Fanconi anemia (FA) is an inherited bone marrow failure syndrome characterized by a variable number of developmental abnormalities, genomic instability, and an increased predisposition to malignancy.1 It is genetically heterogeneous, with 13 subtypes/complementation groups (FA-A, FA-B, FA-C, FA-D1, FA-D2, FA-E, FA-F, FA-G, FA-I, FA-J, FA-L, FA-M, and FA-N) currently recognized. The genes responsible for these subtypes (FANCA, FANCB, FANCC, FANCD1, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCJ, FANCL, FANCM, and FANCN, respectively) have all been identified.
Studies from several research groups over the last 15 years suggest that the proteins encoded by the FA genes are important in DNA repair and therefore in the maintenance of genomic stability.2 However, the precise role of these proteins in the repair of DNA lesions still needs to be fully defined. The molecular mechanism underlying bone marrow failure, the major cause of premature mortality in FA, remains equally unclear.
It has been previously observed that patients with FA3 as well as patients with idiopathic aplastic anemia4 have raised levels of tumor necrosis factor-α (TNF-α) and γ-interferon (γ-IFN). TNF-α is a cytokine with important biological roles in hematopoiesis and apoptosis.5 Soluble mature TNF-α is released from cells by the TNF-α–converting enzyme (TACE) or by the matrix metalloproteinase 7 (MMP-7). The binding of TNF-α to its receptors results in activation of both the mitogen-activated protein kinase (MAPK) stress signaling cascade and the NF-κB transcription factor. Activation of these 2 pathways plays an important role in the recruitment of many other effector molecules, including TNF-α. TNF-α has been shown to have inhibitory effects on normal hematopoietic progenitors, and it has been observed that raised levels of TNF-α are found in several hematopoietic disorders, including idiopathic aplastic anemia and FA. These observations suggest that TNF-α could be a key player in the pathophysiology of bone marrow failure (and other abnormalities) in FA.
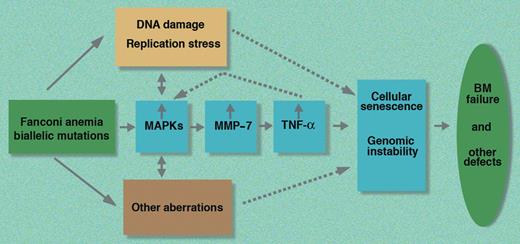
In this issue of Blood, Briot and colleagues provide new and exciting data regarding the origin of TNF-α overproduction in FA. Their studies show that biallelic FA mutations result in aberrant activation of 2 major stress-signaling pathways: NF-κB and MAPK. Using both molecular and pharmacological inhibitors, they show that the ERK pathway, 1 of the 3 major players within the MAPK pathway, is activated and is responsible for the elevated expression of MMP-7 in FA cells. This, in turn, leads to a high rate of TNF-α shedding from the cytoplasmic membrane. Conversely, they show that MMP-7 inhibition normalizes TNF-α levels.
These new data therefore suggest that one of the important aberrations in FA cells is the increased activation of the MAPK–MMP-7–TNF-α axis (see figure). Further studies are necessary to determine the overall net contribution of the up-regulated MAPK–MMP-7–TNF-α axis in the pathophysiology of FA. As it is possible to inhibit this pathway pharmacologically, these new observations provide a putative rationale for developing a new therapeutic approach to the treatment of FA patients based on drugs capable of down-regulating this stress signaling cascade.
A schematic representation of the MAPK–MMP-7–TNF-α stress signaling pathway in FA based on the work of Briot and colleagues. BM indicates bone marrow; MAPKs, mitogen-activated protein kinases; MMP-7, matrix metalloproteinase 7; and TNF-α, tumor necrosis factor-α.
A schematic representation of the MAPK–MMP-7–TNF-α stress signaling pathway in FA based on the work of Briot and colleagues. BM indicates bone marrow; MAPKs, mitogen-activated protein kinases; MMP-7, matrix metalloproteinase 7; and TNF-α, tumor necrosis factor-α.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal