Abstract
Multicentric Castleman disease (MCD) is a devastating human herpesvirus 8 (HHV-8)–related lymphoproliferative disorder that occurs in immunocompromised persons. To determine the role of immune responses in MCD, we studied the frequency, antigenic repertoire, differentiation, and functional profile of HHV-8–specific CD8+ T cells in MCD patients and in human immunodeficiency virus–coinfected asymptomatic HHV-8 carriers (AC). Screening CD8+ T-cell responses with ELISpot interferon-γ (IFN-γ) assays using 56 peptides on 6 latent and lytic HHV-8 proteins showed that MCD and AC patients had responses of similar magnitude and antigenic repertoire and identified a new 10-mer human leukocyte antigen B7 CD8 epitope in K15. Intracellular IFN-γ staining showed significantly more CD45RA−CCR7−CD27− CD8+IFN-γ+ cells (late phenotype) and significantly fewer CCR7−CD27+CD45RA− cells (early and intermediate phenotype) in MCD than in AC patients. This phenotypic shift was not found for Epstein-Barr virus–specific CD8+ T cells tested as controls. HHV-8 viral loads were negatively correlated with early and intermediate effector memory cells. HHV-8–specific T cells were polyfunctional (secretion of IFN-γ, tumor necrosis factor-α, macrophage inflammatory protein-1β, and/or CD107a) in both MCD and AC patients. In conclusion, MCD is not associated with a lack of HHV-8–specific CD8+ T cells or limitation of their functional profile. Their differentiation increases with HHV-8 viral load. These results offer new insight into the pathophysiology of MCD.
Introduction
Multicentric Castleman disease (MCD) is a rare but devastating polyclonal lymphoproliferative disorder observed in human immunodeficiency virus (HIV)–infected and non–HIV-infected elderly persons, most of whom have had Kaposi sarcoma (KS).1 Human herpesvirus-8 (HHV-8; or KS-associated virus) is detected in all HIV-related and approximately 40% of HIV-unrelated MCD cases.2 Clinical MCD exacerbations coincide with HHV-8 replication and high viral loads in blood and affected organs,3,4 and infected cells in MCD lesions preferentially express lytic rather than latent HHV-8 proteins.5 Given that HHV-8 chronic infection is asymptomatic in immunocompetent persons, that MCD is much more prevalent among the immunocompromised, and that exacerbations can regress spontaneously,1,4 immune responses to the virus are likely to play a role in the pathophysiology of MCD. Very high HHV-8 viral loads during MCD exacerbations suggest that immune control of the virus is lost.
T-cell responses and especially CD8+ T cells play a key role in the control of chronic viral infections. A linear differentiation model was proposed for memory or antigen-experienced CD8+ T cells: (1) central memory CD45RA−CCR7+CD27+ cells, which have the ability to migrate to lymph nodes6,7 ; (2) early and intermediate effector memory CD45RA−CCR7−CD27+ cells, with high proliferative capacity and low cytolytic activity8,9 ; (3) CD45RA−CCR7−CD27− cells, so-called late effector memory cells, with strong effector functions such as cytolytic capacity9 ; and (4) CD45RA+CCR7−CD27− cells, known as fully differentiated effector cells, which retain those functional capacities but are stably nonreplicative and apoptosis-resistant memory cells.6,10 This model has been extrapolated from several chronic viral infections (HIV, Epstein-Barr virus [EBV], human cytomegalovirus [HCMV], and hepatitis C virus); each infection elicits a dominant stage of CD8+ T-cell differentiation, influenced by the amount of antigenic exposure.8 In humans, several situations involving viral replication and an absence of immune control during active viral diseases have been associated with either increased (HCMV)11 or decreased (EBV and HIV)12 differentiation of virus-specific memory T cells. In addition to the differentiation of CD8+ T cells, their functional profile (ie, the number of cytokines, chemokines, degranulation markers, or cytotoxic molecules they secrete after antigenic stimulation) seems to play a key role in controlling several chronic viral infections (herpes simplex virus, EBV, HCMV, and HIV).13,14
T-cell responses to HHV-8 have been characterized only in asymptomatic chronically HHV-8–infected patients or Kaposi sarcoma (KS) patients. These responses are functionally cytotoxic15,16 and are directed against several latent and lytic proteins,15-25 but only approximately 15 CD8 and CD4 epitopes have been described so far. T-cell responses to HHV-8 seem to control primary infection,20 are correlated with HHV-8 viral loads in peripheral blood,18 increase during immune restoration with antiretroviral therapy for HIV infection,23,26 and, above all, seem to protect against KS because they are detected more often and in larger quantities in asymptomatic carriers than in KS patients.25,27 HHV-8–specific T cells appear to differentiate toward an effector phenotype in kidney-transplanted patients with controlled HHV-8 infection.27
HHV-8–specific T-cell responses may play a role in the pathogenesis of MCD, but they have not yet been studied in this clinical situation. The association of dysfunction and impaired differentiation of EBV-specific CD8+ T cells and high EBV viral loads with progression to AIDS-related non-Hodgkin lymphomas12 suggest that similar immune disorders may occur during HHV-8–related MCD. Our objectives were to study the magnitude, repertoire, functional profile, and differentiation of HHV-8–specific CD8+ T cells in MCD patients and asymptomatic HHV-8 carriers (AC), with EBV-specific CD8+ T cells studied as a control. We report here that the magnitude and functional profile of HHV-8–specific CD8+ T cells are similar in AC and MCD patients. These results suggest that the pathophysiology of MCD, contrary to KS, does not imply a lack of HHV-8–specific CD8+ T cells or a limitation of their functional profile. MCD patients had more highly differentiated cells as well as higher viral loads than AC patients. This suggests that early and intermediate effector memory HHV-8–specific T cells are correlates of immune control of HHV-8 replication.
Methods
Subjects
The study included 24 patients (23 men and 1 woman) seropositive for HHV-8, classified in 2 groups (Table 1). The first group included 12 subjects with MCD with diagnosis confirmed on peripheral lymph node biopsy; 10 were also HIV-infected, whereas 2 were not but rather had classic MCD. Blood samples were collected from 6 patients during episodes of clinical MCD exacerbation and from 6 patients during remission. Five MCD patients had currently active cutaneous KS, and 2 had KS in remission. The second group comprised AC (HIV-coinfected), matched for CD4 cell count (AC group, n = 12), and without KS or MCD, divided in 2 subgroups: long-term nonprogressors (n = 6, defined as HIV-infected persons who had been untreated and asymptomatic for > 8 years, with CD4 T-cell counts > 600/mm3 and stable for > 5 years28 ) and chronically infected progressors (n = 6) for HIV infection. Peripheral blood mononuclear cells (PBMC) from the AC patients had been used previously for ELISpot interferon-γ (IFN-γ) assays of their HHV-8–specific responses.25 Informed consent to participate in the study was obtained from all patients, and the study complied with the human experimentation guidelines of Pitié-Salpêtrière Hospital. Table 1 summarizes the patients' ages, CD4+ cell counts, and HHV-8 and HIV viral loads. Finally, samples from 7 blood bank donors seronegative for HHV-8 (kindly provided by E. Robinet of the Etablissement Français du Sang, Besançon, France) were tested as a control group.
Patient characteristics
| MCD patients (n=12) . | AC, HIV coinfected (n=12) . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code . | HIV . | Age . | MCD status . | CD4/mm3 . | HIV VL (cp/mL) . | Associated-KS . | HHV-8 VL (cp/1.5 105 PBMC) . | Code . | Age . | CD4/mm3 . | HIV VL (cp/mL) . | HHV-8 VL (cp/1.5 105 PBMC) . |
| MCD MED | + | 39 | A | 260 | ND | - | 365 | AC Prog 210 | 42 | 279 | 70 000 | <10 |
| MCD NIV | + | 38 | A | 180 | ND | - | <10 | AC Prog 238 | 48 | 273 | <800 | ND |
| MCD IOP | + | 56 | A | 171 | 100 000 | A | ND | AC LTNP 9006 | 38 | 258 | 40 000 | <10 |
| MCD RAM | + | ND | A | 92 | ND | A | ND | AC Prog 212 | 41 | 258 | <800 | <10 |
| MCD NEG | + | 37 | A | 34 | ND | A | 240 | AC Prog 204 | 40 | 254 | ND | <10 |
| MCD LOR | + | ND | A | 260 | ND | A | ND | AC Prog REV | 57 | 323 | <50 | <10 |
| MCD FEL | + | 60 | R | 492 | <50 | A | 1068 | AC LTNP 8021 | 36 | 694 | 9800 | 11333 |
| MCD IBM | + | 27 | R | 327 | <20 | - | <10 | AC LTNP 12001 | 33 | 488 | 7800 | <10 |
| MCD REP | + | 41 | R | 305 | ND | R | 837 | AC Prog NAH | 34 | 361 | <20 | <10 |
| MCD QOC | + | 62 | R | 445 | <50 | - | <10 | AC LTNP 4035 | ND | 417 | <20 | <10 |
| MCD UAG | − | 86 | R | 1789 | - | - | 12770 | AC LTNP 2002 | 42 | 1895 | 830 | <10 |
| MCD EUG | − | 66 | R | 789 | - | R | 51 | AC LTNP 11007 | 44 | 709 | 4200 | <10 |
| Median | - | 48a | - | 282b | 50c | - | 240d | 41a | 342b | 830c | 10d | |
| MCD patients (n=12) . | AC, HIV coinfected (n=12) . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code . | HIV . | Age . | MCD status . | CD4/mm3 . | HIV VL (cp/mL) . | Associated-KS . | HHV-8 VL (cp/1.5 105 PBMC) . | Code . | Age . | CD4/mm3 . | HIV VL (cp/mL) . | HHV-8 VL (cp/1.5 105 PBMC) . |
| MCD MED | + | 39 | A | 260 | ND | - | 365 | AC Prog 210 | 42 | 279 | 70 000 | <10 |
| MCD NIV | + | 38 | A | 180 | ND | - | <10 | AC Prog 238 | 48 | 273 | <800 | ND |
| MCD IOP | + | 56 | A | 171 | 100 000 | A | ND | AC LTNP 9006 | 38 | 258 | 40 000 | <10 |
| MCD RAM | + | ND | A | 92 | ND | A | ND | AC Prog 212 | 41 | 258 | <800 | <10 |
| MCD NEG | + | 37 | A | 34 | ND | A | 240 | AC Prog 204 | 40 | 254 | ND | <10 |
| MCD LOR | + | ND | A | 260 | ND | A | ND | AC Prog REV | 57 | 323 | <50 | <10 |
| MCD FEL | + | 60 | R | 492 | <50 | A | 1068 | AC LTNP 8021 | 36 | 694 | 9800 | 11333 |
| MCD IBM | + | 27 | R | 327 | <20 | - | <10 | AC LTNP 12001 | 33 | 488 | 7800 | <10 |
| MCD REP | + | 41 | R | 305 | ND | R | 837 | AC Prog NAH | 34 | 361 | <20 | <10 |
| MCD QOC | + | 62 | R | 445 | <50 | - | <10 | AC LTNP 4035 | ND | 417 | <20 | <10 |
| MCD UAG | − | 86 | R | 1789 | - | - | 12770 | AC LTNP 2002 | 42 | 1895 | 830 | <10 |
| MCD EUG | − | 66 | R | 789 | - | R | 51 | AC LTNP 11007 | 44 | 709 | 4200 | <10 |
| Median | - | 48a | - | 282b | 50c | - | 240d | 41a | 342b | 830c | 10d | |
VL indicates viral load; cp, copies; A, active; R, remission; Prog, progressor for HIV infection; LTNP, long-term nonprogressor for HIV infection; and ND, not determined.
*P = .29, nonparametric Mann-Whitney test.
†P = .35, nonparametric Mann-Whitney test.
‡P = .51, nonparametric Mann-Whitney test.
§P = .02, nonparametric Mann-Whitney test.
HHV-8 serology and viral load
HHV-8 serology detected antibodies against latency-associated nuclear antigen-1 (LANA-1) in an indirect immunofluorescence assay.29 Reactive serum samples with 1:100 dilutions were considered positive. DNA from PBMC was extracted with the QIAamp system (QIAgen, Chatsworth, CA), then assayed with real-time polymerase chain reaction and quantified with fluorescent TaqMan methods on an ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA). This combined the quantification of HHV-8 and albumin gene DNA, as described previously.30 HHV-8 viral load in PBMCs was expressed as the absolute number of viral copies of HHV-8 genome in 150 000 human diploid cells, with a positive threshold of 10 copies/1.5 × 105 PBMCs.
Synthetic peptides
HHV-8 peptides.
Overall, 56 HHV-8 peptides, 9 to 15 mer each, were synthesized (Epytop, Nimes, France) and purified to more than 80% by high-performance liquid chromatography, as described previously25 : 4 HHV-8 T epitopes17,18,23 were synthesized on lytic glycoproteins (Gp) H-ORF22 (GpH59-68), 35/37-ORF K8.1 (Gp35/37131-145 and Gp35/37211-225), and B-ORF8 (GpB492-500); 52 peptides25 were synthesized on 3 latent viral proteins of interest, none with any sequence similarity to EBV genes, and expressed in MCD lesions LANA-1,5 K15,31 and K12.32 Specifically, 15-mer peptides overlapping by 10 amino acids (aa) were synthesized to span the entire K12 sequence (containing the optimal LLNGWRWRL epitope18,22 ) and the first 3 exons of the predominant form33 of the K15 protein. Nine 9- and 10-mer peptides predicted to bind the human leukocyte antigen A2 (HLA-A2) molecule in the LANA-1 sequence34 were synthesized. HHV-8 T-epitopes were tested individually, and peptides from latent proteins were tested in pools of 6 to 10 peptides in ELISpot assays.
EBV peptides.
ELISpot IFN-γ analysis
Ninety-six–well polyvinylidene difluoride-bottomed well plates (Millipore, Molsheim, France) were coated with capture anti-human IFN-γ monoclonal antibody (mAb) (Diaclone, Besançon, France). After saturation, thawed PBMC were added in duplicate or triplicate wells (105 cells per well) and incubated 20 hours at 37°C in 5% CO2 in the presence of peptides at a final concentration of 5 μg/mL when tested individually and of 2 μg/mL when tested in pools. Phytohemagglutinin-p (PHAp; Murex, Paris, France) at a final concentration of 0.5 μg/mL was used as a positive control and medium alone as a negative control. After washing, the second biotinylated anti–IFN-γ mAb (Diaclone, Paris, France) was added, then streptavidin-alkaline phosphatase conjugate (Amerham, Les Ulis, France) and chromogen substrates. After drying, spots were counted with a dissection microscope. The number of peptide-specific T cells, expressed as spot-forming cells (SFC)/106 PBMC, was calculated after subtracting negative control values. ELISpot assay results were considered positive when they exceeded 50 SFC/106 PBMC (ie, the mean response plus 2 standard deviations of blood donors). The median background value for all subjects was 3 (0-30) SFC/106 PBMC (ie, in the same range as interlaboratory background values in ELISpot assays in a recent study).36
CD8+ T-cell depletion
Immunomagnetic beads specific for CD8 (Dynal Biotech, Oslo, Norway) were used for negative selection of CD8+ PBMCs. Frozen PBMCs were thawed and mixed with CD8 beads for 1 hour at 4°C in a 2-mL plastic tube (Becton Dickinson, Meylan, France). The tube was then placed on a magnet for 3 to 5 minutes, and the bead-linked CD8+ -enriched T cells were removed. CD8+ cells accounted for less than 0.1% of the remaining cells. T cells enriched with CD4+ cells were used as effectors in the ELISpot IFN-γ assays.
Cell-surface and intracellular staining by multicolor flow cytometry
Purified PBMC were thawed, resuspended to 2.106 cells/mL, and left to rest 4 hours at 37°C in 5% CO2. One million PBMCs were incubated for 6 hours in the presence of peptides or peptide pools detected as HHV-8 epitopes in the ELISpot assay, at a final concentration of 5 μg/mL for peptides alone or 2 μg/mL for peptide pools, 2 μg/mL for EBV peptide pools, positive control antigen (PHAp, 0.5 μg/mL, Murex or Staphylococcal enterotoxin B, 1 μg/mL; Sigma-Aldrich, St Louis, MO), or RPMI 1640 + alone as a negative control. Cytometric analysis was then performed according to 2 separate protocols: phenotypic differentiation and a functional profile of HHV-8– and EBV-specific T cells. All data files contained at least 75 000 events positive for CD8 fluorescence per lymphocyte gate.
For phenotypic differentiation, 5 μg/mL Brefedin A (Sigma-Aldrich) was added in the last hour of incubation. Cells were harvested, conserved overnight at 4°C, washed in phosphate-buffered saline (PBS) containing 1% bovine serum albumin and 0.1% sodium azide (PBS-BSA), stained with anti–CD8-PC7, CD45RA-ECD (Beckman-Coulter, Fullerton, CA), anti-CCR7-PE (R&D Systems, Minneapolis, MN), and CD27-fluorescein isothiocyanate (FITC; BD Biosciences, San Jose, CA) for 15 minutes, fixed with PBS containing 1% paraformaldehyde (PFA 1%), permeabilized with 0.1% saponin, and stained internally with allophycocyanin (APC)–conjugated IFN-γ mAb (BD Biosciences). After staining, cells were washed and fixed (PFA 1%) and underwent immediate flow cytometric analysis with a 5-color flow cytometer (FC500; Beckman-Coulter). The lymphocyte gate was selected in a forward scatter area (FSC-A) versus side scatter (SSC) plot. IFN-γ production was considered positive if more than 0.1% of the CD8+ cells were IFN-γ positive after subtraction of the negative control values, if IFN-γ production exceeded twice the background values, and if the absolute number of CD8+ IFN-γ+ cells exceeded 200. Phenotypic analysis of virus-specific cells was performed on CD8 high+ IFN-γ+ – gated cells.
For functional profile, cells were incubated with specific antigens, costimulatory antibodies (CD28 and CD49d; 1 μg/mL; BD Biosciences), and anti–CD107a-PECy5 antibody (BD Biosciences) and left for 6 hours at 37°C. Brefeldin A (Sigma-Aldrich; at 5 μg/mL final concentration) and Monensin (Sigma, at 2.5 μg/mL final concentration) were added during the second hour of incubation. Cells were harvested and conserved overnight at 4°C. After washing in PBS, 0.5 mM EDTA, and 1% BSA, cell surfaces were stained for 15 minutes at room temperature in the dark, with anti–CD8-AmCyan, anti–CD3-Pacific Blue (BD Biosciences) and anti–CD4-ECD (Beckman-Coulter). The cells were washed and then fixed and permeabilized with Fix&Perm kit (Invitrogen, Carlsbad, CA) according to instructions. The intracellular staining used the following panel of antibodies: IFN-γ–Alexa700, interleukin-2 (IL-2)–APC, tumor necrosis factor-α (TNFα)–PECy7, macrophage inflammatory protein-1β (MIP1β)—FITC, and CD40L-PE (BD Biosciences). Cells were then washed and fixed (CellFix, BD Biosciences) and underwent flow cytometric analysis on an 11-color flow cytometer (LSR II, BD Biosciences). Initial gating used a FSC-A versus height (FSC-H) plot to remove doublets, and the lymphocyte gate was selected in an FSC-A versus SSC-A plot. Results were considered positive if more than 0.1% of the CD8 high or CD4+ cells were IFN-γ, IL-2, TNF-α, CD107a, or MIP1β positive after subtraction of the negative control values for the corresponding function, if cytokine/chemokine production exceeded twice the background values, and if the absolute number of IFN-γ–, IL-2–, TNF-α–, CD107a–, or MIP1β-positive cells exceeded 200.
Statistical analysis
Statistical analysis was performed with StatView 5.0 software (SAS Institute, Cary, NC). The Mann-Whitney nonparametric test was used to compare groups for CD4 cell counts, HHV-8 viral loads, and specific antiviral T cells. A Spearman nonparametric correlation test was used to examine correlations between individual indicators (proportions of T-cell populations, CD4 cell counts, and HHV-8 viral loads).
Results
Characteristics of MCD patients and AC
We studied 24 HHV-8–infected patients including 12 diagnosed with MCD and 12 HIV-coinfected AC. Patients' ages did not differ between the groups (Table 1). HLA class 1 genotypes were similarly distributed (A2: 8/12 MCD, 8/12 AC; B7: 3/12 MCD, 3/12 AC; A24: 1/12 MCD, 2/12 AC; B44: 4/12 MCD, 1/12 AC). CD4+ cell counts were similar in the MCD and AC groups (median 282 [34-1789]/mm3 and 342 [254-1895]/mm3; P = .35; nonparametric Mann-Whitney test), as were HIV plasma viral loads (median 50 [<20-100 000] and 830 [<20-70 000] copies/mL; P = .51; Table 1). As expected, HHV-8 viral loads in PBMC were higher in MCD (median 240 [<10-12 770] copies/150 000 PBMC) than in AC patients (median 10 [<10-11 333] copies/150 000 PBMC; P = .02; Table 1). HHV-8 viral loads in PBMC did not differ for MCD+ KS+ and MCD+ KS− patients (median 538 [51-1068] and 10 [10-12 770] copies/150 000 PBMC, respectively; P = .32; nonparametric Mann-Whitney test). As reported previously,37 there was no correlation between HHV-8 PBMC and HIV plasma viral loads (P = .62; Spearman nonparametric correlation test).
Similar magnitude of HHV-8–specific T-cell responses in MCD patients and AC
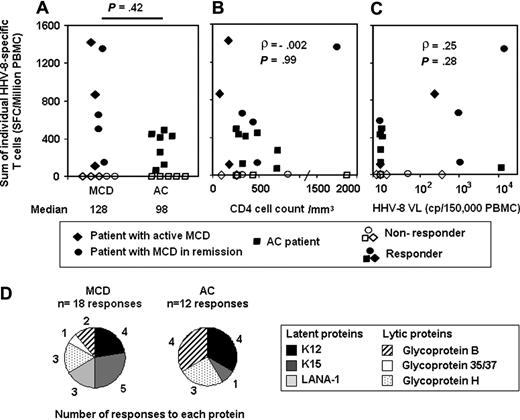
T-cell responses against HHV-8 were tested in ELISpot IFN-γ assays by using 4 epitopes in lytic GpB, GpH, and Gp35/37, 9 optimal peptides predicted according to a theoretical HLA-A2 binding score in the latent LANA-1 protein, and 43 overlapping (by 10 aa) peptides, 10 to 15 mer in length, covering all of the latent protein K12 and exons 1-3 (aa 1-185) in the latent protein K15. All HHV-8 peptides synthesized (n = 56) were tested in 7 HLA-matched HHV-8 seronegative blood donors, and no positive response to any peptide was observed in ELISpot IFN-γ assays (data not shown). All peptides were tested in all MCD and AC patients, and T-cell responses against HHV-8 could be detected in 7 of 12 (58%) MCD (1 classic and 6 HIV-related, 3 active MCD, and 4 in remission) patients and in 7 of 12 (58%) AC subjects (Figure 1A). The magnitude of HHV-8–specific T cells detected was equivalent in both groups (P = 0.42; nonparametric Mann-Whitney test; median response 128 [0-1420] and 98 [0-490] SFC/106 PBMC, respectively), and the magnitude of positive responses was slightly higher in MCD than in AC patients (median 647 [110-1420] and 410 [70-490] SFC/106 PBMC, respectively; P = .08). Magnitude of response did not differ among MCD responders with active disease or in remission (3/6 and 4/6 responders; median 55 and 332 SFC/106 PBMC, respectively; P = .74) or among MCD patients with or without KS (2/5 and 5/7 responders; median 0 and 519 SFC/106 PBMC, respectively; P = .27). The number of HHV-8–specific T cells was not correlated with CD4 cell counts (ρ = −0.002; P = .99; Figure 1B) or with HHV-8 viral load in PBMC (ρ = 0.25; P = .28; Figure 1C), according to the nonparametric Spearman correlation test. EBV pools of optimal epitopes were tested simultaneously in ELISpot assays for each patient as controls for antiviral T-cell responses. All but 2 patients responded to EBV epitopes, and magnitude of response did not differ between MCD and AC patients (median169 and 564 SFC/106 PBMC, respectively; P = .14). These results suggest that MCD patients, either active or in remission, retain as many IFN-γ–secreting HHV-8–specific T cells as AC patients, regardless of either HHV-8 replication or their HIV-induced CD4 cell count deficiency.
Magnitude and antigenic repertoire of HHV-8–specific T cells as assessed by ELISpot IFN assays in patients with MCD (n = 12) and HIV-coinfected AC (n = 12). HHV-8 ELISpot IFN-γ assay used 56 HHV-8–specific peptides, including 4 previously identified epitopes in lytic GpB, GpH, and Gp35/37, 9 optimal peptides predicted in latent LANA-1, and 43 overlapping peptides covering latent K12, and exons 1-3 in latent K15. Results are given as frequencies of SFC/106 PBMC. (A) Individual magnitude of HHV-8–specific T-cell responses in MCD and AC patients. The sum of positive responses (> 50 SFC/106 PBMC) was calculated individually. Solid symbols represent patients with positive responses and empty symbols patients with negative responses. The Mann-Whitney nonparametric test was used to compare groups. (B) No correlation between individual sum of each person's HHV-8–positive T-cell responses and CD4 cell counts in peripheral blood (cells/mm3), calculated with the Spearman nonparametric test in all 24 patients. (C) No correlation between the sum of each person's HHV-8–positive T-cell responses and HHV-8 viral load in PBMC (copies/150 000 PBMC), calculated with the Spearman nonparametric test in 20 patients (4 viral load missing). VL indicates viral load; cp, copies. (D) Antigenic repertoire of HHV-8–specific T-cell responses. Number of positive responses against each protein is shown for MCD and AC group.
Magnitude and antigenic repertoire of HHV-8–specific T cells as assessed by ELISpot IFN assays in patients with MCD (n = 12) and HIV-coinfected AC (n = 12). HHV-8 ELISpot IFN-γ assay used 56 HHV-8–specific peptides, including 4 previously identified epitopes in lytic GpB, GpH, and Gp35/37, 9 optimal peptides predicted in latent LANA-1, and 43 overlapping peptides covering latent K12, and exons 1-3 in latent K15. Results are given as frequencies of SFC/106 PBMC. (A) Individual magnitude of HHV-8–specific T-cell responses in MCD and AC patients. The sum of positive responses (> 50 SFC/106 PBMC) was calculated individually. Solid symbols represent patients with positive responses and empty symbols patients with negative responses. The Mann-Whitney nonparametric test was used to compare groups. (B) No correlation between individual sum of each person's HHV-8–positive T-cell responses and CD4 cell counts in peripheral blood (cells/mm3), calculated with the Spearman nonparametric test in all 24 patients. (C) No correlation between the sum of each person's HHV-8–positive T-cell responses and HHV-8 viral load in PBMC (copies/150 000 PBMC), calculated with the Spearman nonparametric test in 20 patients (4 viral load missing). VL indicates viral load; cp, copies. (D) Antigenic repertoire of HHV-8–specific T-cell responses. Number of positive responses against each protein is shown for MCD and AC group.
Antigenic repertoire of HHV-8–specific T cells in responders and identification of a new 10-mer CD8 epitope in K15 protein
We detected HHV-8–specific T cells in 14 of 24 patients and analyzed the targets of these responses. Responses were observed against all proteins tested: 18 and 12 responses in 7 MCD and 7 AC patients, respectively (Figure 1D). The repertoire of T-cell responses was not very different in both groups, directed mainly against latent proteins K12 (4 MCD and 4 AC responders; median response 1075 [110-1253] and 106 [65-450] SFC/106 PBMC, respectively) and K15 (5 MCD and 1 AC; median response 137 [55-195] and 403 SFC/106 PBMC, respectively), and lytic GpB (2 MCD [80 and 550 SFC/106 PBMC] and 4 AC [median response 170 (70-240) SFC/106 PBMC]) and GpH (3 MCD and 3 AC: 90, 120, and 315; 125, 113, and 220 SFC/106 PBMC, respectively).
Peptides from HHV-8 peptide pools inducing positive responses were tested separately in ELISpot assays, and new epitopes identified in AC patients have been described previously.25 We also identified a new immunogenic peptide in the K15 protein in MCD patients. Pool 4 in K15 protein elicited responses in 4 MCD patients (MCD UAG, FEL, REP, and IOP). Fifteen-mer pool K15-4 peptides (aa 151-185) were tested separately in 2 HLA-B7 responders to the pool (MCD UAG and IOP, 103 and 195 SFC/106 PBMC, respectively) for whom PBMC were available. Only the 15-mer peptide K15166-180 ATVKTGNIKLVSSVS elicited positive response in both patients (300 and 75 SFC/106 PBMC, respectively); the 10-mer peptide K15166-175 ATVKTGNIKL predicted34 to bind HLA-B7 was synthesized and tested in the MCD UAG patient with available PBMC and did elicit a response (123 SFC/106 PBMC). The loss of response to the K15166-175 peptide (20 SFC/106 PBMC) after CD8+ T-cell depletion indicates a CD8+ phenotype for the IFN-γ–secreting T cells. This 10-mer peptide was tested as a negative control in PBMC from an HLA-B7 HHV-8–seronegative blood donor and elicited no response (data not shown). Together, these results suggest that this optimal 10-mer K15166-175 peptide is an HLA-B7–restricted CD8 T epitope.
Advanced differentiation phenotype of effector memory HHV-8–specific CD8+ T cells during MCD
After observing that the magnitude and antigenic repertoire of HHV-8–specific T cells persisted during MCD, we hypothesized that these cells have distinct patterns of differentiation in AC and MCD patients. HHV-8 T-cell responses detected in the ELISpot assay were analyzed in flow cytometry with intracellular IFN-γ staining and the classic cell-surface differentiation markers for CD8+ T cells (CD45RA, CCR7, and CD27; Figure 2A). The median percentage of IFN-γ+ nonstimulated CD8+ cells for all HHV-8 peptide responders was 0.09% (0.01-1.09). Pools of EBV peptides were tested simultaneously as a comparison for phenotypic differentiation of specific antiviral CD8+ T cells. HHV-8–specific CD8+ T-cell phenotypes were analyzed in 6/7 MCD and in 7/7 AC ELISpot responders from whom PBMC were available. The percentage of CD8+ IFN-γ+ HHV-8 peptide–specific T cells ranged from 0.1% to 1.45% of CD8+ T cells (Figure 2A). The phenotype of CD8+ IFN-γ+ HHV-8 peptide–specific T cells was mainly CD45RA−CCR7−CD27− in both the MCD (median 84.5 [68.5-96.9]%) and AC (median 72.5 [62.2-85.1]%) groups. However, the proportion of these late effector memory cells was significantly higher in MCD than in AC patients (P = .03; nonparametric Mann-Whitney test; Figure 2B) and was similar in the MCD patients tested whether they had active disease or were in remission (Figure 2B). Confirming this trend of an advanced differentiated phenotype of HHV-8–specific CD8+ cells in MCD patients, we found significantly fewer early and intermediate effector memory CD45RA−CCR7−CD27+ HHV-8–specific T cells in MCD than in AC patients (median 4.2 [1.4-12.2] and 7.1 [4.1-27.6]%, respectively; P = .03; nonparametric Mann-Whitney test; Figure 2B). Finally, the proportion of fully differentiated effector memory CD45RA+CCR7−CD27− HHV-8–specific T cells was similar in MCD and in AC patients (median 8.1 [0.2-11.7] and 8.9 [5.2-19.3]%, respectively; P = .32; nonparametric Mann-Whitney test; Figure 2B). Figure 2C illustrates in 2 representative patients from each group the shift from CD27+ cells toward CD27− CD8+ IFN-γ+ HHV-8–specific T cells: HHV-8–specific CD8+ T cells are more differentiated in the MCD patient.
Differentiation phenotypes of HHV-8 CD8+ T cells. Flow cytometric analysis of CD45RA, CCR7, and CD27 coexpression gated on IFN-γ–producing CD8+ PBMC after stimulation with appropriate HHV-8 epitopes as detected in the ELISpot-IFN-γ assay, and performed as described in “Methods.” (A) Individual percentage of CD8+ cells producing IFN- as assessed by intracellular cytokine staining after HHV-8 peptide stimulation. PBMC were stimulated by 1-10 HHV-8–specific peptides in the same tube. Each symbol represents a separate patient, with the shape corresponding to the disease form (the 2 rhombuses represent 2 patients with active MCD; the 4 circles, 4 patients with MCD in remission; the 7 squares, 7 AC). (B) Subsets of virus-specific CD8+ T cells producing IFN-γ. Each symbol represents a different patient, with shapes corresponding to disease form as in panel A. Only relevant IFN-γ–secreting CD8+ populations are depicted (CCR7−CD27+CD45RA−, CCR7−CD27−CD45RA−, and CCR7−CD27−CD45RA+ CD8+ IFN-γ+ cells, corresponding to early and intermediate, late effector, and fully differentiated effector memory cells). Median percentages of other populations were lower than 1% of CD8+ IFN-γ+ cells. The Mann-Whitney nonparametric test was used to compare groups. (C) Each display represents cytometric images for CD8hi+ IFN-γ+ cells, and their corresponding complete CCR7, CD27, and CD45RA phenotype. Proportions of cells in each quadrant are presented in the labels. Nonstimulated cells, HHV-8 peptide– and EBV peptide–stimulated cells are depicted, respectively. Each display represents a representative donor from each group.
Differentiation phenotypes of HHV-8 CD8+ T cells. Flow cytometric analysis of CD45RA, CCR7, and CD27 coexpression gated on IFN-γ–producing CD8+ PBMC after stimulation with appropriate HHV-8 epitopes as detected in the ELISpot-IFN-γ assay, and performed as described in “Methods.” (A) Individual percentage of CD8+ cells producing IFN- as assessed by intracellular cytokine staining after HHV-8 peptide stimulation. PBMC were stimulated by 1-10 HHV-8–specific peptides in the same tube. Each symbol represents a separate patient, with the shape corresponding to the disease form (the 2 rhombuses represent 2 patients with active MCD; the 4 circles, 4 patients with MCD in remission; the 7 squares, 7 AC). (B) Subsets of virus-specific CD8+ T cells producing IFN-γ. Each symbol represents a different patient, with shapes corresponding to disease form as in panel A. Only relevant IFN-γ–secreting CD8+ populations are depicted (CCR7−CD27+CD45RA−, CCR7−CD27−CD45RA−, and CCR7−CD27−CD45RA+ CD8+ IFN-γ+ cells, corresponding to early and intermediate, late effector, and fully differentiated effector memory cells). Median percentages of other populations were lower than 1% of CD8+ IFN-γ+ cells. The Mann-Whitney nonparametric test was used to compare groups. (C) Each display represents cytometric images for CD8hi+ IFN-γ+ cells, and their corresponding complete CCR7, CD27, and CD45RA phenotype. Proportions of cells in each quadrant are presented in the labels. Nonstimulated cells, HHV-8 peptide– and EBV peptide–stimulated cells are depicted, respectively. Each display represents a representative donor from each group.
EBV-specific T cells, examined as controls for specific antiviral CD8+ T cells, were detected in 4 MCD and 6 AC patients (percentage of CD8+ IFN-γ+ EBV peptide–specific T cells ranged from 0.14% to 0.71% of CD8+ T cells; data not shown). Late effector memory CD45RA−CCR7−CD27− CD8+ EBV-specific T cells were present in similar quantities in MCD and AC patients (median 67.9 [54.5-77.3] and 75.3 [44.2-89.8]%, respectively; P = .29; nonparametric Mann-Whitney test; data not shown). Figure 2C illustrates EBV-specific CD8+ T-cell phenotypes in 2 representative patients from each group.
These findings suggest that HHV-8–specific CD8+ T cells differentiate more specifically in MCD than in AC patients and that this difference is not related to any global advanced differentiation of antiviral CD8+ cells in MCD patients.
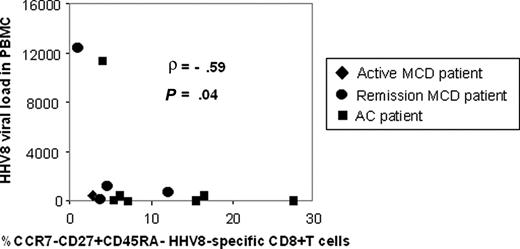
Looking for clinical correlations of this more advanced differentiation of HHV-8–specific CD8+ T cells in MCD patients, we compared the percentage of CCR7−CD27+CD45RA− early and intermediate effector memory HHV-8–specific cells with the individual HHV-8 viral loads in all patients. We found a significant negative correlation between these indicators (ρ = −0.59; P = .04; Spearman nonparametric test; Figure 3). This negative correlation suggests that the greater differentiation of HHV-8–specific CD8+ T cells observed in MCD patients is linked to high antigenic burdens. Together, our data suggest that MCD is associated with more advanced differentiation of HHV-8–specific CD8+ T cells, related to high HHV-8 viral loads.
Negative correlation between percentage of HHV-8–specific CCR7−CD27+CD45RA−/CD8+ IFN-γ+ effector cells and individual HHV-8 viral loads. The percentage of HHV-8–specific CCR7−CD27+CD45RA− early and intermediate effector memory cells in CD8+ IFN-γ+ cells as assessed by intracellular IFN-γ staining after HHV-8 epitope stimulation was negatively correlated with individual HHV-8 viral loads in PBMC for 5 MCD and 7 AC patients (Spearman nonparametric test). Each symbol represents a patient. Rhombus shapes represent patients with active MCD; circles, MCD patients in remission; squares, AC.
Negative correlation between percentage of HHV-8–specific CCR7−CD27+CD45RA−/CD8+ IFN-γ+ effector cells and individual HHV-8 viral loads. The percentage of HHV-8–specific CCR7−CD27+CD45RA− early and intermediate effector memory cells in CD8+ IFN-γ+ cells as assessed by intracellular IFN-γ staining after HHV-8 epitope stimulation was negatively correlated with individual HHV-8 viral loads in PBMC for 5 MCD and 7 AC patients (Spearman nonparametric test). Each symbol represents a patient. Rhombus shapes represent patients with active MCD; circles, MCD patients in remission; squares, AC.
Functional profiles of HHV-8–specific T cells during MCD and HHV-8 asymptomatic infection
Besides looking at the stage of differentiation of HHV-8–specific CD8+ T cells during MCD, we explored their functional profile as another correlate of HHV-8 immune escape. We used an 11-color flow cytometric assay to measure simultaneously and independently degranulation by CD107a mobilization of CD8+ T cells and their intracellular production of IFN-γ, IL-2, TNF-α, and MIP1β after appropriate HHV-8 peptide stimulation. EBV optimal epitopes were used as a control for the T-cell functional profile. Three MCD and 4 AC patients from whom PBMC were available were studied (Table 2). HHV-8–epitope stimulations elicited IFN-γ production in all 7 cases (0.18-1.39% of CD8+ cells). MIP1β production was observed in 6/7 cases (0.52-2.11% of CD8+ T cells), TNF-α production in 4/7 cases (0.15-0.75%), and CD107a mobilization in 4/7 cases (0.32-0.80%). We observed no significant IL-2 CD8+ production nor any CD4+ T-cell response (data not shown). Polyfunctional response (> 1 function) was observed in 2/3 MCD and 4/4 AC patients (Table 2). Polyfunctional responses against EBV peptides were observed as well in 2 MCD and 4 AC patients (data not shown). These results suggest that polyfunctional HHV-8–specific CD8+ T cells are not a correlate of protection against MCD.
Functional profiles of HHV-8–specific CD8+ T cells in MCD patients and AC
| Patient . | Peptide stimulation . | Functions of HHV-8–specific CD8+ T cells* . | HHV-8 viral load (copies/1.5 105 PBMC) . | CD4 cell count/mm3 . | |||||
|---|---|---|---|---|---|---|---|---|---|
| IFN-γ+ . | IL-2+ . | TNF-α+ . | CD107a+ . | MIP1β+ . | Number of functions† . | ||||
| MCD REP | None | 0.01 | 0.12 | 0.01 | 0.16 | 0.09 | |||
| K15 pools, GpH59-68, GpB492-500 | 0.21 | 0.10 | 0.03 | 0.15 | 0.12 | ||||
| Δ | 0.20 | 0.00 | 0.02 | 0.00 | 0.00 | 1 | 837 | 305 | |
| MCD NEG | None | 0.15 | 0.06 | 0.03 | 0.16 | 0.56 | |||
| GpH59-68, GpB492-500 | 1.54 | 0.09 | 0.18 | 0.21 | 1.30 | ||||
| Δ | 1.39 | 0.03 | 0.15 | 0.05 | 0.74 | 3 | 240 | 34 | |
| MCD UAG | None | 0.02 | 0.13 | 0.02 | 0.40 | 0.53 | |||
| K1231-45, K15166-180 | 0.50 | 0.10 | 0.43 | 0.84 | 1.23 | ||||
| Δ | 0.48 | 0.00 | 0.43 | 0.44 | 0.70 | 4 | 12 770 | 1789 | |
| AC 210 | None | 0.06 | 0.28 | 0.02 | 0.99 | 0.21 | |||
| K1231-45, GpH59-68, GpB492-500 | 0.44 | 0.21 | 0.06 | 1.07 | 0.90 | ||||
| Δ | 0.38 | 0.00 | 0.04 | 0.08 | 0.69 | 2 | <10 | 279 | |
| AC 12001 | None | 0.01 | 0.03 | 0.07 | 0.13 | 0.26 | |||
| K1231-45, LANA1116-1124 | 0.19 | 0.08 | 0.11 | 0.45 | 0.78 | ||||
| Δ | 0.18 | 0.05 | 0.04 | 0.32 | 0.52 | 3 | <10 | 488 | |
| AC 11007 | None | 0.12 | 0.19 | 0.11 | 0.80 | 1.28 | |||
| GpH59-68, GpB492-500 | 1.45 | 0.17 | 0.86 | 1.63 | 3.44 | ||||
| Δ | 1.33 | 0.00 | 0.75 | 0.83 | 2.16 | 4 | <10 | 709 | |
| AC NAH | None | 0.06 | 0.12 | 0.06 | 0.43 | 0.76 | |||
| GpH59-68, GpB492-500 | 0.44 | 0.17 | 0.33 | 0.95 | 1.67 | ||||
| Δ | 0.38 | 0.05 | 0.27 | 0.52 | 0.91 | 4 | <10 | 361 | |
| Patient . | Peptide stimulation . | Functions of HHV-8–specific CD8+ T cells* . | HHV-8 viral load (copies/1.5 105 PBMC) . | CD4 cell count/mm3 . | |||||
|---|---|---|---|---|---|---|---|---|---|
| IFN-γ+ . | IL-2+ . | TNF-α+ . | CD107a+ . | MIP1β+ . | Number of functions† . | ||||
| MCD REP | None | 0.01 | 0.12 | 0.01 | 0.16 | 0.09 | |||
| K15 pools, GpH59-68, GpB492-500 | 0.21 | 0.10 | 0.03 | 0.15 | 0.12 | ||||
| Δ | 0.20 | 0.00 | 0.02 | 0.00 | 0.00 | 1 | 837 | 305 | |
| MCD NEG | None | 0.15 | 0.06 | 0.03 | 0.16 | 0.56 | |||
| GpH59-68, GpB492-500 | 1.54 | 0.09 | 0.18 | 0.21 | 1.30 | ||||
| Δ | 1.39 | 0.03 | 0.15 | 0.05 | 0.74 | 3 | 240 | 34 | |
| MCD UAG | None | 0.02 | 0.13 | 0.02 | 0.40 | 0.53 | |||
| K1231-45, K15166-180 | 0.50 | 0.10 | 0.43 | 0.84 | 1.23 | ||||
| Δ | 0.48 | 0.00 | 0.43 | 0.44 | 0.70 | 4 | 12 770 | 1789 | |
| AC 210 | None | 0.06 | 0.28 | 0.02 | 0.99 | 0.21 | |||
| K1231-45, GpH59-68, GpB492-500 | 0.44 | 0.21 | 0.06 | 1.07 | 0.90 | ||||
| Δ | 0.38 | 0.00 | 0.04 | 0.08 | 0.69 | 2 | <10 | 279 | |
| AC 12001 | None | 0.01 | 0.03 | 0.07 | 0.13 | 0.26 | |||
| K1231-45, LANA1116-1124 | 0.19 | 0.08 | 0.11 | 0.45 | 0.78 | ||||
| Δ | 0.18 | 0.05 | 0.04 | 0.32 | 0.52 | 3 | <10 | 488 | |
| AC 11007 | None | 0.12 | 0.19 | 0.11 | 0.80 | 1.28 | |||
| GpH59-68, GpB492-500 | 1.45 | 0.17 | 0.86 | 1.63 | 3.44 | ||||
| Δ | 1.33 | 0.00 | 0.75 | 0.83 | 2.16 | 4 | <10 | 709 | |
| AC NAH | None | 0.06 | 0.12 | 0.06 | 0.43 | 0.76 | |||
| GpH59-68, GpB492-500 | 0.44 | 0.17 | 0.33 | 0.95 | 1.67 | ||||
| Δ | 0.38 | 0.05 | 0.27 | 0.52 | 0.91 | 4 | <10 | 361 | |
Number of CD8+ cells producing IFN-γ, IL-2, TNF-α, CD107a, or MIP1β/number of CD8+ cells, as assessed by intracellular staining.
Positive threshold: >0.1% of CD8+ cells, >twice background values, and >200 absolute number of CD8+ cytokine/chemokine+ cells.
Discussion
Cellular immunity to HHV-8 is poorly understood, and although several reports have studied HHV-8–specific T-cell responses during KS, none addressed the presence, magnitude, phenotypic differentiation, or functional profile of these responses in HHV-8–related lymphoid disorders such as MCD. This study, using AC with naturally low virus replication as controls, was designed to examine HHV-8–specific CD8+ T-cell responses that we suspected were involved in the control of MCD.
We began by studying the magnitude of T-cell responses to HHV-8, using ELISpot IFN-γ assays against the lytic GpB, Gp35/37, GpH, and latent K12, K15, and LANA-1 HHV-8 proteins, with a large panel of peptides. These assays showed that MCD patients have at least as many HHV-8–specific T-cell populations as AC subjects and thus suggest that MCD is not related to any shortfall in the number of these cells. These findings contrast with what we found in KS patients with the same ELISpot assay: there were very few responders, and their level of response was low.25 Thus, it appears that KS and MCD, both HHV-8–related tumors, are not associated with the same cellular immune status against HHV-8 and that specific T cells may be defective during MCD rather than absent from peripheral blood. Interestingly, the repertoire of T-cell responses in both MCD and AC subjects included both latent and lytic viral proteins, although lytic proteins are reported to be overexpressed in MCD.5 However, the residual expression of latent HHV-8 viral proteins in MCD lymph nodes5 may induce a detectable immune responses in peripheral blood.
The qualitative study of HHV-8–specific CD8+ T cells in MCD patients included intracellular IFN-γ staining with phenotypic analysis of classic markers of CD8+ cell differentiation. We showed that HHV-8–specific CD8+ T cells have a significantly more advanced phenotype of differentiation than AC patients and that this advanced differentiation is associated with high antigenic burden (ie, probably with viral immune escape). These results contrast with findings from EBV asymptomatic carriers, who have fewer EBV-specific CD8+CD27+ T cells than patients with EBV-associated AIDS-related non-Hodgkin lymphoma.12 They also differ from findings from HIV-infected patients, for which a lower proportion of HIV-specific CD8+CD27+ T cells was found in patients with delayed progression to AIDS.12 However, our results are consistent with emerging data on chronic viral infections that show, on the contrary, that higher proportions of CD8+CD27+ virus-specific cells are associated with immune protection against active replicating viral diseases. For example, recovery from AIDS-related HCMV-retinitis is associated with higher proportions of HCMV-specific CD27+ T cells than in patients with active retinitis.11 Similarly, the proportion of the HIV Gag-specific CD8+CD27+ subset is negatively correlated with HIV proviral loads and positively correlated with CD4 cell counts in nonprogressors, a finding that again suggests a protective role for CD8+CD27+ cells against HIV disease progression.38 Moreover, these results are consistent with data showing that CD27 expression is determinant in the survival and proliferation of virus-specific T cells39 as well in their effector and proliferative functions, resistance to apoptosis, and their survival advantage, compared with CD8+CD27− T cells.40
Finally, a functional analysis of HHV-8–specific CD8+ T cells in ELISpot responders simultaneously measured CD107a, IFN-γ, IL-2, TNF-α, and MIP1β production after peptide-specific HHV-8 stimulation. Only a small number of patients could be studied because MCD occurs rarely. However, polyfunctional responses were found in asymptomatic carriers, as in MCD patients. This finding suggests that polyfunctional HHV-8 CD8+ T-cell responses are not a correlate of protection against MCD. We therefore demonstrated that MCD patients can show polyfunctional HHV-8–specific CD8 responses, but we cannot rule out the possibility that other MCD patients might have monofunctional responses.
Taken together, our results suggest that HHV-8–related MCD, contrary to KS, is associated with a high magnitude of HHV-8–specific CD8+ T-cell response, which is at least as strong as during asymptomatic HHV-8 carriage. HHV-8–specific CD8+ T cells in patients with MCD are polyfunctional and tend to be more highly differentiated when the antigenic burden is high. Figure 4 proposes a model of T-cell responses to HHV-8 in different clinical situations. It suggests that when HHV-8 infection is asymptomatic, the immune correlates of protection include sufficient magnitude, only slightly differentiated phenotype, and a polyfunctional profile of HHV-8–specific CD8+ T cells that control both HHV-8 replication and the emergence of HHV-8–related tumors. During KS, for unknown reasons, these cells are no longer present in the peripheral blood, and their absence allows moderate viral replication in the blood and proliferation of HHV-8–infected cells in KS lesions.25 During MCD, HHV-8 replicates at high levels and creates an antigen burden stimulating further differentiation of HHV-8–specific T cells, which retain polyfunctional capacities. However, these differentiated cells are unable to control the proliferation of HHV-8–infected cells. This model underlines the crucial role of HHV-8–specific T-cell responses in the emergence of KS but not in MCD because the disease is not linked to absent or dysfunctional HHV-8–specific CD8+ T cells. These data encourage the development of vaccines or immunotherapy strategies to boost HHV-8–specific T-cell responses against HHV-8–infected tumoral cells in KS but not against HHV-8–infected cells in MCD.
Model of CD8+ T-cell responses to HHV-8 in different clinical situations: asymptomatic HHV-8 carriage, KS, and MCD. A model based on the experimental findings of the present study and previous published results25 is proposed. In peripheral blood, both AC and MCD status are associated with a polyfunctional HHV-8–specific CD8+ T-cell response, whereas this response lacks during KS. Cells in MCD tend to be more highly differentiated than in AC and related to high HHV-8 viral loads (*). As in other chronic viral infections, immune protection against HHV-8 seems to be conferred by sufficient magnitude of early or intermediate effector memory antiviral CD8+ T cells. On the contrary, polyfunctional HHV-8–specific CD8+ (black dots) T cells are not correlates of protection against MCD.
Model of CD8+ T-cell responses to HHV-8 in different clinical situations: asymptomatic HHV-8 carriage, KS, and MCD. A model based on the experimental findings of the present study and previous published results25 is proposed. In peripheral blood, both AC and MCD status are associated with a polyfunctional HHV-8–specific CD8+ T-cell response, whereas this response lacks during KS. Cells in MCD tend to be more highly differentiated than in AC and related to high HHV-8 viral loads (*). As in other chronic viral infections, immune protection against HHV-8 seems to be conferred by sufficient magnitude of early or intermediate effector memory antiviral CD8+ T cells. On the contrary, polyfunctional HHV-8–specific CD8+ (black dots) T cells are not correlates of protection against MCD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to the French ALT and IMMUNOCO study groups for providing PBMCs from AC and to Eric Robinet for supplying normal blood donor PBMCs. We thank Catherine Blanc for her advice on 5-color cytometry, Dominique Costagliola for advice on statistical analysis, Ioannis Theodorou for HLA typing, and Jo Ann Cahn for revising our English.
This work was supported by l'Agence Nationale de Recherches sur le Sida (ANRS), by Sidaction Ensemble Contre le Sida, and by Cancéropole Ile-de-France.
National Institutes of Health
Authorship
Contribution: G.C., A.G., and B.A. designed the experiments and investigation; A.G., L.P., A.-S.B., F.A., and A.-G.M. conducted the experiments; E.O., L.G., N.D., and J.C. provided samples and clinical data from HHV-8–infected patients; and A.G. and G.C. analyzed the data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Guislaine Carcelain, MD, PhD, Laboratoire d'Immunologie Cellulaire et Tissulaire du Pr Debré, Unité Inserm U543, Bâtiment CERVI, Hôpital Pitié-Salpêtrière, 47, bd de l'hôpital, 75013, Paris, France; e-mail: guislaine.carcelain@psl.aphp.fr.