (BD Biosciences UK, Oxford, United Kingdom). M-CSF-derived macrophages were plated in a 96-well plate at 105 cells/well and allowed to express adenoviral transgenes for at least 24 hours before stimulation with LPS as previously described.14 For luciferase assays, cells were subjected to 2 subsequent rounds of infections: first with the WT adenoviral constructs, followed by a 2-hour recovery period in complete medium, and second with luciferase adenoviral constructs 24 hours before stimulation.
RNAi
For targeted protein knockdown using RNAi, 5 × 106 freshly elutriated human monocytes were transfected with targeting siRNA or control oligunucleotides (siControl D-001206-13 and human Btk SMARTpool M-003107-01, Dharmacon, IL) at concentration ranges from 100 to 300 nM using the Human Monocyte Nucleofector Kit (Amaxa Biosystems, Cologne, Germany) according to the manufacturer's instructions. After transfection, monocytes were differentiated in the presence of M-CSF as described in “Isolation of monocytes by elutriation and culture of macrophage colony-stimulating factor–derived macrophages.” Protein knockdown was subsequently assessed by Western blotting.
Enzyme linked immunosorbent sandwich assay
The concentrations of TNF, IL-6, IL-8, and IL-10 were determined by ELISA (BD PharMingen, San Diego, CA) according to the manufacturer's instructions. Absorbance was read and analyzed at 450 nm on a spectrophotometric enzyme-linked immunosorbent sandwich assay (ELISA) plate reader (Labsystems Multiskan Biochromic; Labsytems, Basingstoke, United Kingdom) using the Ascent software program.
Western blotting
M-CSF-derived macrophages were plated in a 12-well plate at 106 and either left untreated or infected with adenovirus constructs as before and allowed to express adenoviral transgenes for 24 hours before stimulation. Whole cell protein extracts were prepared as previously described.14 Samples were resolved by 8% SDS-PAGE. Primary antibodies used to visualize the samples were mouse anti-Btk (BD Biosciences) rabbit anti-Bmx (a gift from Michael G. Tomlinson), rabbit anti-Tec (Upstate Biotechnology, Charlottesville, VA), mouse anti-HA (Covance Research Products, Princeton, NJ), and mouse anti–α-tubulin (Sigma Chemical, Poole, United Kingdom). Secondary antibodies used were sheep antimouse and donkey antirabbit (GE Healthcare, Chalfont St Giles, United Kingdom).
Immunoprecipitation and kinase assay
M-CSF macrophages were plated into 10-cm2 cell culture dishes at a density of 5 × 106 cells/dish and infected with HA-tagged Bmx as described in “Western blotting.” After 24 hours, cells were washed and rested in serum-free medium for 2 hours. After stimulation with LPS, cells were subjected to immunoprecipitation and auto-kinase assay as previously described.14,17 Radiolabeled species were visualized by autoradiography using Hyperfilm (GE Healthcare).
Real-time PCR
M-CSF-derived macrophages were plated in a 24-well plate at 5 × 105 and infected as described in “Generation of adenoviral vectors and cell infection.” Cells were treated with LPS for 4 hours before the addition of actinomycin D (2 μg/mL; Sigma Chemical) and harvested at 0, 15, 30, 60, 90, and 120 minutes. Total RNA was extracted using RNeasy Kit (QIAGEN, Dorking, United Kingdom) according to the manufacturer's instructions. All semi-quantitative RT-PCR was performed as previously described.17 All quantifications were normalized to an endogenous control, the housekeeping gene GAPDH, to account for variability in the initial concentration of RNA and the conversion efficiency of the reverse transcription reaction. The analysis of the relative quantitation required calculations based on the threshold cycle (Ct); the cycle number at which the amplification plot crosses a fixed threshold above baseline is defined as Ct. Relative quantitation was performed using the comparative ΔΔCt method according to the manufacturer's instructions.
Luciferase reporter gene assay
After LPS stimulation, cells were washed once in phosphate-buffered saline and lysed with 100 μL CAT lysis buffer. Cell lysates (50 μL) were transferred into a luminometer cuvette strip and 120 μL luciferase assay buffer and 30 μL luciferin added as described previously.14 Luciferase activity was measured in relative luciferase units (RLU) using the PerkinElmer Wallac MicroBeta Tri Lux luminometer (GMI) machine and software.
Electrophoretic mobility shift assay
Electrophoretic mobility shift assay (EMSA) was performed on nuclear extracts from M-CSF macrophages, which were infected with AdBmx and control virus as described in “Generation of adenoviral vectors and all infection.” Nuclear extracts were prepared as described previously.6 The extract was optimized for uniform quantitative loading of protein (5 μg) using the BCA kit for protein estimation (Pierce Biotechnology; Rockford, IL) and DNA binding activity assessed by EMSA as described previously.6 Gels were dried on chromatography paper (Schleicher & Schuell, London, United Kingdom) and exposed to high performance chemiluminescence film (Hyperfilm ECL, GE Healthcare) at −70°C, and phospho imaged using an AGFA Curix 60 Film Processor (Agfa/Gevaert, Brentford, United Kingdom).
Results
Targeted knockdown of Btk protein using RNAi results in reduced TNF, but not IL-6, production
Given that our previous data from XLA cells had shown a defect in LPS-induced TNF, but not IL-6 production, we investigated whether RNAi knockdown of Btk would produce a similar result. Transfection of human macrophages with siRNA targeting Btk resulted in dose-dependent knockdown of Btk protein compared with controls (Figure 1A). Suppression of Btk expression resulted in a significant decrease in LPS-induced TNF expression, with IL-6 production remaining unchanged (Figure 1B).
Targeted RNAi knockdown of Btk protein results in decreased TNF, not IL-6, production in human macrophages after LPS stimulation. Peripheral blood monocytes were transfected with increasing doses of targeting and control siRNA oligonucleotides and differentiated in the presence of 100 ng/mL of M-CSF in culture for 4 days. (A) The expression of Btk was assessed by Western blotting. Densitometry units (mean ± SEM) for 4 separate donors are shown normalized to untreated controls. (B) For analysis of cytokine expression, siRNA-transfected M-CSF macrophages were treated with LPS (10 ng/mL) for 18 hours and supernatants assessed for TNF and IL-6 levels by ELISA. Values are shown as mean plus or minus SEM for 4 separate donors normalized to LPS only controls (**P< .01). (C) PBMCs were prepared from XLA and normal male donors (age range, 17–46 years) as described in “Isolation and culture of PBMCs from XLA patients and control donors.” Cells were left undifferentiated (XLA ■, normal ●) or cultured in the presence of M-CSF (100 ng/mL) for 4 days (XLA □, normal ○) and then stimulated with LPS (10 ng/mL) or Pam3C-SK4 (100 ng/mL). Cytokine production was assessed by ELISA 18 hours after stimulation. Each data point shown represents a single donor (n.s., not significant). (D) Bmx and Tec protein expression in matched undifferentiated and M-CSF-treated XLA PBMCs, normal PBMCs, and blood monocytes was assessed by Western blotting. Blots are representative of 4 separate donors for each matched cell population. Statistical significance was assessed using one-way ANOVA and Bonferroni multiple comparison test.
Targeted RNAi knockdown of Btk protein results in decreased TNF, not IL-6, production in human macrophages after LPS stimulation. Peripheral blood monocytes were transfected with increasing doses of targeting and control siRNA oligonucleotides and differentiated in the presence of 100 ng/mL of M-CSF in culture for 4 days. (A) The expression of Btk was assessed by Western blotting. Densitometry units (mean ± SEM) for 4 separate donors are shown normalized to untreated controls. (B) For analysis of cytokine expression, siRNA-transfected M-CSF macrophages were treated with LPS (10 ng/mL) for 18 hours and supernatants assessed for TNF and IL-6 levels by ELISA. Values are shown as mean plus or minus SEM for 4 separate donors normalized to LPS only controls (**P< .01). (C) PBMCs were prepared from XLA and normal male donors (age range, 17–46 years) as described in “Isolation and culture of PBMCs from XLA patients and control donors.” Cells were left undifferentiated (XLA ■, normal ●) or cultured in the presence of M-CSF (100 ng/mL) for 4 days (XLA □, normal ○) and then stimulated with LPS (10 ng/mL) or Pam3C-SK4 (100 ng/mL). Cytokine production was assessed by ELISA 18 hours after stimulation. Each data point shown represents a single donor (n.s., not significant). (D) Bmx and Tec protein expression in matched undifferentiated and M-CSF-treated XLA PBMCs, normal PBMCs, and blood monocytes was assessed by Western blotting. Blots are representative of 4 separate donors for each matched cell population. Statistical significance was assessed using one-way ANOVA and Bonferroni multiple comparison test.
Bmx protein expression and IL-6 production are independent of M-CSF differentiation
Previous studies showed that Btk and Tec expression levels were low in undifferentiated human PBMCs and monocytes but up-regulated with M-CSF differentiation.14 We therefore investigated whether TLR-induced IL-6 production differed between undifferentiated and M-CSF-treated XLA PBMCs. Studies examining Pam3C-SK4- or LPS-induced IL-6 production in XLA and control PBMCs showed no changes after M-CSF differentiation (Figure 1C). Parallel studies using normal monocytes, PBMCs, and XLA PBMCs showed a constant expression of Bmx in all 3 cell populations (Figure 1D). These findings indicate that Tec is not involved in TLR-induced IL-6 production in undifferentiated cells.
LPS activates Bmx kinase activity in human M-CSF macrophages
Given the correlation between the constant expression of Bmx with the unaffected production of IL-6, we questioned whether there could be a causal link between the two. We used adenoviruses encoding wild-type Bmx (AdBmx) and HA-tagged Bmx (AdBmx HA) to infect human macrophages at increasing multiplicity of infection. HA-tagged Bmx protein levels increased dose-dependently from 50:1 to 150:1 multiplicity of infection in macrophages, with no increase in Bmx expression observable in Ad0-infected controls (Figure 2A). Similar data were obtained for untagged Bmx (data not shown). In the absence of precipitating antibodies to Bmx, we used HA-tagged Bmx to ascertain changes in kinase activity in response to LPS stimulation in human macrophages. As demonstrated in Figure 2B, Bmx auto-kinase activity was increased within 5 minutes in the presence of LPS. Densitometric analysis pooled for 3 separate donors showed a rapid increase in Bmx auto-kinase activity within 5 minutes of LPS stimulation, which returned to just above base level at 10 minutes after stimulation (Figure 2C).
LPS stimulation induces Bmx kinase activity in primary human macrophages. PBMCs were differentiated into macrophages in the presence of 100 ng/mL of M-CSF for 4 days. Cells were infected for 2 hours with adenoviruses overexpressing HA-tagged wild-type Bmx or control adenovirus Ad0 at a multiplicity of infection of 100:1 in serum-free medium. (A) Expression of HA-Bmx at different multiplicities of infection was assessed by Western blotting. (B) For auto-kinase assay, cells were cultured in complete medium and treated with 10 ng/mL of LPS for 0, 5, 10 and 20 minutes. Cells were lysed and HA-Bmx immunoprecipitated from lysates as described in “Immunoprecipitation and kinase assay” and subjected to in vitro kinase assay. (C) Densitometry units (mean ± SEM) pooled for 3 separate donors are shown normalized to untreated controls. Statistical significance was assessed using one-way ANOVA and Bonferroni multiple comparisons test (**P< .01).
LPS stimulation induces Bmx kinase activity in primary human macrophages. PBMCs were differentiated into macrophages in the presence of 100 ng/mL of M-CSF for 4 days. Cells were infected for 2 hours with adenoviruses overexpressing HA-tagged wild-type Bmx or control adenovirus Ad0 at a multiplicity of infection of 100:1 in serum-free medium. (A) Expression of HA-Bmx at different multiplicities of infection was assessed by Western blotting. (B) For auto-kinase assay, cells were cultured in complete medium and treated with 10 ng/mL of LPS for 0, 5, 10 and 20 minutes. Cells were lysed and HA-Bmx immunoprecipitated from lysates as described in “Immunoprecipitation and kinase assay” and subjected to in vitro kinase assay. (C) Densitometry units (mean ± SEM) pooled for 3 separate donors are shown normalized to untreated controls. Statistical significance was assessed using one-way ANOVA and Bonferroni multiple comparisons test (**P< .01).
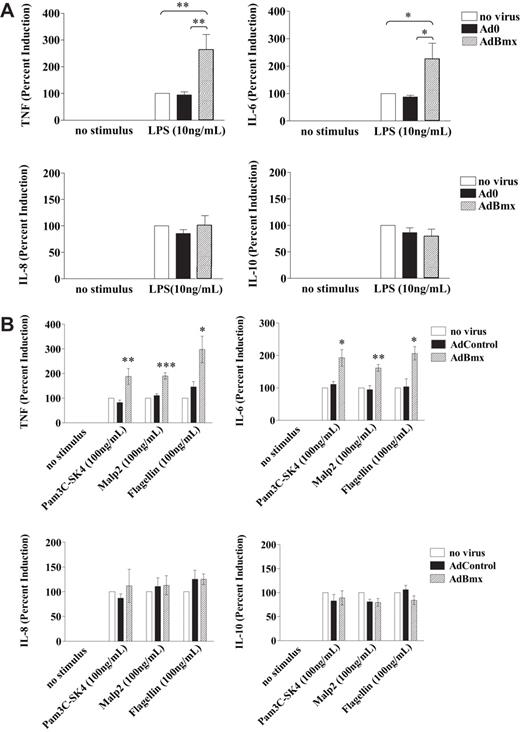
Overexpression of Bmx enhances TLR-induced TNF and IL-6, but not IL-8 or IL-10 production
Infection of macrophages with wild-type AdBmx resulted in a 2- to 3-fold increase in levels of TNF in response to LPS stimulation compared with uninfected and control infected cells (Figure 3A). Wild-type Bmx overexpression also increased IL-6 production by 2- to 3-fold but did not affect the production of IL-8 and IL-10 in response to LPS stimulation (Figure 3A). Similarly, Bmx overexpression also resulted in increased TNF and IL-6, but unchanged IL-8 and IL-10, production in macrophages stimulated with TLR ligands Pam3C-SK4 (TLR1/2), Malp-2 (TLR2/6), and Flagellin (TLR5; Figure 3B).
Bmx regulates TLR-induced TNF and IL-6 production. Macrophages were infected with AdBmx or Ad0 as before. Cells were cultured in complete medium and stimulated with LPS (10 ng/mL), Pam3C-SK4 (100 ng/mL), Malp2 (100 ng/mL), or Flagellin (100 ng/mL) for 18 hours. Cytokine expression in supernatants was assessed by ELISA. (A) LPS-induced TNF, IL-6, IL-8, and IL-10 production was assessed in uninfected, Ad0-infected, and AdBmx-infected macrophages. Values (mean ± SEM) for 4 separate donors normalized to LPS-treated controls are shown. (B) Cytokine production after Pam3C-SK4, Malp2, and Flagellin stimulation was assessed by ELISA. Values (mean ± SEM) for 4 separate donors normalized to Pam3C-SK4- and Malp-2-treated controls are shown. Statistical significance was assessed using Student ttest (*P< .05; **P< .01; ***P< .001).
Bmx regulates TLR-induced TNF and IL-6 production. Macrophages were infected with AdBmx or Ad0 as before. Cells were cultured in complete medium and stimulated with LPS (10 ng/mL), Pam3C-SK4 (100 ng/mL), Malp2 (100 ng/mL), or Flagellin (100 ng/mL) for 18 hours. Cytokine expression in supernatants was assessed by ELISA. (A) LPS-induced TNF, IL-6, IL-8, and IL-10 production was assessed in uninfected, Ad0-infected, and AdBmx-infected macrophages. Values (mean ± SEM) for 4 separate donors normalized to LPS-treated controls are shown. (B) Cytokine production after Pam3C-SK4, Malp2, and Flagellin stimulation was assessed by ELISA. Values (mean ± SEM) for 4 separate donors normalized to Pam3C-SK4- and Malp-2-treated controls are shown. Statistical significance was assessed using Student ttest (*P< .05; **P< .01; ***P< .001).
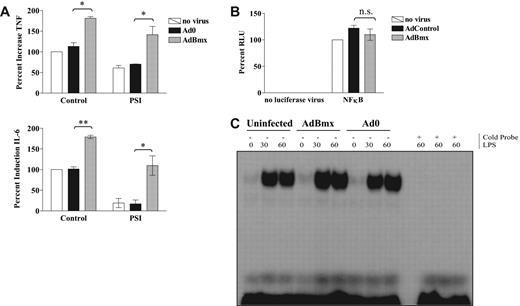
Bmx-dependent IL-6 super-induction does not involve NF-κB activity
LPS-induced production of IL-6 in macrophages depends on activation of the NF-κB,25 and treatment with PSI decreased LPS-induced TNF and IL-6 production by 40% and 80%, respectively, but had no effect on the enhancement induced by AdBmx (Figure 4A). Our hypothesis that Bmx regulates IL-6 production independently of NF-κB activation was further supported by studies showing that overexpression of Bmx had no effect on the LPS response of an NF-κB–driven luciferase reporter gene construct (Figure 4B). Additional studies by EMSA showed that overexpression of Bmx did not increase LPS-induced binding of the NF-κBconsensus oligonucleotide (Figure 4C).
Bmx does not signal upstream of NF-κB. Macrophages were infected for 2 hours with adenoviruses overexpressing wild-type Bmx or control adenovirus Ad0 in serum-free medium. For luciferase assays, cells were subjected to a second round of infections with NF-κB consensus luciferase adenovirus (AdNFκB-luc) before stimulation. Cells were stimulated with 10 ng/mL LPS for 18 hours for ELISA, 4 hours for luciferase reporter gene assays, and 0.5 and 1 hour for EMSA. (A) Uninfected, Ad0-infected, and AdBmx-infected macrophages were left untreated or preincubated with PSI (1 μM) and TNF and IL-6 production assessed by ELISA. Values (mean ± SEM) are presented as percent compared with LPS-treated uninfected controls (100%; *P< .05; **P< .01). (B) Relative luciferase units (RLU) for uninfected, Ad0-infected, and AdBmx-infected cells in the absence (left hand panel) or presence (right hand panel) of AdNFκB-luc after 4 hours of LPS stimulation are shown as mean (± SEM) for 4 separate donors normalized to LPS only controls. n.s, not significant. (C) Nuclear extracts were collected as described in “Electrophoretic mobility shift assays” and assayed for NF-κB DNA binding by EMSA. Statistical significance was assessed by Student ttest.
Bmx does not signal upstream of NF-κB. Macrophages were infected for 2 hours with adenoviruses overexpressing wild-type Bmx or control adenovirus Ad0 in serum-free medium. For luciferase assays, cells were subjected to a second round of infections with NF-κB consensus luciferase adenovirus (AdNFκB-luc) before stimulation. Cells were stimulated with 10 ng/mL LPS for 18 hours for ELISA, 4 hours for luciferase reporter gene assays, and 0.5 and 1 hour for EMSA. (A) Uninfected, Ad0-infected, and AdBmx-infected macrophages were left untreated or preincubated with PSI (1 μM) and TNF and IL-6 production assessed by ELISA. Values (mean ± SEM) are presented as percent compared with LPS-treated uninfected controls (100%; *P< .05; **P< .01). (B) Relative luciferase units (RLU) for uninfected, Ad0-infected, and AdBmx-infected cells in the absence (left hand panel) or presence (right hand panel) of AdNFκB-luc after 4 hours of LPS stimulation are shown as mean (± SEM) for 4 separate donors normalized to LPS only controls. n.s, not significant. (C) Nuclear extracts were collected as described in “Electrophoretic mobility shift assays” and assayed for NF-κB DNA binding by EMSA. Statistical significance was assessed by Student ttest.
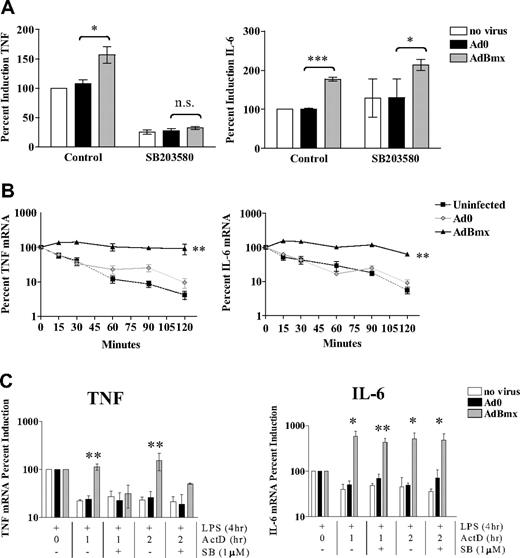
Bmx induces TNF and IL-6 mRNA stabilization via 2 separate pathways
LPS-induced TNF expression is regulated posttranscriptionally via mRNA stabilization, a process that involves the MAPK p38 and targeting of the 3′UTR of the TNFgene.26,,–29 Therefore, we investigated possible involvement of p38 MAPK downstream of Bmx signaling. The p38 MAPK inhibitor SB203580 ablated the enhancing effect of AdBmx on LPS-induced TNF production (Figure 5A). In contrast, SB203580 did not affect IL-6 production in any circumstance (Figure 5A). Studies in RAW 264.7 murine macrophages have suggested a role for the MAPK c-Jun N-terminal kinase (JNK) in translational regulation of LPS-induced TNF production.30 We thus investigated whether JNK and the other MAP kinase, extracellular signal-related protein kinase (ERK), may have a role in LPS-induced IL-6 production. However, neither the ERK inhibitor PD9805931 nor the JNK inhibitor SP60012532 had any effect on LPS induced IL-6 production (data not shown), suggesting that, in contrast to TNF, ERK, and JNK MAPK are not involved in translational regulation of IL-6. We subsequently investigated the effect of Bmx overexpression on TNF and IL-6 mRNA stability using actinomycin D chase studies. Bmx overexpression increased TNF mRNA stability compared with controls (Figure 5B). However, the Bmx effect was not restricted to TNF because there was also stabilization of IL-6 mRNA. Again, the role of p38 MAPK in this process was investigated using SB203580, which abrogated Bmx-induced TNF mRNA stabilization, but did not alter Bmx-induced IL-6 mRNA stabilization after treatment with actinomycin D (Figure 5C).
Bmx increases TNF and IL-6 mRNA stabilization via 2 distinct downstream pathways. (A) Macrophages were left uninfected or infected with Ad0 and AdBmx as before and left untreated or preincubated with SB203580 (1 μM). TNF and IL-6 production was assessed by ELISA. Values (mean ± SEM) are presented as percent compared with LPS-treated uninfected controls (100%). Statistical significance was assessed using Student ttest. n.s., not significant (*P< .05; ***P< .001). (B) Macrophages were left uninfected or infected with Ad0 and AdBmx as before. Cells were stimulated with LPS (10 ng/mL) for 4 hours before addition of actinomycin D (2 μg/mL). TNF and IL-6 mRNA levels at 0, 15, 30, 60, 90, and 120 minutes after addition of actinomycin D were assessed by real-time PCR. Values (mean ± SEM) for 4 separate donors are shown normalized to time 0 of actinomycin D addition (100%) (**P< .01). (C) Macrophages were left uninfected or infected with Ad0 and AdBmx as before and left untreated or preincubated with SB203580 (1 μM). TNF and IL-6 mRNA levels at 1 and 2 hours after addition of actinomycin D in the presence or absence of SB203580 (1 μM) were assessed by real-time PCR. Values (mean ± SEM) for 4 separate donors are shown normalized to time 0 of actinomycin D addition (100%). Statistical significance was assessed by one-way ANOVA and Bonferroni multiple comparison test (*P< .05; **P< .01).
Bmx increases TNF and IL-6 mRNA stabilization via 2 distinct downstream pathways. (A) Macrophages were left uninfected or infected with Ad0 and AdBmx as before and left untreated or preincubated with SB203580 (1 μM). TNF and IL-6 production was assessed by ELISA. Values (mean ± SEM) are presented as percent compared with LPS-treated uninfected controls (100%). Statistical significance was assessed using Student ttest. n.s., not significant (*P< .05; ***P< .001). (B) Macrophages were left uninfected or infected with Ad0 and AdBmx as before. Cells were stimulated with LPS (10 ng/mL) for 4 hours before addition of actinomycin D (2 μg/mL). TNF and IL-6 mRNA levels at 0, 15, 30, 60, 90, and 120 minutes after addition of actinomycin D were assessed by real-time PCR. Values (mean ± SEM) for 4 separate donors are shown normalized to time 0 of actinomycin D addition (100%) (**P< .01). (C) Macrophages were left uninfected or infected with Ad0 and AdBmx as before and left untreated or preincubated with SB203580 (1 μM). TNF and IL-6 mRNA levels at 1 and 2 hours after addition of actinomycin D in the presence or absence of SB203580 (1 μM) were assessed by real-time PCR. Values (mean ± SEM) for 4 separate donors are shown normalized to time 0 of actinomycin D addition (100%). Statistical significance was assessed by one-way ANOVA and Bonferroni multiple comparison test (*P< .05; **P< .01).
Increased Bmx levels regulate mRNA stability of TNF and IL-6 through the 3′UTR
To examine the mechanism of this mRNA stabilization, we used reporter gene constructs for TNFand IL-6 that contained the 5′ promoter with and without the 3′UTRs as described previously14 (Figure 6A). Bmx overexpression only increased reporter gene activity of the reporter constructs that contained the 3′UTR (Figure 6B).
Bmx targets the 3′UTRs of TNF and IL-6 after LPS stimulation. (A) Schematic representation of the human IL-6 5′ promoter-luciferase-3′UTR (AdIL-6 5′/3′) and IL-6 5′ promoter-luciferase (AdIL-6 5′only) adenoviral constructs. (B) Macrophages were left uninfected or infected with Ad0 and AdBmx before a second round of infections with AdTNF 5′/3′, AdTNF5′, AdIL-6 5′/3′, or AdIL-6 5′ luciferase reporter gene constructs and stimulated with LPS (10 ng/mL) for 4 hours. Relative luciferase units (RLU) for uninfected, Ad0-infected, and AdBmx-infected cells in the absence or presence of TNF reporter viruses (top panel) or IL-6 reporter viruses (bottom panel) after 4 hours of LPS stimulation are shown as mean plus or minus SEM normalized to controls for 4 separate donors. Statistical significance was assessed by Student ttest (*P< .05; **P< .01).
Bmx targets the 3′UTRs of TNF and IL-6 after LPS stimulation. (A) Schematic representation of the human IL-6 5′ promoter-luciferase-3′UTR (AdIL-6 5′/3′) and IL-6 5′ promoter-luciferase (AdIL-6 5′only) adenoviral constructs. (B) Macrophages were left uninfected or infected with Ad0 and AdBmx before a second round of infections with AdTNF 5′/3′, AdTNF5′, AdIL-6 5′/3′, or AdIL-6 5′ luciferase reporter gene constructs and stimulated with LPS (10 ng/mL) for 4 hours. Relative luciferase units (RLU) for uninfected, Ad0-infected, and AdBmx-infected cells in the absence or presence of TNF reporter viruses (top panel) or IL-6 reporter viruses (bottom panel) after 4 hours of LPS stimulation are shown as mean plus or minus SEM normalized to controls for 4 separate donors. Statistical significance was assessed by Student ttest (*P< .05; **P< .01).
Discussion
This study has produced several key observations: We have confirmed that Btk regulates TLR-induced TNF, but not IL-6 production, in primary human macrophages after LPS stimulation. Subsequently, we have identified a novel role for Bmx in the regulation of TLR4-induced inflammatory cytokine production. Further investigations into the mechanisms involved show, for the first time, that Bmx overexpression results in increased TNF mRNA stabilization via a p38 MAPK-dependent mechanisms targeting the 3′UTR of the TNFgene. Moreover, our data clearly indicate a novel, thus far undefined, mechanism by which Bmx induces increased IL-6 production through targeting of the 3′UTR and mRNA stabilization. Crucially, increased IL-6 expression downstream of Bmx does not involve changes in NF-κB activity or require activation of the p38 MAPK signaling pathway.
Our observations using targeted knockdown of Btk through RNAi in human macrophages showing decreased TNF, but not IL-6, production in response to LPS further validated our previous findings obtained using both adenoviral overexpression techniques and Btk-deficient XLA cells,14 and have the advantage of reducing potential developmental compensation between Tec kinases. Recent studies have questioned a role for Btk in TLR-induced TNF production and p38 MAPK activation in human XLA cells. Studies by Perez de Diego et al showed that the proportion of cells expressing phosphorylated p38 MAPK and intracellular TNF and IL-6 as assessed by fluorescence-activated cell sorter analyses did not differ between XLA and controls.20 However, this is not in conflict with our previous findings because XLA cells express reduced levels of TNF and also show low levels of p38 MAPK phosphorylation in response to LPS,14,17 indicating no change in the proportion of cells producing TNF downstream of activated p38 MAPK, but a change in quantity of TNF production from the cell population. Sochorova et al showed differential levels of TLR-induced TNF and IL-6 production by XLA-derived dendritic cells compared with controls.21 This discrepancy could be the result of differences in TLR signaling between different cell types. We have previously shown that only undifferentiated XLA PBMCs show impaired TNF responses after TLR stimulation.14 It is therefore possible that, like M-CSF differentiated XLA PBMCs, XLA dendritic cells are able to compensate for the lack of functional Btk by increased expression of related kinases.
Contrasting to its family members Btk and Tec, Bmx protein expression levels were not altered by M-CSF–driven differentiation. In addition, we have shown here, for the first time, that Bmx is activated by LPS stimulation in human macrophages and that overexpression of this kinase leads to increased TNF and IL-6 production. This indicates overlapping roles for Btk and Bmx in the regulation of TNF, and a unique role for Bmx in the regulation of IL-6 in human macrophages. We have previously shown that undifferentiated and M-CSF-differentiated XLA PBMC produce normal levels of IL-6 after TLR4 engagement,17 which can now be explained by the presence of Bmx in these cells. The fact that Bmx may also have an impact on TNF expression would explain why, even in XLA cells, there is still some cytokine production. These findings also explain why Btk siRNA knockdown does not result in a dose-dependent decrease in TNF production, as Bmx could account for some of the TNF production observed in response to LPS.
Our findings for Bmx are important for our current understanding of the inflammatory response because they highlight distinct differences in the regulation of TNF and IL-6 in response to TLR stimulation. Although LPS-induced IL-6 production is sensitive to inhibition of NF-κB activation, signaling downstream of Bmx leading to increased IL-6 production does not involve changes in NF-κB activity, as assessed by inhibitor studies, luciferase reporter gene assays, and EMSA. These findings are supported by the fact that we did not observe any involvement of Btk signaling in NF-κB signaling in primary human macrophages,14 suggesting that these closely related kinases both signal independently of NF-κB in this model system. The apparent lack of involvement of NF-κB downstream is further supported by our studies using the 5′ IL-6 promoter, which contains the IL-6 NF-κBsite and also showed no response to Bmx overexpression. However, we cannot exclude any possible effects of Bmx on IL-6transcription, such as the phosphorylation of histones; this is the subject of future investigations. Differing reports regarding the role of Btk signaling in NF-κB activation in response to TLR engagement have been published in the literature and need to be analyzed in the context of the model systems used. Studies implicating a role for Btk in the regulation of NF-κB, and not p38 MAPK, activation were conducted in nonmyeloid cell lines15 and murine models,16,22 and conflicting findings might thus be the result of differences between species, as well as myeloid and nonmyeloid cell systems. In addition, studies conducted using high concentrations of LFM-A13 as Btk inhibitor need to be reviewed in light of recent findings that this inhibitor is not specific for Btk33,34 and was shown to be toxic at high concentrations in our hands.
Concurring with previous findings by us and others showing various degrees of compensation between Tec kinases,14,35,36 we have now shown that Bmx shows overlapping functions with Btk in targeting the 3′UTR of TNF to increase mRNA stabilization in a p38 MAPK-dependent manner. LPS-induced IL-6 production in human macrophages is not regulated by any of the MAP kinases, as shown by inhibitor studies. Unexpectedly, further investigations into the mechanisms involved in Bmx-dependent regulation of IL-6 revealed that Bmx targets the 3′UTR of the IL-6 gene to increase mRNA stabilization via an as yet undefined, p38 MAPK-independent pathway. It would have been satisfying to support our data from Bmx overexpression with studies on Bmx-deficient cells. However, no human phenotype equivalent to XLA is known for Bmx, and knockdown of Bmx expression in macrophages using siRNA oligonucleotides has thus far not been possible. We have achieved knockdown of Bmx mRNA, but not protein, which might indicate that the half-life of Bmx is too long for RNAi to have any effect on constitutive protein levels. Furthermore, we have shown that the Bmx kinase domain is required for LPS-induced super-induction of TNF and IL-6 using adenoviruses expressing kinase dead (K445E) and kinase deleted (Δ417–675) versions of Bmx (Figure S2, available on the Bloodwebsite; see the Supplemental Materials link at the top of the online article). However, these mutants do not act as dominant negatives, as has been observed by others.37,38
An increasing body of evidence from the laboratory and the clinic indicates that different inflammatory pathologies involve different cytokines and growth factors and that therapies need to be closely tailored toward any particular type of pathologic inflammation. We have shown in this study that 2 major pro-inflammatory cytokines, TNF and IL-6, are differentially regulated by Bmx through downstream targeting of parallel, but distinct, pathways leading to increased mRNA stability of both cytokines. With the clinical focus in anticytokine therapies shifting toward a broader range of targets for different pathologies, it is important to understand which precise mechanisms are involved in the production of different cytokines, and how this might differ depending on cell type and trigger, as this might vary depending on the disease and affected tissues.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Bmx WT, Bmx HA, and Bmx kinase dead (KD) constructs and anti-Bmx antibody were generously provided by Dr Michael Tomlinson (Division of Medical Sciences, The Medical School, University of Birmingham, Birmingham, United Kingdom). Dr David Webster led the collaboration with the Royal Free Medical School, Department of Immunology. XLA blood samples were collected by Dr Lynett Danks. Volunteer venesectionists and control blood donors were employees at the Kennedy Institute of Rheumatology. Dr Andy Clark and Dr Jeremy Turner provided critical feedback.
This work was supported by grants from the Medical Research Council United Kingdom, Arthritis Research Campaign United Kingdom, and Kennedy Institute of Rheumatology Trustees.
Authorship
Contribution: C.D.P. designed and performed research, analyzed data, and wrote the manuscript; B.E.M. and J.P.M. helped with performing research; S.W. provided XLA blood samples; N.J.H. and B.M.J.F. designed research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Brian M. J. Foxwell, Kennedy Institute of Rheumatology, Imperial College London, 1 Aspenlea Road, London, W6 8LH, United Kingdom; e-mail: b.foxwell@imperial.ac.uk.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal