Abstract
In this paper, we report an inhibitor antibody to factor XI (FXI) in a woman with severe inherited FXI deficiency, induced by FXI present in an Rh immune globulin preparation. The patient is homozygous for the Glu117Stop mutation, associated with a FXI level of less than 1 U/dL. Unlike all previously described patients with severe FXI deficiency and an inhibitor, the patient had never been exposed to blood products. Following 3 injections of Rh immune globulin during pregnancy, she developed an inhibitor to FXI (8 Bethesda units) that was shown to bind specifically to FXI and inhibit factor IX cleavage by purified FXIa. The administered Rh immune globulin and 2 other similar products were shown to contain FXI. Clinicians should be aware of the potential for immunization of severely FXI-deficient patients by FXI present in Rh immune globulin preparations.
Introduction
Inherited factor XI (FXI) deficiency is an injury-related bleeding disorder affecting mainly individuals homozygous or compound heterozygous for mutations in the FXI gene. The disorder is particularly common in Ashkenazi and Iraqi Jews.1 Patients with severe inherited FXI deficiency can develop an inhibitor to FXI,2 which complicates their clinical course. Although spontaneous bleeding in FXI inhibitor patients is uncommon, trauma or surgery in such patients can be accompanied by excessive bleeding, manageable only by infusion of activated prothrombin complex concentrate or recombinant factor VIIa. The trigger for development of an inhibitor in 22 previously described or reviewed cases was exposure to blood products and 10 patients genotyped were homozygous for Glu117Stop mutation with baseline FXI levels of less than 1 U/dL.2
In this report, we describe a patient with severe inherited FXI deficiency who developed an inhibitor following injections of Rh immune globulin. We suspected that the Rh immune globulin contained FXI, which elicited the immune response. Herein, we present the characterization of the inhibitor and an analysis of several Rh immune globulin preparations, including the suspect product, for the presence of FXI.
Methods
The Human Subject Ethics Committee of the Sheba Medical Center approved the study. Informed consent was obtained in accordance with the Declaration of Helsinki.
The patient is a 34-year-old Canadian-born woman who has had no bleeding tendency except for heavy menstrual flow since menarche. Tooth extractions were uneventful. Her parents are non-Jewish first cousins of Italian origin. Soon after treatment with clomiphene because of infertility, the patient conceived. Tests showed that she was Rh(D) negative and that she had an unexpectedly prolonged aPTT, shown to be caused by a FXI level less than 1 U/dL. At the 15th and 18th weeks of gestation, she received intramuscularly Rh immune globulin (WinRho SDF; Cangene, Winnipeg, MB), each time at a dose of 300 μg immunoglobulin G (IgG). During the 19th week of gestation, fetal death and placenta previa were diagnosed. Following delivery, a third injection of 300 μg Rh immune globulin was given. Three months later, the patient was shown to have an inhibitor to FXI of 8 Bethesda units (BU). The patient had never been transfused with blood components. One of her brothers bled excessively following tonsillectomy and another brother was asymptomatic. Both had FXI levels of less than 1 U/dL but no inhibitor.
Standard one-stage clotting and Bethesda assays were used to measure FXI activity and FXI inhibitor. DNA was extracted for detection of common Jewish mutations.3
IgG was separated from plasmas of the patient, a healthy control, and a patient with severe inherited FXI deficiency and a known inhibitor to FXI (5 BU) on a protein A column (BioRad, Hercules, CA). All IgG preparations used were adjusted to a concentration of 4 mg/mL containing 1.7 BU factor XI inhibitor both in the patient reported herein and in the patient with a known inhibitor.
Binding of patient or control IgG to microtiter plates coated with purified FXI was performed as described elsewhere.2 The effect of patient and control IgG on activation of factor IX (FIX) by FXIa was tested by incubating 1 mg/mL IgG with TBS-BSA containing 1 nM FXIa for 20 minutes at 22°C followed by addition of 200 nM purified FIX (Enzyme Research, South Bend, IN) and 2.5 mM calcium chloride. The mixture was incubated for 60 minutes at 37°C, during which samples were removed at different time points for Western blotting to analyze cleavage of FIX, representing activation using goat antihuman FIX antibody (Haematologic Technologies, Essex Junction, VT) followed by the peroxidase-labeled anti–goat IgG and chemiluminescence enhancement (ECL) kit (Pierce, Rockford, IL) and x-ray film exposure.
Three Rh immune products were examined for FXI content: anti-D IgG preparation WinRho (lots 0230420 and 0230417), KamRhoD (lot 3145011A; Kamada, Beit-Kama, Israel), and Rhophylac 300 (lot 43110-00002; ZLB Behring, Bern, Switzerland). Measurement of FXI was by enzyme-linked immunosorbent assay (ELISA) using a commercial kit (Affinity Biologicals, Hamilton, ON). A standard curve was constructed by diluting purified FXI (Enzyme Research) in TBS to concentrations of 6.25 to 200 ng/mL. FXI was also analyzed by Western blotting. Samples (30 μL) were first subjected to electrophoresis on 8% LongLife Gels (LifeGels, Frenchs Forest, Australia) and then transferred to polyvinylidene difluoride membrane (Immobilin P; Millipore, Bedford, MA) in transfer buffer (LifeGels). Because IgG and nonreduced FXI have similar molecular weights (160 kDa), samples were tested both in nonreduced and reduced conditions. Detection of FXI was by peroxidase-labeled goat antihuman FXI polyclonal antibody (Affinity Biologicals) displayed by ECL.
Results and discussion
Genotyping analysis disclosed that the patient was homozygous for the Glu117Stop mutation, one of the 2 FXI mutations common in Jews.1 The patient is non-Jewish Italian, and this population also harbors this mutation, with a haplotype identical to the Jewish haplotype.4 The patient is exceptional among previously described patients with inherited FXI deficiency and an inhibitor because she has never been exposed to blood products. Since FXI copurifies with immunoglobulins,5 and FXI and FXIa are present in immunoglobulin concentrates,6,7 we hypothesized that the Rh immune globulin that the patient received contained FXI. This was indeed the case. Western blot analysis revealed the presence of the 160-kDa FXI dimer and of the 80-kDa FXI monomer in nonreduced and reduced samples, respectively, in the WinRho and KamRhoD products but not in the Rhophylac (Figure 1). However, more sensitive measurements of FXI by ELISA revealed FXI in all 3 preparations; the calculated amounts of FXI antigen per recommended injection of Rh immune globulin were 20 ng for Rhophylac, 0.5 μg for KamRhoD, and 5 or 6.5 μg for 2 lots of WinRho.
Western blotting of FXI in purified FXI and 3 Rh immune globulin preparations. Note the presence of FXI 160-kDa dimer and 80-kDa FXI monomer in various concentrations of the WinRho preparation (W) under nonreduced and reduced conditions, respectively. The slanted bands at the higher concentrations of WinRho are caused by overloading the gels by IgG. A faint band is apparent at a high concentration of KamRhoD (K) and is displayed more conspicuously in the reduced gel. No FXI band is demonstrable for Rhophylac (R) but was measurable by ELISA (see “Results and discussion”).
Western blotting of FXI in purified FXI and 3 Rh immune globulin preparations. Note the presence of FXI 160-kDa dimer and 80-kDa FXI monomer in various concentrations of the WinRho preparation (W) under nonreduced and reduced conditions, respectively. The slanted bands at the higher concentrations of WinRho are caused by overloading the gels by IgG. A faint band is apparent at a high concentration of KamRhoD (K) and is displayed more conspicuously in the reduced gel. No FXI band is demonstrable for Rhophylac (R) but was measurable by ELISA (see “Results and discussion”).
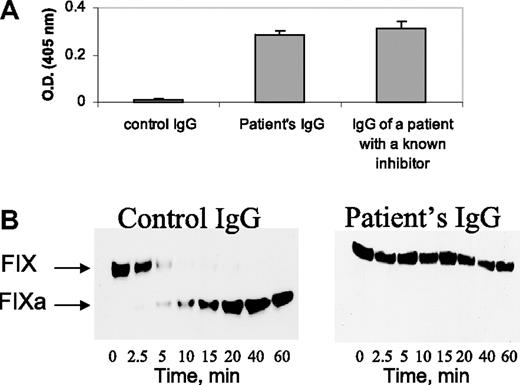
The FXI inhibitor of the patient exhibited characteristics similar to previously described FXI inhibitors2 : (a) The patient's IgG bound to microplates coated with purified FXI, whereas normal IgG did not (Figure 2A); (b) the patient's IgG inhibited FIX activation by purified FXIa in the presence of calcium ions (Figure 2B).
Characterization of the FXI inhibitor. (A) Binding of IgG (4 mg/mL) from a healthy control, the herein reported patient, and a patient with severe inherited FXI deficiency with a known inhibitor to plates coated with purified FXI. (B) Western blots at different time points from reaction mixtures of purified FIX with FXIa and calcium ions, incubated in the presence of control IgG and patient's IgG (4 mg/mL). Note the cleavage of FIX in the presence of control IgG and lack of cleavage in the presence of the patient's IgG.
Characterization of the FXI inhibitor. (A) Binding of IgG (4 mg/mL) from a healthy control, the herein reported patient, and a patient with severe inherited FXI deficiency with a known inhibitor to plates coated with purified FXI. (B) Western blots at different time points from reaction mixtures of purified FIX with FXIa and calcium ions, incubated in the presence of control IgG and patient's IgG (4 mg/mL). Note the cleavage of FIX in the presence of control IgG and lack of cleavage in the presence of the patient's IgG.
Together, these findings suggest that our severely FXI-deficient patient developed an inhibitor to FXI following administration of Rh immune globulin. The size of the population at risk for this problem can be approximated by the following considerations. The estimated proportion of homozygotes for Glu117Stop in Ashkenazi Jews is 1:2128.3 This translates into an estimated 1410 affected individuals in Israel and 2585 in the United States, the countries with the largest populations of Ashkenazi Jews (3 × 106 in Israel and 5.5 × 106 in the United States). Assuming an equal sex distribution, and given the frequency of Rh negativity of approximately 15%, the estimated number of Rh-negative females homozygous for Glu117Stop is 106 in Israel and 194 in the United States. Clearly, only women at the childbearing age will be at risk because Rh immune globulin is most commonly administered during delivery or fetal loss.
Five other populations have been identified with prevalent FXI mutations for which homozygosity leads to severe FXI deficiency: Iraqi Jews, Arabs and Italians harboring the Glu117Stop mutation,3,4,8 French Basques harboring Cys38Arg mutation,9 and Britons carrying the Cys128Stop mutation.10 However, the respective allele frequencies are smaller than in Ashkenazi Jews, and therefore the estimated risk of acquiring a FXI inhibitor would be lower.
This described adverse effect calls for use of Rh immune globulin preparations with the lowest FXI content in patients with FXI levels less than 1 U/dL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to the patient for her generous cooperation. We are also indebted to Mrs Susan Goyer from Cangene, who provided us the 2 lots of WinRho.
National Institutes of Health
Authorship
Contribution: M.Z. preformed the study, designed the research, and wrote the initial draft of the paper; A.Z. designed the study and critically reviewed the paper; J.T. made the diagnosis and critically reviewed the paper; and U.S. designed the study and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Uri Seligsohn, Amalia Biron Research Institute of Thrombosis and Hemostasis, Chaim Sheba Medical Center, Tel-Hashomer, Israel 52621; e-mail: seligson@sheba.health.gov.il.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal