Abstract
Ligation of NKG2D, a potent costimulatory receptor, can be either beneficial or detrimental to CD8+ cytotoxic T cell (CTL) responses. Factors for these diverse NKG2D effects remain elusive. In this study, we demonstrate that 4-1BB, another costimulatory receptor, is an essential regulator of NKG2D in CD8+ T cells. Costimulation of NKG2D caused down-modulation of NKG2D, but induced 4-1BB expression on the cell surface, even in the presence of TGF-β1, which inhibits 4-1BB expression. Resulting NKG2D−4-1BB+ cells were activated but still in an immature state with low cytotoxic activity. However, subsequent 4-1BB costimulation induced cytotoxic activity and restored down-modulated NKG2D. The cytotoxic activity and NKG2D expression induced by 4-1BB on NKG2D+4-1BB+ cells were refractory to TGF-β1 down-modulation. Such 4-1BB effects were enhanced by IL-12. In contrast, in the presence of IL-4, 4-1BB effects were abolished because IL-4 down-modulated NKG2D and 4-1BB expression in cooperation with TGF-β1, generating another CD8+ T-cell type lacking both NKG2D and 4-1BB. These NKG2D−4-1BB− cells were inert and unable to gain cytotoxic activity. Our results suggest that 4-1BB plays a critical role in protecting NKG2D from TGF-β1–mediated down-modulation. Co-expression of NKG2D and 4-1BB may represent an important biomarker for defining competency of tumor infiltrating CD8+ T cells.
Introduction
CD8+ T cells are important for immune defense against cancers.1 Costimulation is a critical process not only for initiating activation of naive T cells but also for differentiating primed cells into effector T cells. T-cell activation and differentiation are tightly regulated by suppressive cytokines such as TGF-β1 and regulatory T cells.2,3 T cell immune responses may reflect a balance between costimulating and suppressing immune reactions. To achieve CD8+ T cell–mediated immune responses, costimulatory effects have to be dominant over immune suppression in response to antigens.
NKG2D, a C-type lectin-like molecule for CD8+ T cells, is a potent costimulatory molecules for promoting immunosurveillance of tumor and infected cells.4,5 In humans, NKG2D is present on most natural killer (NK) cells, CD8+ T cells, and γδT cells.4,6 Human NKG2D interacts with MHC class I chain-related proteins A and B (MICA and MICB). MIC molecules are not detected on most healthy tissues, but are induced by stress such as heat shock, viral infection, or malignant transformation.7,8
Atypically high MICA expression on healthy tissues is linked to immunopathology of diabetes, rheumatoid arthritis, and celiac disease, suggesting involvement of NKG2D in autoimmune pathogenesis.9-12 Similarly, NKG2D ligands (NKG2DL) expressed on tumor cells increase antitumor reactions of tumor-infiltrating cytotoxic T lymphocytes (CTLs) in vivo.8,13-15 This indicates that NKG2D and its ligand are involved in promoting various immune responses. Paradoxically, persistent expression of MICA results in impaired NK and CD8+ T-cell immune responses.16-18 This negative effect of MICA is mainly due to pronounced down-modulation of NKG2D.17,19 Consistent with this, tumor-infiltrating and systemic NK cells and CD8+ T-cells in cancer patients with MICA+ or MICB+ tumors are often functionally compromised.20 In addition, MIC proteins released in the serum as a soluble form (sMIC) from some types of tumors impair NKG2D function by down-regulating NKG2D surface expression on NK and CD8+ T cells.17,19-21
Transforming growth factor-β1 (TGF-β1) is a central molecule responsible for tumor immune escape by down-modulating NKG2D in CD8+ T and NK cells.22 Consistent with this, systemic NKG2D down-modulation is pronounced in subjects with malignancies associated with expression of TGF-β1.17,20,23 We previously demonstrated that 4-1BB, a specific costimulatory receptor primarily for previously primed CD8+ T cells, is a unique costimulatory molecule that reverses TGF-β1-mediated inhibition of CD8+ T-cell responses.24 4-1BB, a member of the tumor necrosis factor receptor family that includes CD40, CD27, CD30, and OX-40, is involved in enhancement of CD8+ T cell–mediated responses to tumor, allografts and virus.25 A major role of 4-1BB is to promote long-term survival and to facilitate development of effector and memory CD8+ T cells.26 The ligand for 4-1BB is expressed on activated dendritic cells (DCs), B cells, and macrophages.27
Unlike constitutive expression of NKG2D on naive human CD8+ T cells, 4-1BB expression is limited to activated or effector/memory T cells. If and how NKG2D and 4-1BB costimulatory receptors cooperate to regulate CD8+ T-cell effector or memory responses has not been addressed. In this study, we evaluated whether NKG2D and 4-1BB cooperate in the development and cytotoxic activity of CD8+ T cells in vitro, especially in the presence of TGF-β1. Our results suggest that NKG2D and 4-1BB are tightly integrated to reverse TGF-β1 inhibition of cytotoxic CD8+ T cells.
Methods
This study was approved by the Indiana University Purdue University at Indianapolis Institutional Review Board.
Reagents
Recombinant human IL-15, IL-12, and TGF-β1 were purchased from PeproTech (Rocky Hill, NJ). Anti-CD3, anti–4-1BB–phycoerythrin (PE), and protein transport inhibitor (GolgiStop) were purchased from BD Biosciences (San Jose, CA). Anti-NKG2D, anti–NKG2D-PE, and anti–NKG2D-APC were purchased from R&D Systems (Minneapolis, MN). Anti–CD8-PE-Cy5.5 and relevant isotype control mouse IgG were purchased from Caltag (Burlingame, CA). Anti–IFN-γ-APC and anti–CD44-FITC were purchased from Miltenyi Biotec (Auburn, CA) and eBioscience (San Diego, CA), respectively. Monoclonal antibody to 4-1BB (mouse IgG1) was purified from ascites collected from Balb/C mice injected intraperitoneally with the hybridoma, 4B4-1.28
Transduction of target cells with MICA
The C1R B lymphoblast cell line was transduced for stable expression of MICA using an improved murine stem-cell virus (MSCV)–based bicistronic MIEG3 retroviral vector with EGFP. MICA gene was inserted into MIEG3 vector using gateway cloning technology (Invitrogen, Carlsbad, CA). Amphotropic retrovirus was generated by transfecting a packaging cell line, Phoenix-Ampho (American Type Culture Collection, Manassas, VA) maintained in Dulbecco modified Eagle medium (DMEM; Invitrogen) containing 10% fetal bovine serum (FBS; Hyclone, Logan, UT) with MICA-MIEG3 or control MIEG3 retroviral vector using LipofectAMINE (Invitrogen). C1R cells were transduced with viral supernatants containing 8 μg/mL polybrene for 5 to 6 hours at 37°C and for an additional 48 hours after viral supernatants were replaced with fresh DMEM. Transduced cells with EGFP fluorescence were sorted by FACS.29
In vitro CD8+ T-cell differentiation
Human umbilical cord blood (CB) was obtained from the Wishard Hospital, Indianapolis, IN. Mononuclear and T cells were isolated as described previously.24 T-cell purity was greater than 85%. CD8+ T cells were purified by negative selection with magnetic beads (CD8+ T cell Isolation Kit II; Miltenyi Biotec) containing antibodies to CD4, CD14, CD16, CD19, CD36, CD56, CD123, TCRγδ, and Glycophorin A, resulting in greater than 95% purity. Purified CD8+ T cells were stimulated with anti-NKG2D and anti-CD3 for 3 days in 5% CO2 at 37°C in complete RPMI 1640 medium containing 10% FBS, 50 μM β-mercaptoethanol, 2 mM glutamine, 50 μg/mL gentamicin, and IL-15 (10 ng/mL) with or without TGF-β1 (10 ng/mL). Anti-NKG2D and anti-CD3 were precoated onto 24-well plates (Costar, Corning, NY) at 5 μg/mL and 0.1 μg/mL, respectively in PBS overnight at 4°C and cells washed 2 times with PBS. In some cases, stimulation cultures included IL-12 (10 ng/mL) or IL-4 (10 ng/mL). Resulting cells were analyzed for surface expression of NKG2D, 4-1BB, and CD8 by flow cytometry. Cells were assessed for cytotoxic activity using 51Cr release assays. For determining effects of anti–4-1BB costimulation, cells were further incubated in plates precoated with either anti–4-1BB (clone 4B4-1) or isotype control IgG (mouse IgG1, MOPC 21) at 10 μg/mL in the presence of anti-CD3 (0.1 μg/mL) with or without TGF-β1. After 3 days, cells were analyzed for expression of NKG2D, 4-1BB, and cytotoxicity. All experiments were performed in the presence of IL-15 (10 ng/mL).
Redirected cytotoxicity assay
For redirected killing assay, C1R B lymphoblasts were used as a target in the 51Cr release assay. Target cells were labeled with 51Cr (MP Biomedicals, Irvine, CA) by incubating 1 × 106 cells in 300 μCi (216 mCi/mg) 51Cr at 37°C for 2 hours in 5% CO2. Free 51Cr was removed by washing cells for 3 times with PBS. Labeled cells were incubated in complete RPMI-1640 medium with anti-CD3 (1 μg/mL) for 30 minutes and plated in 96-well round-bottom plates at a concentration of 1 × 104 cells/mL in triplicate. Effector CD8+ T cells were added at a 2:1 effector/target ratio and incubated at 37°C for 4 hours in 5% CO2. Supernatant was collected and counted using a gamma counter. Percent specific lysis was calculated as percent specific lysis = 100 × [test release − spontaneous release]/[maximal release – spontaneous release].
Results
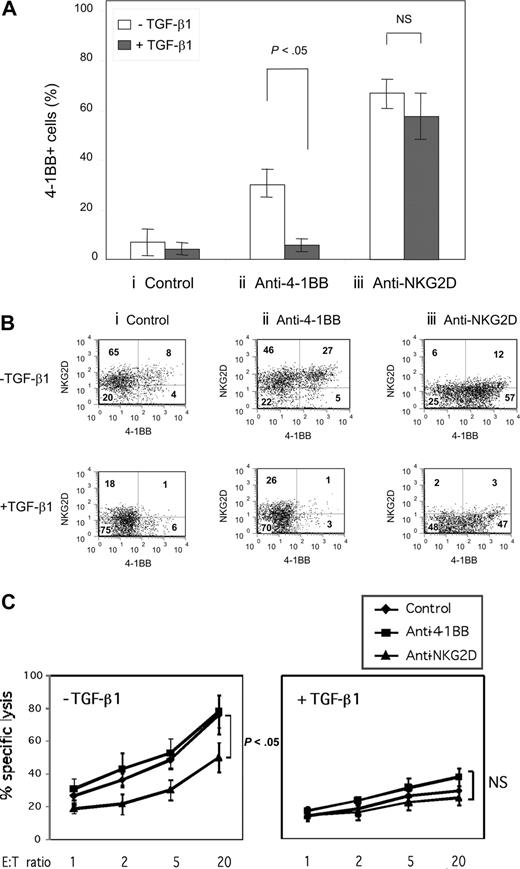
NKG2D costimulation induces 4-1BB while down-regulating NKG2D
Although 4-1BB is readily induced in naive T cells on anti-CD3 stimulation in vitro, it remains unclear what factors are responsible for induction of 4-1BB, especially in an environment enriched with TGF-β1. We asked whether NKG2D, constitutively expressed on naive CD8+ T cells, is involved in induction of 4-1BB especially under immune suppressive conditions of TGF-β1. We took advantage of naive CD8+ T cells isolated from human umbilical CB, which constitutively express NKG2D but lack 4-1BB. Freshly isolated CB CD8+ T cells were stimulated for 3 days with suboptimal anti-CD3 (0.1 μg/mL) in plates coated with isotype control antibody, agonist anti–4-1BB, or anti-NKG2D (10 μg/mL) in the presence or absence of TGF-β1. IL-15 was included in all the cell cultures, because IL-15 enhances differentiation and maintenance of effector/memory CD8+ T cells.30,31 With suboptimal anti-CD3 alone, freshly isolated naive CB CD8+ T cells were induced to express 4-1BB in less than 15% of the cells after 3 days (Figure 1A). Costimulation with anti–4-1BB increased the numbers of cells expressing 4-1BB to about 30%. Induction of enhanced numbers of 4-1BB expressing cells was blocked by TGF-β1. In contrast, costimulation of NKG2D with anti-NKG2D was more effective in inducing 4-1BB than was anti–4-1BB, and the expression of the 4-1BB induced by anti-NKG2D was not inhibited by TGF-β1 (Figure 1A). NKG2D costimulation with anti-NKG2D resulted in an immense down-modulation of NKG2D expression, converting NKG2D+-dominant naive CD8+ T cells to 4-1BB+-dominant cells (Figure 1Biii top panel). Addition of TGF-β1 with anti-NKG2D further reduced NKG2D levels (Figure 1Biii bottom panel). TGF-β1 had an inhibitory effect on NKG2D expression by itself but its effect was less apparent compared with that of anti-NKG2D. Our results suggest that NKG2D plays a role in inducing 4-1BB on a starting population of naive CD8+ T cells particularly under immune-suppressive conditions with TGF-β1, which in the absence of NKG2D costimulation prevents 4-1BB induction.
NKG2D costimulation induces 4-1BB expression, reversing TGF-β1–mediated inhibition. (A) Freshly purified CD8+ T cells from cord blood (CB) were stimulated for 3 days with plate-coated anti-CD3 (0.1 μg/mL) plus either (i) isotype control IgG, (ii) anti–4-1BB, or (iii) anti-NKG2D (10 μg/mL each) in the presence or absence of TGF-β1 (10 ng/mL). All cell cultures contained IL-15 (10 ng/mL). Average percentage of cells expressing 4-1BB was calculated from 4 different CB samples by flow and plotted in a bar graph as mean plus or minus standard deviation (SD). (B) Surface expression of 4-1BB and NKG2D on CD8+ T cells after stimulation as described in panel A was accessed by flow and is displayed in dot plots. Numbers in each quadrant represent percentage of cells expressing NKG2D and/or 4-1BB. Dot plots show one of the representative results from 4 different CB samples. (C) Cytotoxic activity of CD8+ T cells costimulated with isotype control IgG, anti–4-1BB, or anti-NKG2D in the presence and absence of TGF-β1 was measured using the MHC class I chain-related protein A (MICA)–expressing C1R cell line as target cells. All cell cultures contained anti-CD3 (0.1 μg/mL) and IL-15 (10 ng/mL). Percent specific lysis at different effector-to-target ratios was calculated as described in “Redirected cytotoxic assay.” Averaged percentages of specific lysis calculated from quadruplet assay data were further processed to obtain the mean plus or minus SD from 3 independent experiments using 3 CB samples. P values were calculated by Student t test. All the P values at each effector-to-target ratio for the percent specific lysis by the CD8+ T cells costimulated without TGF-β1 were less than .05 indicating the significance of anti-NKG2D effects.
NKG2D costimulation induces 4-1BB expression, reversing TGF-β1–mediated inhibition. (A) Freshly purified CD8+ T cells from cord blood (CB) were stimulated for 3 days with plate-coated anti-CD3 (0.1 μg/mL) plus either (i) isotype control IgG, (ii) anti–4-1BB, or (iii) anti-NKG2D (10 μg/mL each) in the presence or absence of TGF-β1 (10 ng/mL). All cell cultures contained IL-15 (10 ng/mL). Average percentage of cells expressing 4-1BB was calculated from 4 different CB samples by flow and plotted in a bar graph as mean plus or minus standard deviation (SD). (B) Surface expression of 4-1BB and NKG2D on CD8+ T cells after stimulation as described in panel A was accessed by flow and is displayed in dot plots. Numbers in each quadrant represent percentage of cells expressing NKG2D and/or 4-1BB. Dot plots show one of the representative results from 4 different CB samples. (C) Cytotoxic activity of CD8+ T cells costimulated with isotype control IgG, anti–4-1BB, or anti-NKG2D in the presence and absence of TGF-β1 was measured using the MHC class I chain-related protein A (MICA)–expressing C1R cell line as target cells. All cell cultures contained anti-CD3 (0.1 μg/mL) and IL-15 (10 ng/mL). Percent specific lysis at different effector-to-target ratios was calculated as described in “Redirected cytotoxic assay.” Averaged percentages of specific lysis calculated from quadruplet assay data were further processed to obtain the mean plus or minus SD from 3 independent experiments using 3 CB samples. P values were calculated by Student t test. All the P values at each effector-to-target ratio for the percent specific lysis by the CD8+ T cells costimulated without TGF-β1 were less than .05 indicating the significance of anti-NKG2D effects.
NKG2D costimulation is detrimental to cytotoxic activity, although it induces cell activation and 4-1BB expression
As NKG2D costimulation changed expression profiles from NKG2D- to 4-1BB–dominant cells, we examined whether this altered cell phenotype correlated with cytotoxic activity. Chromium release assay was used to determine the cytotoxic activity of cells costimulated with either control IgG, anti–4-1BB or anti-NKG2D. As target cells, we used the C1R lymphoblast tumor cell line transduced with retrovirus containing cDNA coding for MICA, a ligand for NKG2D. As seen in Figure 1C, anti-NKG2D costimulation significantly reduced cytotoxic activity of the cells compared with that of cells treated with control IgG or anti–4-1BB, although anti-NKG2D induced cell activation as evidenced by cell aggregation and induction of 4-1BB (Figure 1A). TGF-β1, which suppressed T-cell activation, inhibited cytotoxic activity regardless of costimulatory treatment. The detrimental effect of NKG2D costimulation on cytotoxic activity to MICA-expressing target may be attributed largely to down-modulation of NKG2D expression. Accordingly, TGF-β1, which suppressed NKG2D expression, inhibited cytotoxic activity regardless of costimulatory treatment.
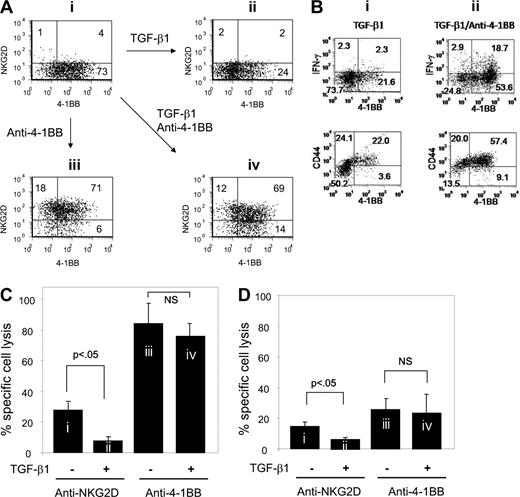
4-1BB restores NKG2D expression down-modulated by NKG2D costimulation
As induction of 4-1BB occurred simultaneously with down-modulation of NKG2D expression (Figure 1A,B), we postulated that 4-1BB costimulation might contribute to NKG2D expression. To test this possibility, we evaluated anti–4-1BB effects on NKG2D expression in CD8+ T cells that were treated with anti-NKG2D in the presence of anti-CD3. These experiments were done in the absence and presence of TGF-β1. Anti–4-1BB treatment dramatically restored the percentage of cells expressing NKG2D from 5 to 89 (compare Figure 2Ai,iii). Induction of NKG2D expression by anti–4-1BB costimu-lation was essentially unaffected by the presence of TGF-β1 (compare Figure 2Aiii,iv). TGF-β1 greatly reduced numbers of cells expressing 4-1BB from 77% to 26% (compare Figure 2Ai,ii). Anti–4-1BB costimulation resulted in increased numbers of cells expressing 4-1BB from 26% to 83% in the presence of TGF-β1 (compare Figure2Aii,iv). This indicates that 4-1BB costimulation reverses the inhibitory TGF-β1 effect on NKG2D and 4-1BB expression.
4-1BB costimulation restores NKG2D expression as well as cytotoxic activity down-regulated by anti-NKG2D costimulation. (A) After anti-NKG2D costimulation, CD8+ T cells were either maintained with anti-NKG2D (i and ii) or costimulated with anti–4-1BB (iii and iv) in the presence (ii and iv) or absence of TGF-β1 (i and iii) for an additional 3 days. Cells were analyzed for NKG2D and 4-1BB expression on the cell surface by flow cytometry. Numbers in each quadrant of dot plots represent percentage of cell populations expressing NKG2D and/or 4-1BB. (B) The cells (i and ii) were prepared as described for the cells in Figure 1Aii,iv and analyzed for the production of IFN-γ and surface expression of CD44. Cell surface was stained with anti–4-1BB-biotin/streptoavidin-FITC, anti–NKG2D-PE, anti–CD8-PE-Cy5.5 and either with anti–IFN-γ-APC for intracytoplasmic IFN-γ or anti–CD44-FITC. For CD44 staining, anti–4-1BB-APC was used instead of anti–4-1BB-biotin. To detect IFN-γ, protein transport inhibitor was added to cell culture 2 hours before cell harvest. IFN-γ and CD44 expression levels were plotted against 4-1BB for the CD8+ T cells (i) and NKG2D+CD8+ cells (ii) gated. (C,D) Cells designated i through iv in panel A were accessed for cytotoxic activity against MIC-vector– (C) and empty vector–transfected C1R target cells (D) as target cells. Averaged percentages of specific lysis calculated from quadruplet assay data were further processed to obtain the mean plus or minus SD from 3 CB samples.
4-1BB costimulation restores NKG2D expression as well as cytotoxic activity down-regulated by anti-NKG2D costimulation. (A) After anti-NKG2D costimulation, CD8+ T cells were either maintained with anti-NKG2D (i and ii) or costimulated with anti–4-1BB (iii and iv) in the presence (ii and iv) or absence of TGF-β1 (i and iii) for an additional 3 days. Cells were analyzed for NKG2D and 4-1BB expression on the cell surface by flow cytometry. Numbers in each quadrant of dot plots represent percentage of cell populations expressing NKG2D and/or 4-1BB. (B) The cells (i and ii) were prepared as described for the cells in Figure 1Aii,iv and analyzed for the production of IFN-γ and surface expression of CD44. Cell surface was stained with anti–4-1BB-biotin/streptoavidin-FITC, anti–NKG2D-PE, anti–CD8-PE-Cy5.5 and either with anti–IFN-γ-APC for intracytoplasmic IFN-γ or anti–CD44-FITC. For CD44 staining, anti–4-1BB-APC was used instead of anti–4-1BB-biotin. To detect IFN-γ, protein transport inhibitor was added to cell culture 2 hours before cell harvest. IFN-γ and CD44 expression levels were plotted against 4-1BB for the CD8+ T cells (i) and NKG2D+CD8+ cells (ii) gated. (C,D) Cells designated i through iv in panel A were accessed for cytotoxic activity against MIC-vector– (C) and empty vector–transfected C1R target cells (D) as target cells. Averaged percentages of specific lysis calculated from quadruplet assay data were further processed to obtain the mean plus or minus SD from 3 CB samples.
4-1BB costimulation promotes differentiation to effector/memory cells after NKG2D costimulation
Because TGF-β1 reduced 4-1BB expression induced by anti-NKG2D costimulation as shown in the cells of Figure 2Aii, we determined whether the cells in which high levels of 4-1BB expression were maintained by subsequent anti–4-1BB costimulation (Figure 2Aiv) displayed characteristic effector and memory phenotypes such as production of IFN-γ and expression of CD44 (Figure 2B). Compared with the cells with TGF-β1 alone (Figure 2Bi), the cells costimulated with anti–4-1BB in the presence of TGF-β1 (Figure 2Bii) showed significantly higher levels of IFN-γ expression. IFN-γ was detected dominantly in the 4-1BB+ cells induced by anti–4-1BB costimulation. Similarly, the cells costimulated with anti–4-1BB in the presence of TGF-β1 (Figure 2Bii) showed higher expression levels of CD44, indicating that 4-1BB costimulation further increased CD44 expression above the levels in the cells with TGF-β1 but without anti–4-1BB (Figure 2Bi). This suggests that NKG2D costimulation may not be sufficient for effector/memory cell differentiation against TGF-β1 inhibition. Subsequent 4-1BB costimulation after NKG2D costimulation may be required to acquire resistance to the inhibitory TGF-β1 effects to achieve effector/memory cell differentiation.
Consecutive costimulation of NKG2D and 4-1BB is necessary for resisting TGF-β1 inhibition of cytotoxic activity
To examine whether NKG2D expression on cells restored by 4-1BB costimulation affected cytotoxicity, we compared the cytotoxic activity of CD8+ T cells costimulated with anti-NKG2D and maintained with anti-NKG2D, with that of cells costimulated with anti–4-1BB after anti-NKG2D costimulation. These experiments were conducted in the presence or absence of TGF-β1. Cells continuously costimulated with anti-NKG2D remained low in cytotoxic activity to C1R target cells expressing MICA (Figure 2C). This cytotoxic activity was further reduced by TGF-β1. In contrast, anti–4-1BB costimulation remarkably increased cytotoxic activity of cells costimulated with anti-NKG2D. Importantly, the cytotoxic activity induced by anti–4-1BB costimulation was not subjected to TGF-β1 inhibition. This indicates that consecutive costimulation may be necessary for CD8+ T cells to become refractory to TGF-β1 inhibition of cytotoxicity. This dramatic increase of cytotoxic activity induced by anti–4-1BB was not detected when control C1R cells transfected with empty retrovirus were used as target cells (Figure 2D). These results support our view that the NKG2D expression restored by 4-1BB costimulation may play a critical role in CD8+ T-cell cytotoxicity to target cells expressing ligands for NKG2D. We still detected lysis of the target cells without MICA transduction by the effector CD8+ T cells we tested regardless of the levels of 4-1BB expression although overall percent lysis levels were considerably lower compared with those of the target cells with MICA transduction. Thus, we do not rule out the possibility that parental C1R cells may also express a residual level of MICA.
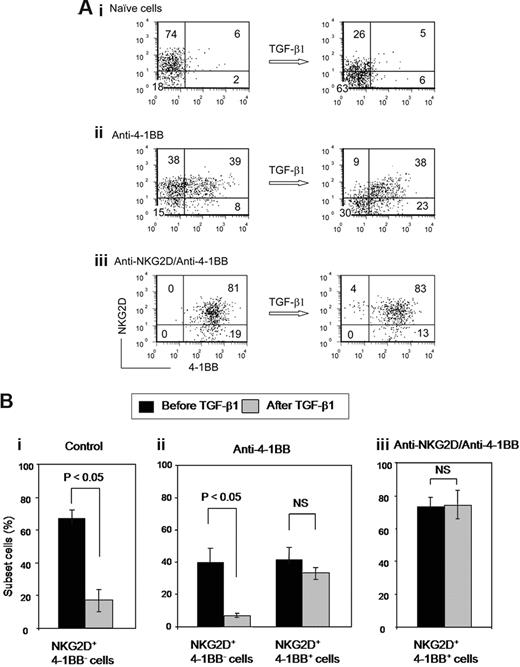
NKG2D coexpressed with 4-1BB is unique for resistance to TGF-β1–mediated down-modulation
The data shown in Figure 2C,D raised a question as to whether expression of NKG2D restored by 4-1BB costimulation might respond to TGF-β1 differently from that of NKG2D on naive CD8+ T cells. Three different cell types with distinct NKG2D and 4-1BB expression profiles were prepared: (1) naive cells, (2) anti–4-1BB costimulated cells, and (3) cells consecutively costimulated with anti-NKG2D and anti–4-1BB. As shown in Figure 3A, NKG2D on naive CD8+ T cells was susceptible to TGF-β1 inhibition. TGF-β1 lowered the percentage of cells expressing NKG2D from 80% to 31% (Figure 3Ai). When we compared effects of TGF-β1 on NKG2D expression in activated cells that were NKG2D+ 4-1BB+ or NKG2D+ 4-1BB−, we observed different effects. Cells expressing NKG2D and 4-1BB demonstrated striking resistance to TGF-β1 down-modulation of NKG2D (Figure 3Aii). In contrast, NKG2D+ 4-1BB− cells were responsive to TGF-β1 down-modulation of NKG2D. Consistent with these results, NKG2D on cells coexpressing 4-1BB, as generated by consecutive costimulation with anti-NKG2D and anti–4-1BB, was almost completely protected from the TGF-β1 induced down-modulation of NKG2D (Figure 3Aiii). Statistical evaluation of the results in Figure 3A for 4 independent experiments verified the specific TGF-β1 effect on down-modulating NKG2D expression in NKG2D+4-1BB− but not NKG2D+4-1BB+ cells (Figure 3B). This demonstrates that cells co-expressing NKG2D with 4-1BB are unique for resistance against TGF-β1-mediated down-modulation of NKG2D. In summary, NKG2D and 4-1BB are interdependent for the protection of their expression from TGF-β1 inhibition; NKG2D costimulation induces 4-1BB expression and 4-1BB costimulation restores the NKG2D expression down-modulated by NKG2D ligation or against TGF-β1–mediated inhibition. Resulting cells expressing both NKG2D and 4-1BB produced by 2 consecutive costimulations were refractory to TGF-β1 inhibition thereby maintaining high level of cytotoxic activity. Thus far, we have not detected evidence of a physical association between these receptors via immunoprecipitation and immunoblotting experiments.
NKG2D coexpressed with 4-1BB is unique for being refractory to TGF-β1 down-modulation. (A) CD8+ T cells were costimulated for 3 days with (i) isotype control IgG, (ii) anti–4-1BB, or (iii) subsequent anti–4-1BB costimulation after anti-NKG2D costimulation. Costimulated cells were further treated with or without TGF-β1 for 3 days in the presence of anti-CD3 and IL-15 and analyzed for expression of NKG2D and 4-1BB by flow cytometry. Numbers in each quadrant represent percentage of cells expressing NKG2D and/or 4-1BB. (B) Differential responses to TGF-β1 in NKG2D+4-1BB− and NKG2D+4-1BB+ cells were analyzed. Percentage of respective subset cells before and after TGF-β1 treatment in the costimulated cells described in panel A were calculated as mean plus or minus SD from 4 different CB samples.
NKG2D coexpressed with 4-1BB is unique for being refractory to TGF-β1 down-modulation. (A) CD8+ T cells were costimulated for 3 days with (i) isotype control IgG, (ii) anti–4-1BB, or (iii) subsequent anti–4-1BB costimulation after anti-NKG2D costimulation. Costimulated cells were further treated with or without TGF-β1 for 3 days in the presence of anti-CD3 and IL-15 and analyzed for expression of NKG2D and 4-1BB by flow cytometry. Numbers in each quadrant represent percentage of cells expressing NKG2D and/or 4-1BB. (B) Differential responses to TGF-β1 in NKG2D+4-1BB− and NKG2D+4-1BB+ cells were analyzed. Percentage of respective subset cells before and after TGF-β1 treatment in the costimulated cells described in panel A were calculated as mean plus or minus SD from 4 different CB samples.
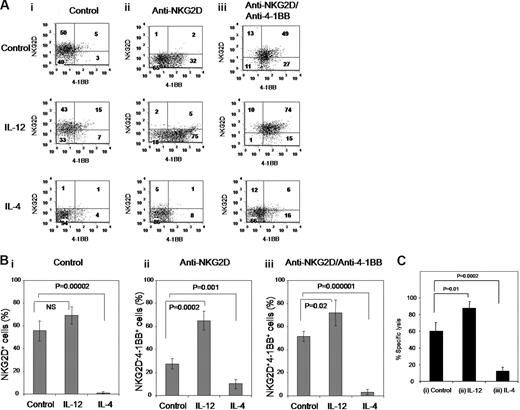
IL-4 acts as a strong suppressor in combination with TGF-β1 by inhibiting expression of NKG2D and 4-1BB, as well as cytotoxic activity
Because our data indicate that the CD8+ T cells coexpressing NKG2D and 4-1BB showed potent cytotoxic activity as well as resistance to TGF-β1–mediated inhibition, we asked whether cytokines such as IL-12 and IL-4, respective type 1 and type 2 cytokines, affected generation of cytotoxic NKG2D+4-1BB+ cells. Cells were costimulated with anti-NKG2D, followed subsequently with anti–4-1BB to generate NKG2D+4-1BB+ cells from naive CD8+ T cells in the presence of IL-12 or IL-4. Cells harvested from each costimulation step: (1) freshly isolated naive CD8+ T cells, (2) anti-NKG2D costimulated cells, and (3) anti–4-1BB costimulated cells after anti-NKG2D costimulation (as depicted in Figure 3) were examined for NKG2D and 4-1BB expression (Figure 4A). Because effects of 4-1BB on CD8+ T-cell cytotoxicity become more obvious in cells treated with TGF-β1 (Figure 3), experiments were performed in the presence of TGF-β1. IL-12 enhanced 4-1BB expression especially with anti-NKG2D costimulation from 34% to 80% (Figure 4Aii with IL-12). IL-12 also significantly increased the NKG2D+4-1BB+ cell production generated by consecutive costimulation with anti-NKG2D and anti–4-1BB increasing this dual expressing cell type from 49% to 74% (Figure 4Aiii with IL-12 compared with control medium). In contrast to IL-12, IL-4 showed remarkable inhibitory effect on both NKG2D and 4-1BB expression. In the presence of IL-4, the majority of cells expressed neither NKG2D nor 4-1BB, with low numbers of NKG2D+4-1BB+ cells even after consecutive costimulation with anti-NKG2D and anti–4-1BB (Figure 4Aiii with IL-4). These results were confirmed in 4 independent experiments (Figure 4B).
IL-12 and IL-4 affect NKG2D and 4-1BB expression. (A) CD8+ T cells were sequentially costimulated with isotype control IgG (i) or anti-NKG2D (ii) for 3 days, or with anti–4-1BB for an additional 3 days after 3-day anti-NKG2D costimulation (iii). Costimulation was undertaken in the presence and absence of IL-12 (10 ng/mL) or IL-4 (10 ng/mL). All the cell cultures contained anti-CD3, IL-15 and TGF-β1. Surface expression of NKG2D and 4-1BB is displayed in dot plots. Numbers in for each quadrant represent percentage of cells expressing NKG2D and/or 4-1BB. (B) Percent NKG2D+, NKG2D−4-1BB+ and NKG2D+4-1BB+ subsets in the respective CD8+ T-cell groups costimulated with (i) control IgG, (ii) anti-NKG2D, and (iii) anti–4-1BB after anti-NKG2D in the presence or absence of IL-12 or IL-4 are compared as mean plus or minus SD from 4 different CB samples. (C) CD8+ T cells consecutively costimulated with anti–4-1BB and anti-NKG2D with no cytokine (i) or with either IL-12 (ii) or IL-4 (iii) were measured for percentage of cell lysis using the MIC-expressing C1R cell line as target cells. Effector to target cell ratio was 2. These results are average plus or minus SD from 4 different CB samples.
IL-12 and IL-4 affect NKG2D and 4-1BB expression. (A) CD8+ T cells were sequentially costimulated with isotype control IgG (i) or anti-NKG2D (ii) for 3 days, or with anti–4-1BB for an additional 3 days after 3-day anti-NKG2D costimulation (iii). Costimulation was undertaken in the presence and absence of IL-12 (10 ng/mL) or IL-4 (10 ng/mL). All the cell cultures contained anti-CD3, IL-15 and TGF-β1. Surface expression of NKG2D and 4-1BB is displayed in dot plots. Numbers in for each quadrant represent percentage of cells expressing NKG2D and/or 4-1BB. (B) Percent NKG2D+, NKG2D−4-1BB+ and NKG2D+4-1BB+ subsets in the respective CD8+ T-cell groups costimulated with (i) control IgG, (ii) anti-NKG2D, and (iii) anti–4-1BB after anti-NKG2D in the presence or absence of IL-12 or IL-4 are compared as mean plus or minus SD from 4 different CB samples. (C) CD8+ T cells consecutively costimulated with anti–4-1BB and anti-NKG2D with no cytokine (i) or with either IL-12 (ii) or IL-4 (iii) were measured for percentage of cell lysis using the MIC-expressing C1R cell line as target cells. Effector to target cell ratio was 2. These results are average plus or minus SD from 4 different CB samples.
To establish a causal link between expression profiles of NKG2D and 4-1BB, and cytotoxic activity, we evaluated cells treated with IL-12 or IL-4 in the presence of TGF-β1 during consecutive costimulation with anti-NKG2D and anti–4-1BB for cytotoxic activity. IL-12, which increased numbers of NKG2D+4-1BB+ cells, enhanced cytotoxic activity. In contrast, IL-4, which abolished production of the NKG2D+4-1BB+ cells, ablated cytotoxic activity (Figure 4C). These correlations suggest that NKG2D+4-1BB+ cells may be a primary cell type responsible for destroying MIC-expressing target cells especially under immune suppressive conditions with TGF-β1. Conversely, NKG2D−4-1BB− cells may represent noncytotoxic cells.
Discussion
Activated CD8+ T cells are predominantly responsible for antigen-specific clearance of tumor cells.32 TGF-β1 controls T-cell homeostasis by directly inhibiting T-cell activation and proliferation.2 CD8+ T cells may inevitably encounter ubiquitously existing TGF-β1 in vivo during their immune surveillance.33,34 To efficiently respond to tumor antigens and overcome suppression by TGF-β1, CD8+ T cells might require strong multiple costimulation. NKG2D and 4-1BB are the most prominent costimulatory receptors for CD8+ T cells. A highlight of our findings is the importance of cooperative NKG2D and 4-1BB costimulation to block suppressive TGF-β1 effects on CD8+ T-cell cytotoxicity as well as expression of costimulatory receptors.
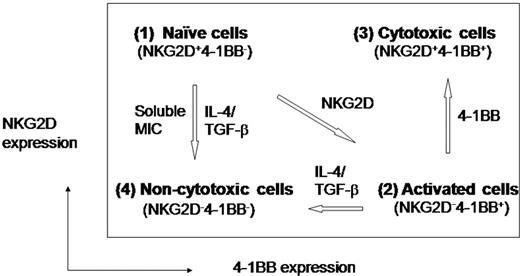
In the present paper, we characterized 4 CD8+ T-cell types phenotypically with distinct expression profiles of NKG2D and 4-1BB, and also functionally for their cytotoxic activities. These characteristics are summarized in Figure 5: (1) NKG2D+4-1BB− cells manifested a naive cell phenotype with little cytotoxic activity, primarily due to their immaturity; (2) NKG2D−4-1BB+ cells produced by NKG2D costimulation exhibited a highly activated phenotype, but remained low in cytotoxicity probably due to loss of surface NKG2D; (3) NKG2D+4-1BB+ cells, produced by consecutive costimulation with anti–4-1BB after anti-NKG2D, acquired potent cytotoxic activity and were refractory to TGF-β1 inhibition; and (4) NKG2D−4-1BB− cells were generated from either naive or activated cells by respectively losing surface expression of NKG2D or 4-1BB. This cell type developed by a combination of IL-4 and TGF-β showed little cytotoxic activity and was unable to gain cytotoxicity via either NKG2D or 4-1BB costimulation.
Diagrammatic expression profiles of NKG2D and 4-1BB in conjunction with cytotoxic effector status. (1) Naive cells: naive CD8+ T cells express NKG2D, but no 4-1BB on the cell surface. They have NKG2D susceptible to down-modulation in the presence of TGF-β1 and IL-4 becoming cells lacking both NKG2D and 4-1BB. (2) NKG2D-costimulated cells: these cells lack NKG2D and express induced 4-1BB, representing activated yet immature cells with the capacity for cytotoxic activity. (3) Cytotoxic cells: 4-1BB costimulation of NKG2D-costimulated cells results in another cell type expressing both NKG2D and 4-1BB on the cell surface. These cells are highly cytotoxic and are refractory to TGF-β1 inhibition. (4) Incapacitated cells: these cells lack both NKG2D and 4-1BB and manifest no cytotoxic activity. These cells may be derived from naive or NKG2D costimulated cells by losing NKG2D or 4-1BB, respectively, in the presence of soluble MIC, IL-4, and TGF-β1.
Diagrammatic expression profiles of NKG2D and 4-1BB in conjunction with cytotoxic effector status. (1) Naive cells: naive CD8+ T cells express NKG2D, but no 4-1BB on the cell surface. They have NKG2D susceptible to down-modulation in the presence of TGF-β1 and IL-4 becoming cells lacking both NKG2D and 4-1BB. (2) NKG2D-costimulated cells: these cells lack NKG2D and express induced 4-1BB, representing activated yet immature cells with the capacity for cytotoxic activity. (3) Cytotoxic cells: 4-1BB costimulation of NKG2D-costimulated cells results in another cell type expressing both NKG2D and 4-1BB on the cell surface. These cells are highly cytotoxic and are refractory to TGF-β1 inhibition. (4) Incapacitated cells: these cells lack both NKG2D and 4-1BB and manifest no cytotoxic activity. These cells may be derived from naive or NKG2D costimulated cells by losing NKG2D or 4-1BB, respectively, in the presence of soluble MIC, IL-4, and TGF-β1.
NKG2D is an activating receptor for NK and CD8+ T cells and enhances CD8+ T-cell responses to tumor.18 In some cases, sustained NKG2DL expression substantially impairs NK responses and represses antitumor CTL functions,35,36 because ligation of NKG2D causes its rapid internalization from the cell surface.8 Because cell activation and NKG2D down-modulation occurs simultaneously as a result of NKG2D ligation, the effects of NKG2D on NK and CTL antitumor responses have been controversial. We believe that outcomes of NKG2D ligation may depend on how effectively NKG2D down-modulation is countered and/or restored. Our results suggest that 4-1BB may be involved in restoration of down-modulated NKG2D. Unlike NKG2D on naive CD8+ T cells, NKG2D induced by 4-1BB costimulation after its down-modulation was refractory to TGF-β1–induced down-modulation. The mechanism by which NKG2D induced by 4-1BB costimulation on NKG2D+4-1BB+ cells acquires resistance to the suppressive effects of TGF-β1 is not clear.
4-1BB is rarely detected on CD8+ T cells purified from peripheral blood. Although 4-1BB is induced in vitro by anti-CD3 stimulation, factors responsible for inducing 4-1BB in vivo remain unclear. TGF-β1 has sweeping inhibitory effects on the immune system, negatively affecting many immune cell types, including CD8+ T and NK cells.34 Thus, it is conceivable that effective costimulatory input may be essential for naive CD8+ T cells to initiate T-cell activation, inducing 4-1BB especially under overwhelmingly immune-suppressive conditions with TGF-β1. CD28, a prototype costimulatory molecule is not effective in inducing 4-1BB expression in the presence of TGF-β1 because of its susceptibility to TGF-β1–mediated suppression.24 Our study indicates that NKG2D, which is constitutively expressed on human naive CD8+ T cells, is an efficient costimulatory receptor for inducing 4-1BB on naive CD8+ T cells especially in the presence of TGF-β1. However, NKG2D costimulation with anti-NKG2D attenuated cytotoxicity with concurrent NKG2D down-modulation, although it activated naive CD8+ T cells inducing 4-1BB with suboptimal anti-CD3 even in the presence of TGF-β1. For these reasons, we believe that 4-1BB might also play an important role in compensating for NKG2D down-modulation. Levels of 4-1BB expression in response to anti-NKG2D costimulation often fluctuated especially in the presence of TGF-β1 because 4-1BB expression was dictated by a delicate balance between anti-NKG2D and TGF-β1 with strong opposite activities in 4-1BB induction. Clearly, IL-12 and IL-4 affected the balance of anti-NKG2D and TGF-β1 effects on 4-1BB expression: IL-12 promoted anti-NKG2D induced 4-1BB expression counterbalancing inhibitory TGF-β1 effect, while IL-4 promoted inhibitory TGF-β1 effect on 4-1BB expression preventing NKG2D expression (Figure 4A,B).
Similar to 4-1BB, inflammatory factors such as IL-1537 or poly(I:C)18 are also involved in restoration of down-regulated NKG2D expression. In addition, certain pathologic conditions such as rheumatoid arthritis11 and celiac disease12,38 may affect NKG2D expression. As reported by others,22,39 NKG2D restoration may be blocked by TGF-β1–mediated down-modulation of NKG2D. We previously demonstrated that 4-1BB signals can block TGF-β1 effects at a level of intracellular signaling.24 Although 4-1BB costimulation induced IFN-γ, the 4-1BB effect on blocking TGF-β1 suppression may not be mediated by IFN-γ because IL-12, although even more potent in inducing IFN-γ, does not mimic the 4-1BB effect on negating TGF-β1 suppression (Kim et al Figure 5A24 ). Hence, activating innate immunity,18,40-42 or proinflammatory cytokines such as IL-12 and IL-15, might play an important regulatory role in NKG2D-mediated NK and CTL responses in vivo through 4-1BB induction, especially in an environment enriched with TGF-β1.
The NKG2D−4-1BB− cell type can be derived from naive CD8+ T cells by induced loss of NKG2D, and also from NKG2D costimulated CD8+ T cells by loss of 4-1BB. For both pathways, IL-4 and TGF-β1 may accelerate generation of NKG2D−4-1BB− cells. We previously reported that IL-4 down-regulated 4-1BB expression.24 In this study, we identified IL-4 as a strong suppressor of NKG2D expression as well (Figure 4A). In contrast to IL-4, IL-12–mediated enhancement of NKG2D+4-1BB+ cells may be largely mediated through its ability to increase 4-1BB expression. Consistent with this, synergistic effects of IL-12 with 4-1BB on antitumor CD8+ T-cell responses have been recognized by others in mouse models.43,44
TGF-β1 ubiquitously exists in the body. We found that effects of IL-4 on generation of NKG2D−4-1BB− CD8+ T cells became more apparent in the presence of TGF-β1. For example, IL-4 showed only moderate effects on NKG2D expression by itself, but IL-4 cooperated with TGF-β1 to induce noncytotoxic NKG2D−4-1BB− cells. Enhancing effects of IL-12 on anti–4-1BB-induced cytotoxic NKG2D+4-1BB+ cells also became more apparent when TGF-β1 was present. Thus, type 1 and 2 cytokines may influence CD8+ T-cell functions by shifting a subtle balance between stimulatory NKG2D and 4-1BB, and suppressive TGF-β1.
Some tumor cells shed a soluble form of MIC, a ligand for NKG2D, into the circulation.17 Soluble MIC may internalize NKG2D from the surface of CD8+ T cells without induction of 4-1BB, thereby resulting in production of non-cytotoxic NKG2D−4-1BB− cells. Tumors might use soluble MIC to evade antitumor immune responses by converting tumor-infiltrating CD8+ T cells to irreversibly inactivated NKG2D−4-1BB− cells. TGF-β1secreted by tumors might cooperate with soluble MIC to produce NKG2D−4-1BB− cells by down-modulating NKG2D and 4-1BB expression. TGF-β1 is also involved in expansion of CD25+CD4+ regulatory T cells that might inhibit antitumor CTL responses.3,45,46 It remains to be seen whether CD25+CD4+ regulatory T cells affect development of NKG2D+4-1BB+ CTLs.
Many current strategies for tumor vaccines largely focus on expanding tumor antigen-specific CTLs.47 Future protocols for T-cell therapy of tumors may need to be designed to improve quality rather than quantity of tumor-specific CD8+ T cells, primarily to resist immune suppressors such as TGF-β1 and soluble MIC. 4-1BB has been successfully used to improve CTL and NK- cell responses to poorly immunogenic tumors in mouse models,48 although exactly how 4-1BB enhances efficacy of antitumor CTL and NK-responses has not been fully elucidated. Our results suggest that development of tumor-specific NKG2D+4-1BB+ CTLs refractory to TGF-β1 immune suppression could represent a primary target for 4-1BB–mediated tumor therapy in humans. If this is the case, expression profiles of NKG2D and 4-1BB on tumor-infiltrating CTLs could be useful parameters for defining the quality of CTLs during tumor evasion of immune surveillance.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the nurses and staff at Wishard Hospital, Indianapolis, IN, for coordinating human umbilical cord blood donations.
This work was supported by US Public Health Service grants RO1 HL56416 and RO1 HL67384, and a Project in PO1 HL53586 to H.E.B. from the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: Y.-J.K. designed and performed research, analyzed data, and prepared the manuscript. M.-K.H. constructed cell lines used for cytotoxic assays. H.E.B. edited the manuscript and funded the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Young-June Kim, Walther Oncology Center, Indiana University School of Medicine, Research Institute No. 2 Building, 950 W Walnut Street, Room 302, Indianapolis, IN 46202-5181; e-mail: yjkim@iupui.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal