Abstract
Studies have shown that the immune system can recognize self-antigens under conditions (eg, cell injury) in which the self-tissue might elaborate immune-activating endogenous danger signals. Uric acid (UA) is an endogenous danger signal recently identified to be released from dying cells. Prior work has shown that UA activates immune effectors of both the innate and adaptive immune system, including neutrophils and cytotoxic T-cell immunity. However, it was unclear whether UA could enhance antibody immunity, which was examined in this study. When added to dying tumor cells or with whole protein antigen, UA increased IgG1-based humoral immunity. Further, UA blocked growth of tumor in subsequent tumor challenge experiments, which depended on CD4, but not CD8, T cells. Sera derived from UA-treated animals enhanced tumor growth, suggesting it had little role in the antitumor response. UA did not signal for T-cell expansion or altered tumor-infiltrating leukocyte populations. Consistent with the lack of T-cell expansion, when applied to dendritic cells, UA suppressed T-cell growth factors but up-regulated B cell–activating cytokines. Understanding the nature of endogenous danger signals released from dying cells may aid in a better understanding of mechanisms of immune recognition of self.
Introduction
A hallmark of the immune system is the keen ability to distinguish between infected and noninfected self.1 Immune responses to infected tissues are not due merely to the presence of a microbial-derived antigen but rather the presentation of that antigen to the immune system in the context of another microbial-derived molecule termed a pathogen-associated molecular pattern (PAMP).2 Unlike antigens, PAMPs are not typically peptides or proteins, but rather molecules such as nucleic acids or glycolipids that are not readily subject to change through mutations. There are several PAMPs including lipopolysaccharide (LPS), unmethylated cytosine-guanosine repeats (CpGs), and double-stranded RNA motifs. PAMPs are recognized by one of several mammalian receptors, most being from the toll-like receptor (TLR) family of pattern-recognition receptors (PRRs).3 Proof that the immune system relies heavily on PAMPs for the decision about whether to activate comes from studies in which PAMPs are used as adjuvants in vaccines that target self-antigens. For example, cancer vaccines containing a combination of bacterial CpGs along with HER-2/neu-derived peptide epitopes are able to readily overcome tolerance and to provide long-term protection against spontaneous tumor development in the neu-transgenic mouse model of breast cancer.4 Despite the extensive understanding of PAMPs, it is now clear that the adaptive immune system can become activated against self under sterile conditions such as malignancy, cell death, or cell injury.5 There are a few stimuli (ie, endogenous danger signals) that have been characterized and identified in recent times that may be capable of driving sterile immune responses.6,7 One such group of endogenous danger signals is the purines, which include both uric acid (UA) and adenosine triphosphate (ATP).7-10
UA has received attention recently due to a series of reports from Rock and colleagues who have recently identified UA as a danger signal that is released from dying cells (Chen et al5,11 ; Shi et al8,9 ). These findings are now being corroborated by studies from other groups. For example, Hu et al found that UA was released from tumor cells undergoing immune rejection and that it had a significant role in the rejection process.12 UA is a natural product of the purine metabolic pathway and although found in extracellular fluids (eg, blood and interstitial spaces), its release from cells is thought to result in crystallization, which creates the immune bioactive form of UA.7,8 Crystalline UA has been shown to activate innate immune effectors including dendritic cells (DCs) and macrophages.8,13 Prior studies have shown that its ability to activate innate effectors can lead ultimately to activation of antigen-specific cytotoxic T-cell immunity.8
What has not been addressed is whether UA can also enhance antibody immunity. The primary reason to suggest a role of UA in antibody immunity is that sterile immune responses, such as those seen in rheumatoid arthritis and malignancy are often associated with the development of autoantibodies.14,15 In the current study, we investigated whether UA could augment antigen-specific antibodies in 2 separate model systems. In the first model, UA was added to dying cells that were injured using irradiation, with the intent of mimicking the purported physiologic role of UA. In the second, the ovalbumin foreign antigen model was used. In both models, it was found that crystalline UA led to augmented antibody immunity in the absence of significant tumor-specific T-cell expansion.
Methods
Animals
C57BL/6, OT-I, Balb/c, DO11.10, and FVB/N-TgN (MMTVneu)-202Mul (neu-tg mouse) mice were from the Jackson Laboratory (Bar Harbor, ME). OT-I mice are transgenic for a H-2Kb–restricted T-cell receptor that is specific for the chicken ovalbumin epitope Oval(257-264); DO11.10 mice are transgenic for a I-Ad–restricted TCR recognizing Oval(323-339). Only female mice (8-12 weeks old) were used, in accordance with institutional guidelines.
Reagents
UA crystals (> 99% purity; Sigma-Aldrich, St Louis, MO) were prepared as described before.8 Fluorochrome-conjugated antibodies targeting CD3, CD4, and CD8, CD19, CD14, NKT1.1, and CD11C were from BD Pharmingen (San Diego, CA). Ovalbumin and basic reagents were from Sigma Chemical (St Louis, MO). Synthetic peptides, Oval(323-339) and Oval(257-264), were synthesized by Sigma Genosys (The Woodlands, TX). Rat neu peptide, p420-429, was synthesized by the Mayo Clinic (Rochester, MN). Use of the neu peptide p420-429 H-2q tetramer has been previously described.16 The anti–IL-4/biotinylated anti–IL-4 and anti–IFN-γ/biotinylated anti–IFN-γ cytokine antibody pairs were from Endogen (Pierce, Rockford, IL) and BD Pharmingen, respectively. Recombinant murine granulocyte/macrophage-colony stimulating factor (GM-CSF) was obtained from R&D Systems (Minneapolis, MN). The IL-2 immunotoxin, denileukin diftitox (ie, ONTAK), was obtained from Ligand Pharmaceuticals (San Diego, CA).16 Foxp3 antibody was obtained from eBiosciences (San Diego, CA).
Tumor growth
Mouse mammary carcinoma (MMC) cell line was established from a spontaneous tumor from the neu-tg mice as previously described.17 For in vivo tumor growth, mice were inoculated with 5 × 106 MMC cells subcutaneously on the middorsum. Tumors were measured every few days with calipers, and tumor volume was calculated as the product of length × width × height × 0.5236. The numbers of mice used in each experiment are stated in “Results.” For in vitro experiments, 105 MMC cells were plated in 6-well plates with media alone or with various concentrations of sera. Proliferation analysis was done as previously described.18 In some cases, the tumors were removed for assessment of intracellular trafficking of leukocytes. Leukocyte content was compared between tumors of similar sizes to minimize potential variations due to tumor size such as necrosis and hypoxia.
Adoptive transfer and immunizations
Splenocytes from oval-TCR transgenic donor mice were adoptively transferred into the tail vein of recipient mice one day prior to the immunization. Balb/c mice received 2 × 106 DO11.10 splenocytes per mouse; C57BL/6 received 10 × 106 OT-I splenocytes. Mice were immunized intradermally with 10 μg ovalbumin (or appropriate ovalbumin peptide in the case of TCR transgenic T cells) and 5 μg GM-CSF or 25 μg UA crystals per mouse. Six days later, mice were killed and the Balb/c splenocytes were stained with anti-DO11.10 TCR mab KJ1-26 (Caltag, Burlingame, CA), while B6 splenocytes were stained with (SIINFEKL)-H2-Kb tetramer (Beckman Coulter, Fullerton, CA) followed by flow cytometric analysis. For the antibody studies, Balb/c mice were immunized with whole ovalbumin protein as described for C57BL/6 mice. For dying tumor cell studies in the neu-tg mouse, MMC cells (5 × 106/mouse) were irradiated (150 gray) and injected with or without UA. Two injections were given approximately 7 to 14 days apart, subcutaneously. Seven to 14 days following the last injection, either live MMC tumor cells were injected or splenocytes, lymph node cells, and blood were harvested for examination by flow cytometry. To deplete T regulatory cells (Tregs) prior to immunization, the animals were pretreated with denileukin diftitox as previously described.16 In some cases, mice were pretreated with monoclonal antibodies to deplete either CD8+ or CD4+ T cells prior to challenge with dying tumor with UA as previously described.19
ELIspot assays
Enzyme-linked immunosorbent spot (ELIspot) analysis was conducted essentially as previously described.20 Splenocytes isolated from treated animals were exposed to media alone, freeze-thawed MMC tumor cells (1 tumor cell per 4 splenocytes), neu peptide p420-429 (10 μg/mL), or concanavalin A (5 μg/mL, concanavalin A, ConA) for 48 hours.
Isolation and in vitro culture of splenic dendritic cells
Minced spleens were incubated with collagenase D (37°C, 30 minutes) and passed through a 70-μm cell strainer. DCs were purified with a CD11c cell purification kit to greater than 90% purity (Miltenyi, Bergisch Gladbach, Germany). TCR transgenic T cells were purified with CD4+ or the CD8+ T-cell isolation kits that produce untouched T cells (Miltenyi). Purified DCs were exposed for 2 hours to peptide antigens and 10 μg/mL UA (in RPMI-1640, 2 mM l-glutamine, 25 mM HEPES, 10% FCS, 50 mM 2-mercaptothanol, and 1% penicillin and streptomycin) after which the media were removed and the DCs washed. TCR-transgenic T cells were then added to the DCs for 48 hours at 37°C after which the T cells were again repurified and placed back into T-cell media for an additional 48 hours. The T cell–conditioned supernatants were then collected and examined for cytokine content using multiplexed microsphere analysis as described in “Multiplexed microsphere cytokine immunoassay.” In some cases, DCs were not exposed to T cells but rather allowed to remain in media for 48 hours after which DC-conditioned media were removed and assessed for cytokine content.
Enzyme-linked immuno sorbent assay
For direct enzyme-linked immunosorbent assays (ELISAs), Maxisorp ELISA plates (Nalge Nunc International, Rochester, NY) were coated overnight at 4°C with 5 μg/mL ovalbumin or 10 μg/mL freeze-thawed tumor lysates in 0.05 M carbonate-bicarbonate buffer containing and blocked with 1% BSA. Sera were applied at a 1:120 dilution followed by incubation for 1 hour at room temperature (RT) with horseradish peroxidase–conjugated antibodies against mouse IgG, IgG1, or IgG2a (Zymed, South San Francisco, CA). Serum was omitted from control wells to determine background signals, which were subtracted from each experimental value. In other cases, standard curves were prepared to convert the optical density signals into an estimated antibody concentration by directly plating several concentrations of purified IgG, IgG1, or IgG2a onto the ELISA plates. The plates were developed using tetramethylbenzadine (TMB), which was stopped by the addition of 1 N sulfuric acid. The plates were read at 450 nm on a Victor V 1420 Multilabel Reader (Perkin Elmer, Waltham, MA).
For sandwich ELISA, plates were coated with 100 μL/well of rabbit polyclonal antibodies to rat neu (ab36728; Abcam, Cambridge, MA). After blocking and washing, 20 μg/well of tumor freeze-thawed lysates or assay buffer was added to each well and incubated at RT for 2 hours. After washing, mouse sera were added to the plate at a 1:40 dilution in triplicate and incubated for 1 hour at RT. All other steps were performed as described above.
Multiplexed microsphere cytokine immunoassay
Cytokines (IL-12, IL-2, IFN-γ, IL-4, and IL-5) were measured using multiplex microspheres per the manufacturer's direction (BioRad, San Diego, CA). Briefly, 100 μL Bio-Plex assay buffer (BioRad) was added to each well of a MultiScreen MABVN 1.2-μm microfiltration plate (Millipore, Billerica, MA) followed by the addition of 50 μL of the multiplex bead preparation. Following washing of the beads with 100 μL wash buffer, 50 μL of the samples (ie, cell culture supernatants) or the standards was added to each well and incubated with shaking for 30 minutes at room temperature. Standard curves were generated with a mixture of cytokine standards and 8 serial dilutions ranging from 0 to 32 000 pg/mL. The plate was washed 3 times followed by incubation of each well in 25 μL premixed detection antibodies for 30 minutes with shaking. The plate was washed and 50 μL streptavidin solution was added to each well and incubated for 10 minutes at RT with shaking. The beads were given a final washing and resuspended in 125 μL Bio-Plex assay buffer. Cytokine levels in the sera were quantitated by analyzing 100 μL of each well on a Bio-Plex using Bio-Plex Manager software (version 4; BioRad).
Flow cytometry
Cell surface molecules and Foxp3 staining and flow cytometry were done essentially as described by Knutson et al.16 For flow cytometric analysis, a similar number of events, typically 100 000, were collected for all groups.
Immunoprecipitation and Western blotting
Cell lysate preparation, immunoprecipitations, and Western blot analysis were done as previously described by Knutson et al.21 Sera collected from treatment mice were diluted 50-fold and mixed with 400 μg MMC tumor cell lysates.
Statistical analyses
Statistical analyses were performed using GraphPad InStat for Windows 95/NT (GraphPad Software, San Diego, CA). Data were analyzed using Mann-Whitney or Student t tests unless otherwise stated, and the results were considered statistically significant if P was less than .05.
Results
UA signals for enhanced antitumor immunity
The danger signaling properties of UA were examined in a tumor protection model (Figure 1). Crystalline UA, when added with dying (irradiated) tumor cells, led to subsequent suppression of tumor growth in a dose-dependent manner when given prior to tumor challenge, indicating augmentation of memory immune responses. For example, on day 38 after tumor challenge, the size of the tumors in mice pretreated with dying tumor cells containing 100 μg UA was 143 ± 95 mm3 (mean ± SEM, n = 5), which was significantly smaller than control tumors (25 μg UA only) at the same time point (972 ± 83 mm3; P < .001) and smaller than tumors from animals treated with tumors only (695 ± 200 mm3; P = .019). UA doses of 100 or 20 μg UA led to similar tumor suppression (P > .05). UA was ineffective at the 10-μg dose, and at this dose the mean tumor size was not significantly different from control (P > .05). UA at lower doses (eg, 1 μg; data not shown) had no impact on tumor growth challenge, likely due to rapid dissolution. In addition to suppressing the growth rate, the inclusion of UA was able to improve overall survival as shown in Figure 1B. Animals that received 20 μg or 100 μg UA had a survival that was more than 35 days longer than that observed in the control mice and approximately 14 days greater than animals treated with tumor cells alone. Depletion of CD4 T cells, but not CD8 T cells, prior to immunization reversed the tumor-suppressive effects of UA (Figure 1C). In fact, CD8 T-cell depletion provided enhanced antitumor activity. Despite its effects on suppressing tumor growth when used in the prevention setting, when injection was begun after the tumors had formed, UA was unable to suppress tumor growth when combined with dying tumor cells (data not shown). Thus, UA added to dying tumor cells augments CD4 T helper cell–dependent antitumor immune responses.
Uric acid signals for enhanced antitumor immunity. (A) Tumor growth measurements for animals treated with dying tumor cells, either alone or along with various concentrations of uric acid (UA) prior to tumor challenge. Control animals (none) received 25 μg UA. Each data point is a mean (± SEM) tumor measurement calculated from 6 animals per group. A similar experiment yielded similar results. (B) Survival curves for mice, treated the same as in panel A, over a course of 75 days. The experiment is representative of 2 similar independent experiments. (C) Tumor growth measurements in animals treated with dying tumor cells and 25 μg UA alone or following depletion of either CD8 or CD4 T cells. Control animals received no treatments. Measurements are the mean (± SEM) of 4 animals per group. A repeat experiment gave identical results.
Uric acid signals for enhanced antitumor immunity. (A) Tumor growth measurements for animals treated with dying tumor cells, either alone or along with various concentrations of uric acid (UA) prior to tumor challenge. Control animals (none) received 25 μg UA. Each data point is a mean (± SEM) tumor measurement calculated from 6 animals per group. A similar experiment yielded similar results. (B) Survival curves for mice, treated the same as in panel A, over a course of 75 days. The experiment is representative of 2 similar independent experiments. (C) Tumor growth measurements in animals treated with dying tumor cells and 25 μg UA alone or following depletion of either CD8 or CD4 T cells. Control animals received no treatments. Measurements are the mean (± SEM) of 4 animals per group. A repeat experiment gave identical results.
UA signals for an enhanced IgG1 antibody response
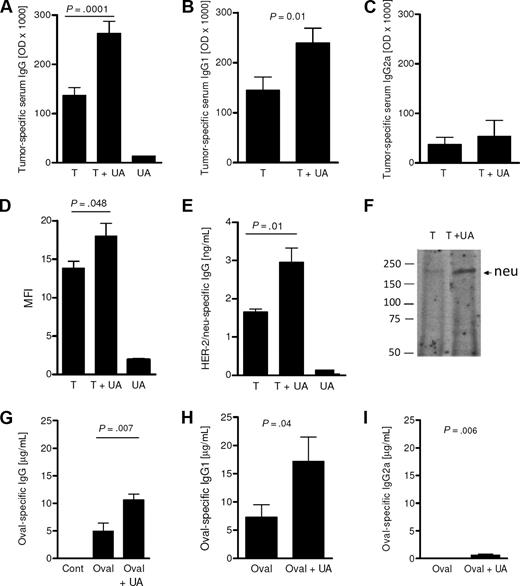
The ability of UA to boost IgG antibody immunity was next examined. As shown in Figure 2A, injection of dying tumor cells alone generated a strong IgG antibody response in the absence of exogenous UA. The inclusion of UA (25 μg) along with the dying tumor cells further boosted this antibody response compared with animals injected with 25 μg UA alone. This level of tumor-specific antibody was significantly higher and nearly double the antibody level achieved following injection of dying tumor cells alone (P= .001, n = 12). The increase in tumor-specific IgG antibody was due, at least in part, to a significant increase in tumor-specific IgG1. As shown in Figure 2B, the levels of IgG1 attained with the inclusion of UA were significantly higher compared with the levels achieved with the injection of tumor cells alone (P= .01). In contrast, UA did not induce a significant increase in tumor-specific IgG2a (Figure 2C; P > .05). Flow cytometric analysis confirmed the finding of elevated IgG by demonstrating that serum from animals injected with tumor cells and UA had higher levels of antibodies capable of binding to live tumor cells as assessed by flow cytometry compared with mice pretreated with either UA alone or with dying tumor cells alone (Figure 2D). Binding to tumor-specific antigens was analyzed by neu-specific ELISA and immunoprecipitation. Figure 2E shows that sera from mice exposed to UA and tumor cells have increased levels of neu-specific antibody compared with mice treated with tumor alone. This increase was confirmed by immunoprecipitation assays (Figure 2F). To examine whether UA was able to augment antibody responses in the absence of dying tumor cells, we immunized Balb/C mice with ovalbumin protein with or without UA. Sera taken 28 days after immunization showed that the inclusion of UA with ovalbumin resulted in significantly higher levels of ovalbumin-specific IgG (P < .05; Figure 2G). Again, as with dying tumor cells, IgG subtype analysis revealed that UA induced an increase in IgG1 (Figure 2H). There was a minor, albeit significant (P= .006), increase in IgG2a induced by UA (Figure 2I). Collectively, these findings show that UA, whether added with self- or foreign antigens, augments IgG1-based antibody immunity.
UA signals for enhanced tumor-specific IgG1 antibodies. (A) The tumor-specific antibody concentration in the serum 14 days after 2 injections of dying tumor cells alone (T), tumor cells with UA (T + UA) or with UA alone (UA). Each bar represents the mean (± SEM) of 12 replicates calculated from the optical densities (× 1000) in the ELISA assay. Similar to panel A, panels B and C show the levels of IgG1 and IgG2a, respectively. Each mean (± SEM) is calculated from 2 to 7 replicates. (D) The mean fluorescent intensity (MFI) of tumor cells stained with IgG from sera of animals. Each bar represents the mean (± SEM) of 3 replicates; *P = .05. (E) Rat neu-specific IgG levels in animals treated as determined by capture sandwich ELISA. Each bar is the mean (± SEM) of 3 determinations. (F) Western immunoblot analysis of rat neu immunoprecipitated with sera. Numbers shown are the position of the molecular weight markers (KDa). (G-I) The serum levels of total IgG (F), IgG1 (G), and IgG2a (H) specific for ovalbumin from control mice (Cont), ovalbumin-immunized (Oval), or OVAL/UA-immunized (Oval + UA) mice (n = 5-7). The levels of IgG and IgG1 obtained following ovalbumin immunization were not significantly different (P > .05).
UA signals for enhanced tumor-specific IgG1 antibodies. (A) The tumor-specific antibody concentration in the serum 14 days after 2 injections of dying tumor cells alone (T), tumor cells with UA (T + UA) or with UA alone (UA). Each bar represents the mean (± SEM) of 12 replicates calculated from the optical densities (× 1000) in the ELISA assay. Similar to panel A, panels B and C show the levels of IgG1 and IgG2a, respectively. Each mean (± SEM) is calculated from 2 to 7 replicates. (D) The mean fluorescent intensity (MFI) of tumor cells stained with IgG from sera of animals. Each bar represents the mean (± SEM) of 3 replicates; *P = .05. (E) Rat neu-specific IgG levels in animals treated as determined by capture sandwich ELISA. Each bar is the mean (± SEM) of 3 determinations. (F) Western immunoblot analysis of rat neu immunoprecipitated with sera. Numbers shown are the position of the molecular weight markers (KDa). (G-I) The serum levels of total IgG (F), IgG1 (G), and IgG2a (H) specific for ovalbumin from control mice (Cont), ovalbumin-immunized (Oval), or OVAL/UA-immunized (Oval + UA) mice (n = 5-7). The levels of IgG and IgG1 obtained following ovalbumin immunization were not significantly different (P > .05).
UA neither augmented T-cell expansion nor altered tumor-infiltrating leukocyte levels
It had been anticipated that because UA resulted in elevated tumor-specific IgG1 antibody immunity that Th2 T cells would also be elevated. However, IL-4 ELIspot showed that the number of tumor-specific IL-4–secreting cells in the spleen of mice treated with both tumor and UA were not augmented compared with spleens of mice treated with tumor alone as shown in Figure 3A (P > .05), although tumor alone induced strong Th2 T-cell responses. Tumor cells neither alone nor with UA were able to induce tumor-specific IFN-γ–secreting T cells as assessed by ELIspot (data not shown). UA also did not induce increased levels of neu-specific IFN-γ–secreting T cells (Figure 3B) nor did the numbers of neu-peptide–specific CD8 T cells increase in response to UA as assessed by tetramer analysis (Figure 3C).
UA did not augment T-cell expansion or alter tumor-infiltrating leukocytes levels. (A) The levels of IL-4–secreting cells that responded to either ConA or tumor cell lysates. Splenocytes for the ELIspot assay were obtained from animals injected with tumor cells alone (▭), tumor cells with 25 μg UA ( ), and 25 μg UA alone (
), and 25 μg UA alone ( ). Each bar represents the mean (± SEM) of 12 determinations. (B) The levels of IFN-γ–secreting T cells responding to no antigen, ConA, or neu-derived MHC class I peptide p420-429. Splenocytes were derived from animals that received dying tumor cells with various levels of UA. Each bar is the mean of 9 determinations. (C) The numbers of neu peptide p420-429 tetramer-positive T cells in draining nodes or splenocytes from mice depicted in panel F. Each is the mean (± SEM) of triplicate determinations calculated from 3 mice. (D,E) The frequencies of Oval(323)-specific CD4+DO11.10 T cells (D) and Oval(257)-specific CD8+OT-I T cells (E) as percentage of all CD3+ splenocytes in untreated control (Cont), ovalbumin cognate peptide/UA (UA), or cognate ovalbumin peptide/GM-CSF (GM). Each bar represents the mean (± SEM) of 7 replicates and represents 2 identical experiments, which yielded similar results. (F-L) The levels of various leukocytes in tumors from mice that received either no treatment (Cont) or pretreatment with tumor cells and 25 μg UA (T + UA), prior to tumor injection. Each bar is the mean (± SEM) of 3 replicates. Each graph represents a unique intratumoral leukocyte population from control, untreated mice and from mice pretreated with dying tumor cells containing UA. All are calculated from 100 000 total events. Results are expressed as the percentage of total cells, both tumor cells and leukocytes, collected following tumor mincing.
). Each bar represents the mean (± SEM) of 12 determinations. (B) The levels of IFN-γ–secreting T cells responding to no antigen, ConA, or neu-derived MHC class I peptide p420-429. Splenocytes were derived from animals that received dying tumor cells with various levels of UA. Each bar is the mean of 9 determinations. (C) The numbers of neu peptide p420-429 tetramer-positive T cells in draining nodes or splenocytes from mice depicted in panel F. Each is the mean (± SEM) of triplicate determinations calculated from 3 mice. (D,E) The frequencies of Oval(323)-specific CD4+DO11.10 T cells (D) and Oval(257)-specific CD8+OT-I T cells (E) as percentage of all CD3+ splenocytes in untreated control (Cont), ovalbumin cognate peptide/UA (UA), or cognate ovalbumin peptide/GM-CSF (GM). Each bar represents the mean (± SEM) of 7 replicates and represents 2 identical experiments, which yielded similar results. (F-L) The levels of various leukocytes in tumors from mice that received either no treatment (Cont) or pretreatment with tumor cells and 25 μg UA (T + UA), prior to tumor injection. Each bar is the mean (± SEM) of 3 replicates. Each graph represents a unique intratumoral leukocyte population from control, untreated mice and from mice pretreated with dying tumor cells containing UA. All are calculated from 100 000 total events. Results are expressed as the percentage of total cells, both tumor cells and leukocytes, collected following tumor mincing.
UA did not augment T-cell expansion or alter tumor-infiltrating leukocytes levels. (A) The levels of IL-4–secreting cells that responded to either ConA or tumor cell lysates. Splenocytes for the ELIspot assay were obtained from animals injected with tumor cells alone (▭), tumor cells with 25 μg UA ( ), and 25 μg UA alone (
), and 25 μg UA alone ( ). Each bar represents the mean (± SEM) of 12 determinations. (B) The levels of IFN-γ–secreting T cells responding to no antigen, ConA, or neu-derived MHC class I peptide p420-429. Splenocytes were derived from animals that received dying tumor cells with various levels of UA. Each bar is the mean of 9 determinations. (C) The numbers of neu peptide p420-429 tetramer-positive T cells in draining nodes or splenocytes from mice depicted in panel F. Each is the mean (± SEM) of triplicate determinations calculated from 3 mice. (D,E) The frequencies of Oval(323)-specific CD4+DO11.10 T cells (D) and Oval(257)-specific CD8+OT-I T cells (E) as percentage of all CD3+ splenocytes in untreated control (Cont), ovalbumin cognate peptide/UA (UA), or cognate ovalbumin peptide/GM-CSF (GM). Each bar represents the mean (± SEM) of 7 replicates and represents 2 identical experiments, which yielded similar results. (F-L) The levels of various leukocytes in tumors from mice that received either no treatment (Cont) or pretreatment with tumor cells and 25 μg UA (T + UA), prior to tumor injection. Each bar is the mean (± SEM) of 3 replicates. Each graph represents a unique intratumoral leukocyte population from control, untreated mice and from mice pretreated with dying tumor cells containing UA. All are calculated from 100 000 total events. Results are expressed as the percentage of total cells, both tumor cells and leukocytes, collected following tumor mincing.
). Each bar represents the mean (± SEM) of 12 determinations. (B) The levels of IFN-γ–secreting T cells responding to no antigen, ConA, or neu-derived MHC class I peptide p420-429. Splenocytes were derived from animals that received dying tumor cells with various levels of UA. Each bar is the mean of 9 determinations. (C) The numbers of neu peptide p420-429 tetramer-positive T cells in draining nodes or splenocytes from mice depicted in panel F. Each is the mean (± SEM) of triplicate determinations calculated from 3 mice. (D,E) The frequencies of Oval(323)-specific CD4+DO11.10 T cells (D) and Oval(257)-specific CD8+OT-I T cells (E) as percentage of all CD3+ splenocytes in untreated control (Cont), ovalbumin cognate peptide/UA (UA), or cognate ovalbumin peptide/GM-CSF (GM). Each bar represents the mean (± SEM) of 7 replicates and represents 2 identical experiments, which yielded similar results. (F-L) The levels of various leukocytes in tumors from mice that received either no treatment (Cont) or pretreatment with tumor cells and 25 μg UA (T + UA), prior to tumor injection. Each bar is the mean (± SEM) of 3 replicates. Each graph represents a unique intratumoral leukocyte population from control, untreated mice and from mice pretreated with dying tumor cells containing UA. All are calculated from 100 000 total events. Results are expressed as the percentage of total cells, both tumor cells and leukocytes, collected following tumor mincing.
Again, these findings were verified using the ovalbumin model. Specifically, DO11.10 TCR transgenic ovalbumin-specific T cells were used to better track T-cell expansion. As shown in Figure 3D, the addition of UA to the ovalbumin immunization used did not change the frequency of ovalbumin-specific CD4+ T cells, which were at similar levels to those in animals that received ovalbumin alone. As a positive control, GM-CSF promoted a strong expansion to more than 2% of all T cells. Similar data were obtained following the infusion of MHC class I–restricted OT-1 CD8 T cells (Figure 3E). To determine whether enhanced tumor rejection was due to increased immune effectors in the tumor microenvironment, tumors either from mice pretreated with dying tumor cells and UA or from untreated control mice were removed, and the tumor-infiltrating leukocyte population was examined. So as to avoid any mass-related differences, leukocyte infiltration was evaluated in similar-size tumors (“Methods”). Thus, when harvested after the harvesting of control tumors (mean size: 466 ± 29 mm3), the mean tumor size from the mice that were pretreated was 544 (± 18) mm3. As shown in Figure 3F-L, the levels of CD4+ T cells, CD8+ T cells, CD19+ B cells, CD14+ macrophages, CD4+CD25+Foxp3+ Tregs, CD8+CD25+Foxp3+ Tregs, and CD11C+ DCs in the mice treated with dying tumor cells and UA were similar (P > .05) relative to tumors derived from untreated animals. There were no measurable levels of natural killer (NK) cells in the tumors of either group (data not shown).
UA favors an IL-5–based Th2 immune response
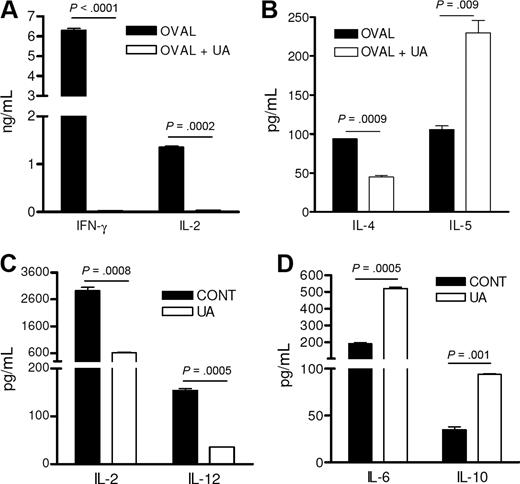
Increased antibody production in the absence of T-cell expansion suggested that UA somehow skewed the T-cell and DC responses more toward a Th2 phenotype ultimately favoring a B-cell response. This was confirmed with in vitro studies that showed that DO11.10 CD4 T cells that had been stimulated by ovalbumin and UA-pulsed DCs failed to produce IFN-γ and IL-2 (Figure 4A) but maintained expression of IL-4 and even demonstrated elevated secretion of IL-5, a B-cell–stimulating cytokine known to augment antibody production (Figure 4B). DCs, prepared from mice immunized with ovalbumin and UA DCs had reduced IL-2 and IL-12 production compared with DCs from animals treated with ovalbumin alone (Figure 4C). In contrast, these same DCs demonstrated elevated expression of the B-cell growth factors, IL-6 and IL-10 (Figure 4D).
UA favors an IL-5–based Th2 immune response. (A,B) The cytokine concentrations of cell culture supernatants from purified antigen-stimulated DO11.10 CD4 T cells stimulated by DCs stimulated with either control media (control) or 10 μg/mL UA (UA) and ovalbumin antigen (OVAL). Each represents the mean (± SEM) cytokine concentration from duplicate wells. Experiment was reproduced twice with similar results. (C,D) Cytokine concentrations of cell culture supernatants from purified CD11C+ splenic dendritic cells stimulated with UA (UA) or media alone (CONT). Each represents the mean (± SEM) cytokine concentration from duplicate wells. Experiment was reproduced twice with similar results.
UA favors an IL-5–based Th2 immune response. (A,B) The cytokine concentrations of cell culture supernatants from purified antigen-stimulated DO11.10 CD4 T cells stimulated by DCs stimulated with either control media (control) or 10 μg/mL UA (UA) and ovalbumin antigen (OVAL). Each represents the mean (± SEM) cytokine concentration from duplicate wells. Experiment was reproduced twice with similar results. (C,D) Cytokine concentrations of cell culture supernatants from purified CD11C+ splenic dendritic cells stimulated with UA (UA) or media alone (CONT). Each represents the mean (± SEM) cytokine concentration from duplicate wells. Experiment was reproduced twice with similar results.
Antibodies induced by UA may not be therapeutic or contribute to the antitumor immune response
To examine whether the antibodies induced by UA were therapeutic by themselves, sera collected from animals treated with tumor and UA, treated with tumor alone, or not treated were injected along with the live tumor cells at the time of tumor challenge. As shown in Figure 5A, although UA increased the levels of antibodies, these antibodies had no impact on tumor growth when used in vivo. Tumors also retained expression of the neu antigen (data not shown). However, when serum derived from animals treated with irradiated tumor cells and UA was applied to tumor cells in vitro, there was a clear enhancement of tumor growth, likely due to an increased level of agonistic antibodies that recognize and cross-link growth factors such as neu (Figure 5B). Such cross-linking may result in activation and increased proliferation. Thus, antibodies generated by UA appear to be neither therapeutic nor tumor inhibitory.
UA may induce growth-promoting antibodies. (A) In vivo tumor growth of MMC tumor cells following intratumoral injection (day 0) of sera from naive animals or from animals from either pretreated with irradiated tumor with (T + UA) or without (T) uric acid. Calculated from 3 separate tumors for each data point. Curves overlap. (B) The thymidine incorporation in live MMC tumor cells exposed to media (■) or various concentrations of serum from animals treated with UA alone (U alone, ▴), irradiated tumor alone (T, ♦), or with UA (T + UA, •). Each determination represents the mean (± SEM) of 3 determinations; *P = .01.
UA may induce growth-promoting antibodies. (A) In vivo tumor growth of MMC tumor cells following intratumoral injection (day 0) of sera from naive animals or from animals from either pretreated with irradiated tumor with (T + UA) or without (T) uric acid. Calculated from 3 separate tumors for each data point. Curves overlap. (B) The thymidine incorporation in live MMC tumor cells exposed to media (■) or various concentrations of serum from animals treated with UA alone (U alone, ▴), irradiated tumor alone (T, ♦), or with UA (T + UA, •). Each determination represents the mean (± SEM) of 3 determinations; *P = .01.
Treg depletion augments UA induced antitumor responses
Our group has previously observed that regulatory T cells interfere with the endogenous cell–mediated immune responses against breast cancers in the neu-tg mouse. In the current study, we observed that UA led to an increase in both peripheral CD4+ and CD8+ Tregs (ie, Foxp3+CD25+) (Figure 6A). To determine whether Tregs were involved in preventing T-cell expansion, Tregs were depleted with denileukin diftitox (ONTAK, On) prior to the injection of tumor, with and without UA.16 As shown in Figure 6B and consistent with our prior studies, Treg depletion with denileukin diftitox led to an expected increase in the level of neu-specific CD8 T cells, as we have previously shown.16 But, the addition of UA did not further augment CD8 T-cell expansion, suggesting that although UA itself fails to augment T-cell expansion, it does not actively block it. In addition, prior Treg depletion did not impair the ability of UA to induce antitumor responses as shown in Figure 6C. In contrast, both Treg depletion and UA collaborated for enhanced tumor suppression, as was anticipated.
UA does not prevent increased T-cell immunity induced by other means. (A) The peripheral levels of Tregs (CD4+CD25+Foxp3+ or CD8+CD25+Foxp3+) in animals injected with dying tumor cells alone (T) or with tumor cells with UA (T + UA). Each bar is the mean (± SEM; *P < .05) of 3 mice. (B) Levels of neu peptide tetramer+ CD8+CD62Llo T cells (ie, effector or effector memory) in control animals, or animals injected with tumor cells (T) with or without UA (UA) or ONTAK (On). Each bar is the mean (± SEM) of 3 to 4 mice. (C) The tumor growth rates in mice pretreated with tumor cells alone and tumor cells with or without UA, ONTAK, or both. Each data point is the mean (± SEM) of 3 to 5 mice. *P < .05 compared with all other groups.
UA does not prevent increased T-cell immunity induced by other means. (A) The peripheral levels of Tregs (CD4+CD25+Foxp3+ or CD8+CD25+Foxp3+) in animals injected with dying tumor cells alone (T) or with tumor cells with UA (T + UA). Each bar is the mean (± SEM; *P < .05) of 3 mice. (B) Levels of neu peptide tetramer+ CD8+CD62Llo T cells (ie, effector or effector memory) in control animals, or animals injected with tumor cells (T) with or without UA (UA) or ONTAK (On). Each bar is the mean (± SEM) of 3 to 4 mice. (C) The tumor growth rates in mice pretreated with tumor cells alone and tumor cells with or without UA, ONTAK, or both. Each data point is the mean (± SEM) of 3 to 5 mice. *P < .05 compared with all other groups.
Discussion
Recent evidence shows that mammalian immune systems have evolved mechanisms to respond to uninfected dying or injured cells through a variety of novel ligands.6,8,9,22-26 The underlying reason for the evolution of such mechanisms for responding to endogenous ligands remains controversial, but one possibility is that they are needed to detect potentially infected cells for which there are no associated PAMPs or other adjuvants.27 Alternatively, sterile immune responses may be involved in normal tissue homeostasis by providing mechanism to recruit in phagocytic cells of the innate immune system such as macrophages.28 Regardless of the biologic rationale, further characterization of sterile immunity should have important implications for the treatment of autoimmunity and potentially malignancy. While it is known that UA signaling leads to activation of innate immunity and T lymphocytes, little is known about whether antibody production ensues. We had speculated that since autoantibodies were so prevalent in several diseases associated with sterile immune responses that responses to endogenous danger signals also lead to autoantibody production under some conditions. Indeed, the present studies show that the recently identified danger signal, crystalline UA, when added to either dying tumor cells or soluble protein antigen was able to augment antibody immunity. Specifically, it was found that UA increased IgG1-based antibody immunity to both self- and foreign antigens, suggesting that Th2 immunity was the favored response. As previously reported, when UA was used as an adjuvant, the increased immunity generated resulted in enhanced protection against tumor. However, unexpectedly, the antitumor response depended on CD4 T cells but not CD8 T cells.
In the mouse, the predominant Ig produced following the emergence of a Th2-directed immune response is IgG1. In contrast, the major Ig produced following induction of Th1 immunity is IgG2a. While our study showed little or no increased production of IgG2a, we cannot rule out the possibility that UA influences other antibody subsets (eg, IgG2b, IgG3). In both the dying tumor cell model and the foreign antigen models used in the current study, UA induced significant increases in the IgG1 response, suggesting that the purine is also able to augment Th2 immunity in addition to its reported abilities to augment Th1-based cell-mediated immunity. The observations of increased IL-5 production by ovalbumin-specific CD4 T cells and the increased production of B-cell activators (IL-10 and IL-6) by UA-treated DCs support this conclusion. There are several biologic functions of IgG1 in the mammal that could potentially be beneficial to a cell injury or death response, including complement activation, interference with cell signaling, and opsonization of particles for enhanced phagocytosis.29 One could envision that such a response may be useful to neutralize viruses that do not express traditional PAMPs. However, it was observed that the augmented antibody response induced by UA had little impact on tumor growth in vivo alone. In contrast, the antibodies seem to be detrimental and counterproductive. Despite that, we cannot rule out the possibility that, in vivo, the antibodies could activate tumor-destruction mechanisms, such as antigen-dependent cellular cytotoxicity, that were offset by the tumor-promoting effects.
It was speculated that the predominant augmentation of IgG1 immunity by UA may have been accompanied by increased numbers of Th2 T-cell responses. Although dying tumor cells elicited a strong increase in tumor antigen–specific Th2 immunity, the addition of UA failed to augment these numbers, suggesting that there were factors other than increased numbers of IL-4–secreting T cells that contributed to the increased IgG response. One possibility is increased elaboration of B-cell activation cytokines (eg, IL-5 and IL-4). Based on the studies with purified TCR transgenic Th cells (ie, D011.1 T cells), IL-4 release was not increased, but rather there was a slight decline in favor of enhanced IL-5 production in response to UA stimulation. While our studies did not reveal the mechanisms behind increased IgG1 immunity, such a cytokine could favor B1 B-cell activation and maturation.30 B1 B cells are a subset of B cells that specifically responds to IL-5 with increased proliferation and IgG production.30-32 B1 B cells are associated primarily with the production of autoantibodies targeting self-antigens such as phosphatidylcholine, immunoglobulins (eg, rheumatoid factor), and DNA.33,34 Although fairly high levels of tumor-binding antibodies were observed in the current study, it is unclear yet as to what antigens are being targeted by the augmented immune response, with the exception that a small fraction bound to rat neu.
Another key finding from our study is that, in both the neu-tg mouse and the TCR-transgenic T-cell models, UA did not result in expansion of cell-mediated immunity (eg, cytotoxic T lymphocytes [CTLs] and IFN-γ) as there was neither an increase in tumor antigen–specific T cells in the dying tumor cell model nor an increase in OT-1 T cells following immunization with ovalbumin. Despite that, the enhanced antitumor activity was completely reversed with the depletion of CD4 T cells, which would have been expected given the important role of CD4 T cells in regulating the antibody responses. In fact, the tumor growth was substantially greater in the CD4-depleted mice compared with untreated mice, suggesting that CD4 T cells have an active role in modulating tumor growth in the absence of prior immunization with irradiated tumor cells. Unlike the effects observed in the CD4-depleted animals, CD8 depletion did not reverse the effects of UA, but rather enhanced the effects. Since it was observed that the inclusion of UA led to increased peripheral Tregs, it was speculated that these specialized regulatory T cells were involved in blocking the expansion of cell-mediated immunity, consistent with their roles in maintaining peripheral tolerance.16,35,36 However, Treg depletion studies with denileukin diftitox, which we previously showed to be effective in depleting Tregs and elevating cell-mediated immunity, failed to bear this out.16
Our inability to see expansion of CD8 T-cell immunity could be explained by the fact that we did not use the same immunization strategies as described in previous studies. In one of the prior studies by Shi and colleagues, it was found that UA when administered with purified, latex bead–bound gp120 antigen led to an increase in the cytotoxic T-cell activity response compared with animals immunized with the antigen alone.8 The ability of UA to enhance expansion of cytotoxic T cells was shown in another study, by the same group who observed that intraperitoneal pretreatment of RIP-mOVA mice with both allopurinol and uricase reduced in vivo cell division of infused OT-1 T cells, secondarily to UA depletion.9 There are potential reasons we failed to see enhanced T-cell expansion. First, our study did not use antigens linked to latex beads, the latter of which could alter immune responses. Alternatively, we used concentrations of UA that were lower in many cases. It is possible that UA is a more versatile danger signal than either the current study or past studies suggest with capabilities of supporting both Th1 and Th2 T-cell immune responses, depending on the extent of damage or injury. Lower levels of UA may indicate natural cell senescence in which antibodies could be induced to facilitate phagocytosis by macrophage for reversal of the tissue pathology (ie, healing) and tissue maintenance. Consistent with this reasoning, Martinon et al recently showed that UA crystals also directly activate macrophages.13 Alternatively, the higher levels as used by Shi and colleagues may reflect massive damage indicative of an infection.
The finding of increased Tregs following the application of UA is also another novel finding. Repeatedly, it has been found that Tregs are elevated in patients with a variety of different types of cancers.36 Despite these observations, many things remain unclear about the nature of elevated levels of Tregs in patients with cancer, including the factors that signal for Treg expansion. Based on the current study and the prior studies by Shi et al, it could be speculated that danger signals, including UA, that emanate from the dying tumor cells lead to the peripheral increases in Tregs.8,27 Despite these increases, the clinical significance of elevated peripheral Tregs in cancer patients remains uncertain. What is known is that the tumor burden directly correlates with increased peripheral Tregs in patients with melanoma, and renal and gastric cancers, strongly implicating danger signaling (UA?) from the tumor.37,38 These peripheral increases in Tregs may result in the often-observed migration of Tregs into the tumor microenvironment, which has been linked to cancer pathogenesis in several cancers such as ovarian cancer.39
In conclusion, in the current study we observed that the danger signal UA could, in addition to its previously reported ability to augment CTLs, induce IgG1 responses, suggesting that it can also augment humoral immune responses. Understanding natural mechanisms of danger signaling, apart from exogenous microbial-derived signals (eg, CpGs) may have important implications for vaccines, tumor immunity, and autoimmunity.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors gratefully acknowledge the assistance of Dr George Vielhauer.
This work was supported by the Mayo Clinic Comprehensive Cancer Center and National Cancer Institute grants K01-CA100764 and R01-CA113861 (K.L.K).
National Institutes of Health
Authorship
Contribution: M.D.B. and W.M.W. performed research and analyzed data; C.J.K., C.L.E., J.K., and E.A.G. per-formed research; K.R.K. designed research and wrote the paper; M.L.D. provided materials and resources; and K.L.K. designed and performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Keith L. Knutson, Mayo Clinic College of Medicine, 342C Guggenheim, 200 First St SW, Mayo Clinic, Rochester, MN 55905; e-mail: knutson.keith@mayo.edu.
References
Author notes
M.D.B. and W.M.W. contributed equally to this work.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal