Microbial products, including lipopolysaccharide (LPS), an agonist of Toll-like receptor 4 (TLR4), regulate the lifespan of dendritic cells (DCs) by largely undefined mechanisms. Here, we identify a role for calcium-calmodulin–dependent kinase IV (CaMKIV) in this survival program. The pharmacologic inhibition of CaMKs as well as ectopic expression of kinase-inactive CaMKIV decrease the viability of monocyte-derived DCs exposed to bacterial LPS. The defect in TLR4 signaling includes a failure to accumulate the phosphorylated form of the cAMP response element-binding protein (pCREB), Bcl-2, and Bcl-xL. CaMKIV null mice have a decreased number of DCs in lymphoid tissues and fail to accumulate mature DCs in spleen on in vivo exposure to LPS. Although isolated Camk4−/− DCs are able to acquire the phenotype typical of mature cells and release normal amounts of cytokines in response to LPS, they fail to accumulate pCREB, Bcl-2, and Bcl-xL and therefore do not survive. The transgenic expression of Bcl-2 in CaMKIV null mice results in full recovery of DC survival in response to LPS. These results reveal a novel link between TLR4 and a calcium-dependent signaling cascade comprising CaMKIV-CREB-Bcl-2 that is essential for DC survival.
Introduction
Dendritic cells (DCs) are antigen presenting cells (APCs) that circulate in the blood and are also present in peripheral tissues and lymphoid organs. They are able to sustain and polarize the primary adaptive immune response and are involved in the mechanisms of tolerance toward self-antigens.1,–3 These cells recognize microbial products by using a variety of molecules expressed on their surface that enable them to detect infections in the periphery. Among these molecules, the Toll-like receptors (TLRs) bind pathogen-derived molecules to trigger the activation programs of DC, thus inducing the release of cytokines and driving DC migration to the T-cell zone.4,–6 Visualization of cellular interactions in intact lymphoid tissues reveals that DC–T cell conjugates must remain stable for up to 2 days for lymphocytes to become fully activated.7 Therefore, the lifespan of DC is an essential factor in controlling the number of viable antigen-bearing DC in the T-cell zone, and in turn, to regulate the quality and magnitude of the adaptive immune response
Agonists of TLR, including the Gram-negative bacterial lipopolysaccharide (LPS), control survival of DC by mechanisms only partially defined.8 LPS signals DC via TLR4, an interaction that requires the lipopolysaccharide-binding protein and MD2, a TLR4-associated molecule.4,6 Two distinct biochemical pathways are activated by this interaction. The “MyD88-dependent” cascade, involving Toll-interleukin-1 receptor domain adaptors MyD88 and Mal, regulates activation of the NF-kκB transcription factor and drives the synthesis of cytokines and the terminal differentiation program. The triggering of the “MyD88-independent” pathway requires TRIF and TRAM (a second set of Toll-interleukin-1 receptor domain adaptors) and stimulates phosphorylation and dimerization of IRF-3, a key event regulating the synthesis of interferon-γ. Several reports have suggested that TLR4 agonists activate antiapoptotic as well as proapoptotic pathways.8,,,–12 Recently, it has been proposed that LPS controls accumulation of both proapoptotic and antiapoptotic members of the Bcl-2 family of proteins and in so doing regulates the lifespan of DC.8
Calcium (Ca2+) is a pervasive intracellular second messenger that initiates signaling cascades, leading to essential biologic processes such as secretion, cell proliferation, differentiation, and movement.13 In DCs, many critical functions involve Ca2+ signaling. For example, apoptotic body engulfment and processing are accompanied by a rise in intracellular Ca2+ and are dependent on external Ca2+.14 In addition, chemotactic molecules produce Ca2+ increases in DC,15,,–18 suggesting the involvement of a Ca2+-dependent pathway in the regulation of DC migration. The role of a Ca2+-dependent pathway in the mechanism regulating DC maturation is suggested by the opposite effects induced by Ca2+ ionophores or chelation of extracellular Ca2+ on this process.19,–21
Many of the effects of Ca2+ are mediated via Ca2+-induced activation of the ubiquitous Ca2+ receptor calmodulin (CaM).22 In turn, Ca2+/CaM stimulates a plethora of enzymes including those that comprise the family of multifunctional, serine-threonine kinases (CaMKs), 2 of which are CaMKII and CaMKIV.23 These protein kinases have different tissue distributions, as CaMKII is ubiquitous24 whereas CaMKIV is tissue-selective, and expressed primarily in brain, thymus, testis, ovary, bone marrow, and adrenal glands.25 Whereas CaMKIV is expressed in immature thymocytes and mature T cells, it is absent in B cells. Previous studies have revealed roles for CaMKIV in regulating thymic selection as well as activation of naive and memory T cells. Moreover, CaMKIV plays a role in regulating the survival of hematopoietic progenitor cells.26 Importantly, in addition to a rise in intracellular Ca2+, activation of CaMKIV requires phosphorylation by an upstream CaMKK, leading to the suggestion that these 2 Ca2+/CaM-dependent enzymes constitute a “CaM kinase cascade.”
In this study, we demonstrate that CaMKIV is expressed in DC and plays a key role in the pathway linking the TLR4 with the control of DC lifespan by regulating the temporal expression of Bcl-2. These findings, which have been confirmed in human monocyte–derived DCs as well as in DCs derived from mice null for CaMKIV, reveal the importance of a CaMK cascade in mediating DC survival.
Methods
Mice and DCs
Mice were housed and maintained in the Levine Science Research Center Animal Facility located at Duke University under a 12-hour light, 12-hour dark cycle. Food and water were provided ad libitum, and all care was given in compliance within National Institutes of Health (NIH) and institutional guidelines on the use of laboratory and experimental animals under an approved Duke Institutional Animal Care and Use Committee protocol.
Camk4−/− mice were generated as previously described.27 The BCL-2 transgenic mice (a kind gift from Dr Tannishtha Reya, Duke University) have been previously described.28
BCL-2tg/tg/Camk4−/− mice were generated by crossing BCL-2tg/tg with Camk4+/− mice to generate BCL-2tg/tg/Camk4+/− hybrids. These hybrids were crossed to generate the BCL-2tg/tg/Camk4−/− mice used in our experiments. All mice were screened by PCR to confirm the presence of the BCL-2 transgene and the absence of the Camk4 gene.
Mouse DCs were isolated from spleen, thymus, and lymph nodes of 4- to 8-week-old mice. CD11c+ cells were positively selected using an anti-CD11 antibody (Miltenyi Biotech, Calderara di Reno, Italy). The purity of DC determined by flow cytometry was 80%-92%.
Human DCs were generated from CD14+ monocytes isolated from peripheral blood of healthy donors (Miltenyi Biotech) cultured for 5 days in RPMI 1640 (Invitrogen, Carlsbad, CA), 10% fetal calf serum, 50 ng/mL granulocyte macrophage colony stimulating factor (GM-CSF; Schering-Plough, Kenilworth, NJ), and 250 ng/mL interleukin-4 (PeproTech, Rocky Hill, NJ). Phenotype was evaluated by cytometry. LPS was from Sigma (St Louis, MO).
Measurement of viability
The percentage of apoptotic cells was quantified using annexin V fluorescein isothiocyanate (FITC) kits (Bender MedSystem, Vienna, Austria) according to the manufacturer's instructions. Viable cells were evaluated by the exclusion of Trypan blue using a kit from Invitrogen.
Protein and RNA analyses
Immunoblots were performed as described.29 Calpain inhibitors ALLM and ALLN were obtained from Calbiochem (San Diego, CA). Primary antibodies were: anti-CaMKII (Santa Cruz Biotech, Santa Cruz, CA), anti-CaMKIV (BD, San Jose, CA and Acris, Hidden Hausen, Germany), anti-actin (Sigma), anti-pCREB (phosphorylated form of the cAMP response element-binding protein), anti-pAkt, anti-Bcl-2 family proteins (Cell Signaling, Danvers, MA), anti-human Bcl-2 (BD). Binding was detected by horseradish peroxidase-conjugated secondary antibody and chemiluminescence (Amersham Pharmacia Biotech, Chalfont, United Kingdom). NIH Scion Image software version 1.61 (Bethesda, MD) was used to quantify bands.
RNA was isolated by using Trizol kits (Invitrogen), and first strand cDNA prepared by using SuperScript III (Invitrogen), according to the manufacturer's directions. PCR-based gene expression analysis was performed as reported elsewhere.27 The sequences of all the primers used in this study are available on request.
Immunocytochemistry
CD14+ monocytes were resuspended at 106 cells/mL in regular medium supplemented with IL-4 (1000 IU/mL, Immunotools, Friesoythe, Germany) and GM-CSF (50 ng/mL, Schering-Plough) and adhered to microscope slides coated with 0.05 mg/mL of poly-L-lysine in 24-well plates. DCs were fixed and permeabilized with the Cytofix/Cytoperm reagent (Becton Dickinson, Milan, Italy) according to the manufacturer's instruction and left in 3% bovine serum albumin solution in phosphate-buffered saline for 30 minutes at room temperature. DCs were then incubated with a rabbit polyclonal antibody to CaMKIV (0.5 μg/mL Acris Antibodies), stained with Alexa Fluor 594 goat anti-rabbit IgG (0.5 μg/mL Molecular Probes, Eugene, OR) and counterstained with Hoechst 33342 (Vector). Images were acquired with a DMIRE2 inverted confocal microscope (Leica Microsystems, Wetzlar, Germany) using a 40× lens at 40×/1.25 NA oil objective and processed using LCS software version 2.61 (Leica Microsystems). Internal photon multiplier tubes collected images in 8-bit, unsigned images at a 400-Hz scan speed. Hoechst 33342 fluorescence (Invitrogen) was excited with a mode-locked titanium-sapphire laser (Chameleon; Coherent, Santa Clara, CA; excitation wavelength: 740 nm, emission range: 410-470 nm). Two-photon intensity input was regulated with an amplitude modulator linked to the Leica Software System. Alexa Fluor 594 (Invitrogen) was excited by a helium-neon laser line (excitation wavelength: 543 nm, emission range: 600-700 nm). Line profiles of acquired images were performed with LCS 2.61 image analysis software (Leica Microsystems).
Flow cytometry
Antibodies used for human DC analysis: FITC-anti-CD14, phycoerythrin (PE)-anti-CD86, PE-CD1a, FITC-anti-CD83. Mouse DC staining were performed with: FITC-anti-I-A, PE-anti-CD8α, APC-anti-CD11c, FITC-anti-CD86, PE-anti-tumor necrosis factor (TNF), FITC-anti-IL-6. All of these antibodies were purchased from BD Pharmingen.
Lentiviral infection
The lentiviral constructs were generated and characterized by Kitsos et al.26 Briefly, CaMKIV-WT and CaMKIV-K71M cDNA were cloned into Lenti-IRES-GFP vectors, and high titer control and recombinant viruses were prepared by pseudo-typing with VSV.G using a quadruple transfection protocol in 293T cells according to Follenzi et al.30 Approximately 5 × 106 of immature monocyte-derived DCs were infected with the appropriate lentivirus at a multiplicity of infection of 5.2 days after infections GFP+ cells were sorted by flow cytometry and cultured for an additional 18 hours in the presence of LPS (1 μg/mL), or left in regular medium.
Results
CaMKIV accumulates during differentiation of human monocyte–derived DCs
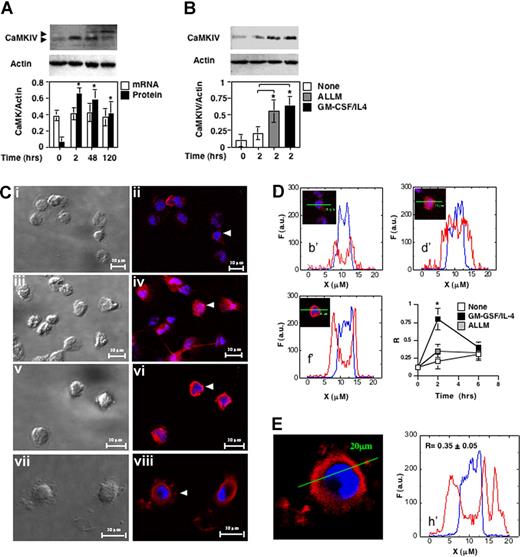
CD14+ cells were cultured in the presence of optimal amounts of GM-CSF and IL-4 and at different time points aliquots of cells were lysate to measure CaMKIV accumulation. Immunoblots showed a barely detectable amount of CaMKIV in freshly isolated monocytes (Figure 1A). However, within 2 hours after cytokine exposure CaMKIV was robustly up-regulated and remained so after 48 hours. After 120 hours of stimulus, cells have acquired the phenotype typical of immature DCs (CD14− CD1a+ CD86+ CD83−; data not shown) and still expressed CaMKIV. No significant modulation in the amount of CaMKI occurred during the monocyte differentiation process (data not shown). Parallel analysis showed that CaMKIV mRNA remained stable during the differentiation process. Based on these findings, we reasoned CaMKIV expression likely to be largely regulated by a posttranscriptional mechanism.
CaMKIV accumulates during differentiation of monocyte-derived dendritic cells. (A) CD14+ mononuclear cells were cultured in the presence of GM-CSF and IL-4. Whole-cell lysates were prepared at the indicated times and analyzed by immunoblot with specific antibodies (CaMKIV and actin). Aliquots of cells were used to measure CaMKIV and actin mRNA levels by quantitative reverse transcription–polymerase chain reaction. Bottom panel shows mean (± SD) of optical density measurements expressed as the ratio between the CaMKs and actin bands (n = 4). *P < .01. (B) Calpain regulates CaMKIV accumulation in differentiating monocytes. CD14+ mononuclear cells were cultured in the presence of ALLM, a selective calpain inhibitor (ALLM), or GM-CSF and IL-4 and analyzed for CaMKIV expression by immunoblot (top). The bottom panel shows mean (± SD) of the optical density measurements expressed as the ratio between the CaMKIV and actin bands (n = 4). *P < .01. (C-E) Intracellular distribution of CaMKIV in differentiating monocytes. (C) Transmission and confocal fluorescent immunocytochemistry images of CaMKIV expression in monocytes cultured for: 2 hours in regular medium (i,ii); 2 hours in the presence of GM-CSF/IL-4 or ALLM (iii, iv, v, and vi, respectively); and 120 hours in the presence of GM-CSF/IL-4 (vii,viii). (D) Line profiles of cells indicated by white arrows in the corresponding subpanels in panel C. The line segment is 20 μM; F indicates the fluorescence intensity in arbitrary units (a.u.). The bottom right graph shows the ratio (R) between the mean fluorescence intensity of Alexa Fluor 594 (CaMKIV) and Hoechst 33342 (nuclear staining) in the nuclear region along different line profiles. Means (± SD) represent 20 independent line profiles. *P < .01. (E) Expression and line profile analysis of CaMKIV in monocytes treated for 120 hours with GM-CSF/IL-4..
CaMKIV accumulates during differentiation of monocyte-derived dendritic cells. (A) CD14+ mononuclear cells were cultured in the presence of GM-CSF and IL-4. Whole-cell lysates were prepared at the indicated times and analyzed by immunoblot with specific antibodies (CaMKIV and actin). Aliquots of cells were used to measure CaMKIV and actin mRNA levels by quantitative reverse transcription–polymerase chain reaction. Bottom panel shows mean (± SD) of optical density measurements expressed as the ratio between the CaMKs and actin bands (n = 4). *P < .01. (B) Calpain regulates CaMKIV accumulation in differentiating monocytes. CD14+ mononuclear cells were cultured in the presence of ALLM, a selective calpain inhibitor (ALLM), or GM-CSF and IL-4 and analyzed for CaMKIV expression by immunoblot (top). The bottom panel shows mean (± SD) of the optical density measurements expressed as the ratio between the CaMKIV and actin bands (n = 4). *P < .01. (C-E) Intracellular distribution of CaMKIV in differentiating monocytes. (C) Transmission and confocal fluorescent immunocytochemistry images of CaMKIV expression in monocytes cultured for: 2 hours in regular medium (i,ii); 2 hours in the presence of GM-CSF/IL-4 or ALLM (iii, iv, v, and vi, respectively); and 120 hours in the presence of GM-CSF/IL-4 (vii,viii). (D) Line profiles of cells indicated by white arrows in the corresponding subpanels in panel C. The line segment is 20 μM; F indicates the fluorescence intensity in arbitrary units (a.u.). The bottom right graph shows the ratio (R) between the mean fluorescence intensity of Alexa Fluor 594 (CaMKIV) and Hoechst 33342 (nuclear staining) in the nuclear region along different line profiles. Means (± SD) represent 20 independent line profiles. *P < .01. (E) Expression and line profile analysis of CaMKIV in monocytes treated for 120 hours with GM-CSF/IL-4..
Pharmacologic inhibition of calpain activity leads to the rapid accumulation of CaMKIV
Previous studies have suggested that accumulation of CaMKIV in neuronal cells is regulated by a Ca2+-sensitive protease, calpain.31 Thus, we evaluated CaMKIV expression in fresh isolated monocytes and in monocytes cultured for 2 hours in regular medium, with or without ALLM, a cell-permeable calpain inhibitor, or the cytokine cocktail composed of GM-CSF and IL-4. The immunoblot in Figure 1B reveals that the exposure to ALLM or GM-CSF/IL-4 resulted in a statistically significant and comparable accumulation of CaMKIV. This contention was confirmed using an additional calpain inhibitor ALLN (data not shown). Because the anti-CaMKIV antibody used recognizes the entire p55 molecule,31 the barely detectable amount of p55 observed in untreated monocytes as well as the ability of calpain to increase its expression led us to hypothesize that a protease-dependent mechanism was likely to play a role in the control of CaMKIV accumulation in myeloid cells.
Confocal analysis of CaMKIV expression
To analyze the intracellular distribution of CaMKIV in differentiating monocytes, we used 2-photon confocal microscopy (Figure 1C-E). The image analysis confirmed a low level of CaMKIV in untreated monocytes and revealed that this kinase was primarily localized in close proximity to the nuclear membrane (Figure 1Ci,ii). Exposure to the cytokine cocktail or to ALLM induced a rapid increase in CaMKIV (Figure 1Ciii-vi). However, whereas inhibition of calpain activity did not stimulate nuclear accumulation of this kinase, a large amount of CaMKIV is detected in nuclei of monocytes exposed to GM-CSF/IL-4 for 2 hours. (Figure 1Ciii,iv). Finally, in monocytes treated with cytokines for 120 hours, conditions that generate a phenotype typical of DC, CaMKIV is detected predominantly in the perinuclear region as well as in spotted zones in proximity to plasma membranes (Figure 1Cvii,viii).
A quantitative 20-μm line profile analysis of the acquired images confirmed a very low nuclear/perinuclear ratio of CaMKIV in unstimulated monocytes (Figure 1D, b′). This ratio is similar to that observed in freshly isolated cells and did not increase on culture in the absence of differentiating stimuli or in the presence of calpain inhibitors (Figure 1D, c′ and d′). Kinetic analysis showed that at a later time point (6 hours) the nuclear accumulation of CaMKIV in cytokine-treated cells decreases and CaMKIV returns to be localized mainly in the perinuclear region (Figure 1D bottom right). The quantitative analysis of DC images confirmed that, at this stage of differentiation, CaMKIV is located predominantly outside the nucleus (Figure 1E).
CaMKs regulate differentiation and survival of monocyte-derived DCs
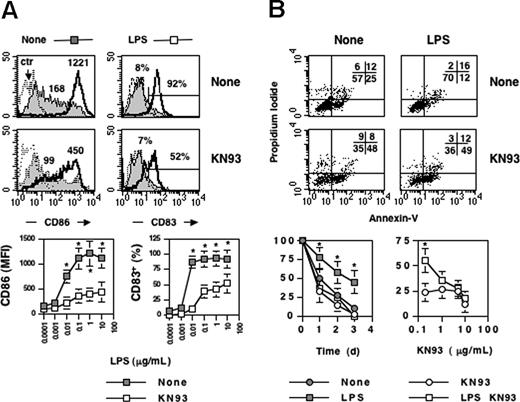
To examine potential roles of the multifunctional CaMKs in the activation process of DCs, we tested the ability of KN93, a selective inhibitor of the multifunctional CaMKs (CaMKI, CaMKII, CaMKIV), to alter terminal differentiation and/or survival of DCs exposed to LPS. As shown in Figure 2A, KN-93 interfered with up-regulation of CD83 and CD86 induced by LPS (Figure 2A). To analyze the effect of KN93 on survival, we exposed DCs to increasing concentrations of the kinase inhibitor and double-stained cells at different time points with annexin-V and propidium iodide. Finally, we quantified the number of double-negative viable cells by flow cytometry or by using a trypan blue-exclusion assay (Figure 2B top and bottom, respectively). The exposure of DCs to LPS normally increases their lifespan: 50% of DCs treated with LPS were still viable after 2 days of culture compared with 25% of cells left in regular medium alone (Figure 2B). Probably because of its inhibitory effect on all 3 multifunctional CaMKs (I, II, and IV), high doses of KN93 (> 5 μM) also led to a decrease in the survival of unstimulated DCs (Figure 2B top and bottom left). However, at lower doses this drug exerted its effect preferentially on the LPS-stimulated DCs by preventing the prosurvival ability of the bacterial endotoxin with barely detectable effects on the viability of unstimulated DCs (Figure 2B bottom right). Of note KN92, a KN93 derivative that is 10-fold less potent that KN93 as a CaMK inhibitor, had no effect on differentiation and survival of DC at a concentration equivalent to the effective dose of KN93 (data not shown). These results suggest the importance of multifunctional CaM kinases in LPS-mediated DC survival.
CaMKs regulate terminal differentiation and survival of monocyte-derived dendritic cells. Immature monocyte–derived DCs were cultured untreated or stimulated with LPS- (1 μg/μL) in the presence or absence of KN93 (10 μM), a selective inhibitor of the multifunctional CaMKs. After 24 hours, cells were recovered and double-stained with anti-CD86/anti-CD83 antibodies or with Annexin V/propidium iodide (A,B top). (A) Bottom: effects of KN93 on CD83 and CD86 expression as a function of the LPS dose. Mean (± SD) represents 6 independent experiments. (B) Bottom: effects of KN93 on survival of LPS-stimulated DC (LPS, 10 μg/mL) as a function of time or KN93 dose (left or right, respectively). Viability was calculated by trypan blue exclusion. Mean (± SD) represents 6 independent experiments.*P < .01. Values in panel A represent the mean fluorescente intensity of CD86 and the percentage of CD83 positive cells. Ctr refers to profiles of unstained cells. Values in panel B represent the percentage of cells in each quadrant.
CaMKs regulate terminal differentiation and survival of monocyte-derived dendritic cells. Immature monocyte–derived DCs were cultured untreated or stimulated with LPS- (1 μg/μL) in the presence or absence of KN93 (10 μM), a selective inhibitor of the multifunctional CaMKs. After 24 hours, cells were recovered and double-stained with anti-CD86/anti-CD83 antibodies or with Annexin V/propidium iodide (A,B top). (A) Bottom: effects of KN93 on CD83 and CD86 expression as a function of the LPS dose. Mean (± SD) represents 6 independent experiments. (B) Bottom: effects of KN93 on survival of LPS-stimulated DC (LPS, 10 μg/mL) as a function of time or KN93 dose (left or right, respectively). Viability was calculated by trypan blue exclusion. Mean (± SD) represents 6 independent experiments.*P < .01. Values in panel A represent the mean fluorescente intensity of CD86 and the percentage of CD83 positive cells. Ctr refers to profiles of unstained cells. Values in panel B represent the percentage of cells in each quadrant.
The ectopic expression of kinase-inactive CaMKIV decreases the viability of LPS-stimulated DCs
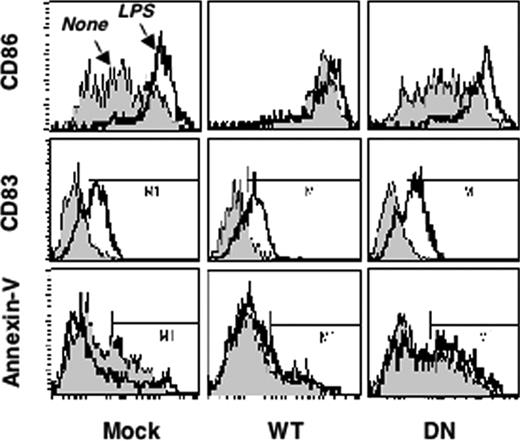
Our experiments using KN93 indicated that CaMKs play an important role in the activation programs triggered by TLR4 stimulation. However, because of the ability of KN93 to equivalently inhibit CaMKI, CaMKII, and CaMKIV, it is impossible to identify the relevant multifunctional CaMK family members. To directly investigate a role for CaMKIV in DC activation, we infected human immature monocyte–derived DCs with lentiviral vectors encoding wild-type or kinase-inactive Camk4 (Lenti-IRES-GFP CaMKIV-WT and CaMKIV-K71M, respectively). Aliquots of DC were also infected with the control virus (Lenti-IRES-GFP). After 2 days, GFP+ cells were sorted, washed, and cultured for additional 24 hours in the presence or absence of LPSs (1 μg/mL), before being analyzed by flow cytometry (Figure 3; Table 1). Although DCs infected with CaMKIV-K71M or control viruses (DN and Mock, respectively) left in regular medium expressed comparable amounts of CD86, infection of the cells with the CaMKIV-WT virus (WT) induced a significant up-regulation of this costimulatory molecule. However, neither CaMKIV-WT, nor CaMKIV-K71M, nor control virus interfered with LPS-induced increases in the surface level of CD86 or CD83 (Figure 3).
CaMKIV regulates survival of monocyte–derived DCs. Monocyte-derived DCs were infected with Lenti-IRES-GFP lentivirus expressing Camk4, Camk4-WT, or Camk4-K71M (Mock, WT, and DN, respectively). After 48 hours, cells were cultured for an additional 18 hours in the presence of LPS (1 μg/mL) or left untreated. Top: fluorescence-activated cell sorting (FACS) profiles of DC stained with CD86, CD83, and Annexin-V.
CaMKIV regulates survival of monocyte–derived DCs. Monocyte-derived DCs were infected with Lenti-IRES-GFP lentivirus expressing Camk4, Camk4-WT, or Camk4-K71M (Mock, WT, and DN, respectively). After 48 hours, cells were cultured for an additional 18 hours in the presence of LPS (1 μg/mL) or left untreated. Top: fluorescence-activated cell sorting (FACS) profiles of DC stained with CD86, CD83, and Annexin-V.
Effects of CaMKIV on DC activation markers
| . | Mock . | WT . | DN . |
|---|---|---|---|
| Regular medium | |||
| CD86 (MFI) | 350 ± 125 | 950 ± 320* | 270 ± 160 |
| CD83, % | 10 ± 5 | 8± 2 | 7 ± 3 |
| A-V, % | 55 ± 14 | 28± 17* | 63 ± 22 |
| Viability, % | 35 ± 12 | 67± 16* | 32 ± 18 |
| LPS | |||
| CD86 (MFI) | 1140 ± 250 | 1250 ± 305 | 1070 ± 360 |
| CD83, % | 52 ± 13 | 48± 20 | 51 ± 18 |
| A-V, % | 28 ± 10 | 23± 15 | 65 ± 19* |
| Viability, % | 65 ± 11 | 62± 15 | 30 ± 16* |
| . | Mock . | WT . | DN . |
|---|---|---|---|
| Regular medium | |||
| CD86 (MFI) | 350 ± 125 | 950 ± 320* | 270 ± 160 |
| CD83, % | 10 ± 5 | 8± 2 | 7 ± 3 |
| A-V, % | 55 ± 14 | 28± 17* | 63 ± 22 |
| Viability, % | 35 ± 12 | 67± 16* | 32 ± 18 |
| LPS | |||
| CD86 (MFI) | 1140 ± 250 | 1250 ± 305 | 1070 ± 360 |
| CD83, % | 52 ± 13 | 48± 20 | 51 ± 18 |
| A-V, % | 28 ± 10 | 23± 15 | 65 ± 19* |
| Viability, % | 65 ± 11 | 62± 15 | 30 ± 16* |
Means are plus or minus SD. MFI indicates mean fluorescence intensity; and A-V, annexin-V.
indicates statistical significance (n = 3); P < .01.
The effect of ectopic CaMKIV expression on DC survival was evaluated at 24 hours by both annexin-V staining and the trypan blue-exclusion assay. As shown in Figure 3, in mock-infected DC, LPS treatment led to a significant decrease in the percentage of annexin-V-positive cells and induced a parallel increase in the percentage of viable cells (trypan blue unstained cells), compared with DCs cultured in regular medium. Overexpression of CaMKIV-WT in DCs induced a detectable antiapoptotic effect (Figure 3). Contrariwise, CaMKIV-K71M did not affect viability of untreated DCs but abrogated the antiapoptotic effect induced by LPS, suggesting that the kinase-inactive protein might play a dominant/negative role in this instance. These results clearly implicate CaMKIV in survival of human monocyte–derived DC and suggest that the absence of CaMKIV in mice should negatively impact the number of DC cells.
Camk4−/− mice contain a decreased number of DC
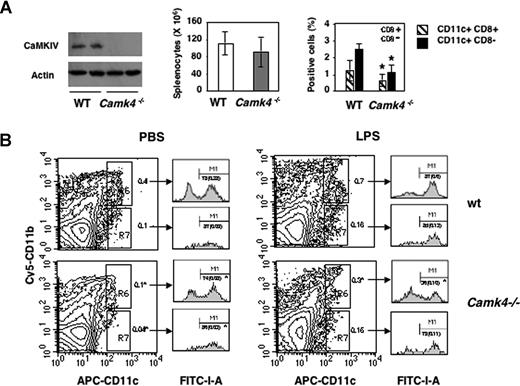
To evaluate the hypothesis, we analyzed spleen-derived DCs in WT and Camk4−/− mice. Splenocytes from Camk4−/− and WT mice were isolated, counted, and stained with anti-CD11c and CD8α antibodies. Immunoblots were performed to measure CaMKIV expression (Figure 4A left). Camk4−/− and WT mice contained comparable numbers of splenocytes (Figure 4A middle), but the former genotype showed a significant decrease in the percentage of both CD11c+ CD8α+ and CD8α− subsets (Figure 4A right). Similar results were found on analysis of CD11c+ cells present in the lymph nodes of WT versus CaMKIV null mice (data not shown).
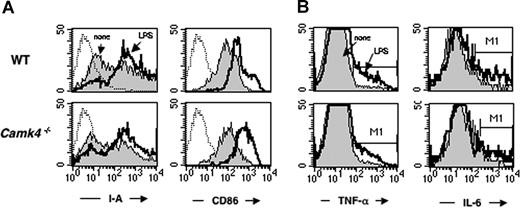
The number of splenic mature DCs is reduced in Camk4−/− mice. Splenocytes from normal or Camk4−/− mice were counted and stained for CD11c and CD8. (A) Left panel: immunoblots show CaMKIV and actin expression in splenocytes isolated from 2 mice from each genotype. Middle: bar graph reports mean (± SD) of the total number of splenocytes (n = 15 mice per genotype). Right: percentage of WT and Camk4−/− CD11c + subsets. Bars graphs show mean (± SD) representing 15 mice per genotype. *P < .01. (B) LPS-induced mature DC accumulation is impaired in Camk4−/− mice in vivo. LPS or phosphate-buffered saline (PBS) was injected into Camk4−/− and control WT mice. Eighteen hours later, splenocytes were isolated and triple-stained with anti-CD11b, -CD11c and -I-A antibodies. For the typical dot plot profiles, the inset values show the percentage of cells in the R6/R7 gates (CD11bhigh/CD11chigh and CD11blow/CD11chigh, respectively. FACS profile histograms show I-A expression. Inset values refer to the percentage of I-Ahigh cells in the R6/R7 gates. Values in parenthesis display the percentage of CD11bhigh/CD11chigh/I-Ahigh and CD11blow/CD11chigh/I-Ahigh in whole splenocytes. *P < .01.
The number of splenic mature DCs is reduced in Camk4−/− mice. Splenocytes from normal or Camk4−/− mice were counted and stained for CD11c and CD8. (A) Left panel: immunoblots show CaMKIV and actin expression in splenocytes isolated from 2 mice from each genotype. Middle: bar graph reports mean (± SD) of the total number of splenocytes (n = 15 mice per genotype). Right: percentage of WT and Camk4−/− CD11c + subsets. Bars graphs show mean (± SD) representing 15 mice per genotype. *P < .01. (B) LPS-induced mature DC accumulation is impaired in Camk4−/− mice in vivo. LPS or phosphate-buffered saline (PBS) was injected into Camk4−/− and control WT mice. Eighteen hours later, splenocytes were isolated and triple-stained with anti-CD11b, -CD11c and -I-A antibodies. For the typical dot plot profiles, the inset values show the percentage of cells in the R6/R7 gates (CD11bhigh/CD11chigh and CD11blow/CD11chigh, respectively. FACS profile histograms show I-A expression. Inset values refer to the percentage of I-Ahigh cells in the R6/R7 gates. Values in parenthesis display the percentage of CD11bhigh/CD11chigh/I-Ahigh and CD11blow/CD11chigh/I-Ahigh in whole splenocytes. *P < .01.
The injection of LPSs in WT resulted in a significant increase in the percentage of cells with a phenotype typical of mature myeloid DC: CD11chigh/CD11bhigh/I-Ahigh (0.28 ± 0.05 vs 0.65 ± 0.07, untreated vs LPS-treated; Figure 4B right). Otherwise, this treatment did not induce similar changes in Camk4−/−: the CD11chigh/CD11bhigh/I-Ahigh population failed to accumulate in response to LPSs and only 37% of the CD11chigh/CD11bhigh subset, compared with the 87% detected in WT mice, expressed high levels of I-A molecules. Therefore, genetic ablation of CaMKIV led to a marked defect in the accumulation of cells showing typical markers of mature myeloid DC in response to LPSs.
The CD11chigh/CD11blow population contains a mixture of DCs at different stages of differentiation, including DC precursors (DCp), immature DC (iDC), and plasmacytoid DC (pDC), which display different abilities to replicate and differentiate in basal condition as well as in response to LPSs.32 Our data show a significant decrease in the percentage of CD11chigh/CD11blow cells in untreated Camk4−/− mice (0.1% vs 0.04%, WT and Camk4−/−, respectively). However, although LPSs did not induce significant changes in the percentage of CD11chigh/CD11blow cells, this did occur in Camk4−/− mice (Figure 4B). Thus, in WT, the CD11chigh/CD11blow population seems to be made up predominantly of LPS-unresponsive DC subsets (ie, pDC). However, in Camk4−/− mice, the CD11chigh/CD11blow cells appear to be mainly derived from LPS-responsive DC subsets (ie, DCp, iDC). These data suggest that CaMKIV may be involved in either the developmental program of other DC subsets (ie, pDC) or in the control of the proliferative capacity of DC precursors.
Genetic ablation of CaMKIV does not prevent the ability of DC to differentiate and secrete cytokines in response to LPSs
The involvement of CaMKIV in LPS signaling was evaluated in vitro using purified DCs. To this end, CD11c+ cells were positively selected from splenocytes of Camk4−/− and WT mice before being cultured in the presence or absence of LPSs (10 μg/mL). After 24 hours, we measured the cell surface expression of I-A and CD86 by flow cytometry as well as the intracellular levels of TNF-α and IL-6 by immunocytochemistry (Figure 5A,B, respectively; Table 2). LPS treatment induced a comparable increase of I-A and CD86 expression in WT and Camk4−/− DC (Figure 5A). Moreover, cells from both genotypes accumulated comparable levels of IL-6 and TNF-α in response to LPSs (Figure 5B). Therefore, we conclude that CaMKIV is largely dispensable for this branch of the LPS signaling pathway.
CaMKIV is not required for terminal differentiation and cytokine synthesis induced by LPS. Isolated CD11c+ splenic DCs from WT and Camk4−/− were exposed to LPS (10 μg/mL) or left untreated (none). After 16 hours, cells were recovered and double-stained for I-A and CD86 (A). Aliquots of cells were stained for the presence of intracellular TNF-α and IL-6 (A,B).
CaMKIV is not required for terminal differentiation and cytokine synthesis induced by LPS. Isolated CD11c+ splenic DCs from WT and Camk4−/− were exposed to LPS (10 μg/mL) or left untreated (none). After 16 hours, cells were recovered and double-stained for I-A and CD86 (A). Aliquots of cells were stained for the presence of intracellular TNF-α and IL-6 (A,B).
Effects of LPS on CamK4−/− DC (n = 6)
| Markers . | wt . | Camk4−/− . | P . |
|---|---|---|---|
| Regular medium | |||
| I-A (MFI) | 658 ± 107 | 538 ± 97 | .09 |
| CD86 (MFI) | 150 ± 57 | 128 ± 50 | .54 |
| TNF-α, % | 2.3 ± 0.5 | 2.6 ± 0.6 | .46 |
| IL-6, % | 2.6 ± 0.5 | 3.0 ± 0.4 | .18 |
| LPS | |||
| I-A (MFI) | 1241 ± 289 | 938 ± 190 | .08 |
| CD86 (MFI) | 657 ± 159 | 830 ± 127 | .08 |
| TNF-α, % | 11 ± 3 | 15 ± 3 | .49 |
| IL-6, % | 13 ± 3 | 15 ± 6 | .48 |
| Markers . | wt . | Camk4−/− . | P . |
|---|---|---|---|
| Regular medium | |||
| I-A (MFI) | 658 ± 107 | 538 ± 97 | .09 |
| CD86 (MFI) | 150 ± 57 | 128 ± 50 | .54 |
| TNF-α, % | 2.3 ± 0.5 | 2.6 ± 0.6 | .46 |
| IL-6, % | 2.6 ± 0.5 | 3.0 ± 0.4 | .18 |
| LPS | |||
| I-A (MFI) | 1241 ± 289 | 938 ± 190 | .08 |
| CD86 (MFI) | 657 ± 159 | 830 ± 127 | .08 |
| TNF-α, % | 11 ± 3 | 15 ± 3 | .49 |
| IL-6, % | 13 ± 3 | 15 ± 6 | .48 |
Means are plus or minus SD (n = 6).
I-A indicates major histocompatibility complex class II molecules; and IL-6, interleukin-6.
DCs from CaMKIV null mice fail to increase CREB phosphorylation in response to LPSs
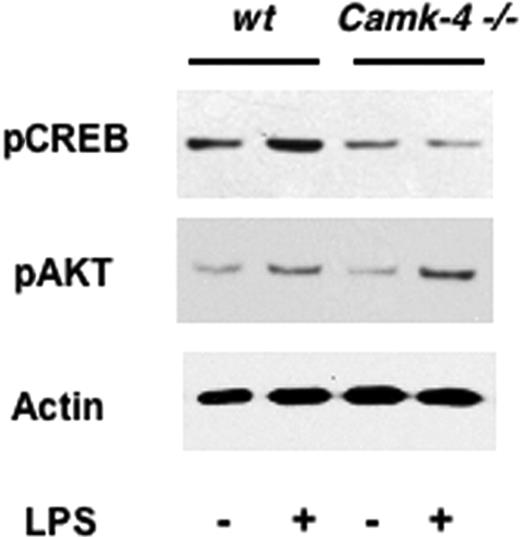
To investigate the role of CaMKIV in the early events induced by LPS signaling, we compared the levels of pCREB and pAKT in DCs isolated from WT and Camk4−/− mice cultured for 1 hour in the presence or absence of the bacterial endotoxin. Although a comparable up-regulation in the levels of pAKT was observed in DCs isolated from both genotypes, the ablation of CaMKIV prevented the increase in pCREB in response to LPSs (Figure 6; Table 3). This finding suggests a role for CaMKIV in the CREB-dependent pathway by which TLR4 regulates DC survival.
CaMKIV is required to link TLR4 signaling with pCREB accumulation. CD11c + DCs were isolated from spleens of WT or Camk4−/− mice and cultured in the presence or absence of LPS (10 μg/mL) for 1 hour. Whole lysates were separated by SDS-PAGE and immunoblotted with the reported antibodies (pCREB, pAKT, actin, LPS). A typical immunoblot analysis is shown.
CaMKIV is required to link TLR4 signaling with pCREB accumulation. CD11c + DCs were isolated from spleens of WT or Camk4−/− mice and cultured in the presence or absence of LPS (10 μg/mL) for 1 hour. Whole lysates were separated by SDS-PAGE and immunoblotted with the reported antibodies (pCREB, pAKT, actin, LPS). A typical immunoblot analysis is shown.
TLR4 signaling in CamK4−/− DC
| . | wt . | Camk4−/− . |
|---|---|---|
| Regular medium | ||
| pCREB | 0.32 ± 0.1 | 0.19 ± 0.05* |
| pAKT | 0.09 ± 0.04 | 0.07 ± 0.06 |
| LPS | ||
| pCREB | 0.51 ± 0.12 | 0.15 ± 0.03* |
| pAKT | 0.17 ± 0.05 | 0.23 ±0.06 |
| . | wt . | Camk4−/− . |
|---|---|---|
| Regular medium | ||
| pCREB | 0.32 ± 0.1 | 0.19 ± 0.05* |
| pAKT | 0.09 ± 0.04 | 0.07 ± 0.06 |
| LPS | ||
| pCREB | 0.51 ± 0.12 | 0.15 ± 0.03* |
| pAKT | 0.17 ± 0.05 | 0.23 ±0.06 |
Means are plus or minus SD of the optical density measurements expressed as the ratio between pCREB or pAKT.
indicates statistical significance (n = 3); P < .01.
CaMKIV regulates survival of DC
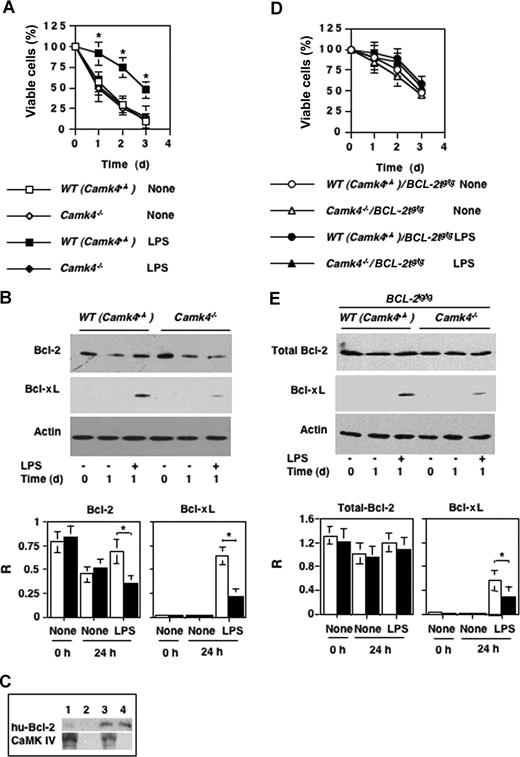
To evaluate whether CaMKIV plays a direct role in regulating the survival of DCs, we isolated CD11c+ from Camk4−/− and WT mice and measured their ability to survive in vitro in the absence or presence of LPSs. At different time points, cell viability was tested by trypan blue exclusion (Figure 7A). The number of viable DCs remaining in the culture in the absence of treatment decreased progressively as a function of days in culture and the time course was similar in WT and Camk4−/− cells (Figure 6). On the other hand, whereas LPS clearly increased viability of WT cells, it failed to alter the lifespan of Camk4−/− DC (Figure 7A).
CaMKIV regulates lifespan and Bcl-2 family protein accumulation. CD11c + DCs were positively selected from WT (Camk4+/+), Camk4−/−, BCL-2tg/tg transgenic, and Camk4−/−/BCL-2tg/tg hybrid mice cultured in the presence or absence of LPS (10 μg/mL). (A,D) Viability was assayed by trypan blue exclusion at daily intervals. The results represent mean and SD of 6 independent experiments. (B,E) Typical results obtained by immunoblot analysis. Bar graphs show mean (± SD) of the optical density measurements expressed as the ratio between Bcl-2 or Bcl-xL and actin bands (n = 6). (C) Immunoblot shows the typical expression of CaMKIV and hu-Bcl-2 detected in WT (Camk4+/+), Camk4−/−, BCL-2tg/tg transgenic, and Camk4−/−/BCL-2tg/tg hybrid mice (lanes 1, 2, 3, and 4, respectively). *P < .01.
CaMKIV regulates lifespan and Bcl-2 family protein accumulation. CD11c + DCs were positively selected from WT (Camk4+/+), Camk4−/−, BCL-2tg/tg transgenic, and Camk4−/−/BCL-2tg/tg hybrid mice cultured in the presence or absence of LPS (10 μg/mL). (A,D) Viability was assayed by trypan blue exclusion at daily intervals. The results represent mean and SD of 6 independent experiments. (B,E) Typical results obtained by immunoblot analysis. Bar graphs show mean (± SD) of the optical density measurements expressed as the ratio between Bcl-2 or Bcl-xL and actin bands (n = 6). (C) Immunoblot shows the typical expression of CaMKIV and hu-Bcl-2 detected in WT (Camk4+/+), Camk4−/−, BCL-2tg/tg transgenic, and Camk4−/−/BCL-2tg/tg hybrid mice (lanes 1, 2, 3, and 4, respectively). *P < .01.
To begin to evaluate the mechanism by which CaMKIV might participate in LPS-initiated signaling, we quantified the expression of Bcl-2 family proteins. CD11c+ cells were isolated by positive selection from spleens of WT and Camk4−/− mice. Freshly isolated WT and Camk4−/− DCs expressed comparable amounts of Bcl-2 but undetectable levels of Bcl-xL (Figure 7B). Although the amount of Bcl-2 decreased similarly in cells of both genotypes cultured for 24 hours, LPS prevented the decrease in WT but not Camk4−/− DCs. In addition, LPS induced accumulation of Bcl-xL in WT cells, and this effect was markedly decreased in Camk4−/− DCs (Figure 7B).
Transgenic expression of Bcl-2 reverses the ability of CaMKIV-null DC to survive
To analyze the contribution of the decreased amount of Bcl-2 to survival, we generated BCL-2tg/tg/Camk4−/− mice by crossing BCL-2tg/tg mice with Camk4−/− mice to generate BCL-2tg/tg/Camk4−/− hybrid mice that overexpress Bcl-2 in a CaMKIV-null background. The immunoblot in Figure 7C shows a typical result obtained in mice carrying the 4 different genotypes. CD11c+ cells were recovered by positive selection from spleens of BCL-2tg/tg and BCL-2tg/tg/Camk4−/− mice, cultured in the presence or absence of LPSs and analyzed for viability as described previously (Figure 7D). DCs from BCL-2tg/tg and BCL-2tg/tg/Camk4−/− mice cultured in regular medium show a comparable and prolonged lifespan. Furthermore, regardless of genotypes, the presence of LPS in the culture medium did not result in a significant increase in the number of viable cells (Figure 7D).
The immunoblot in Figure 7E shows the typical expression of total Bcl-2 and Bcl-XL in these transgenic mouse strains. Regardless of CaMKIV expression but correlated with the presence of the human Bcl-2 transgene, mice carrying the hybrid genotypes show a high level of total Bcl-2 protein that was barely altered by LPS treatment (Figure 7E). Because most of the effect exerted by the LPS-CaMKIV pathway on Bcl-2 was at the transcriptional level (data not shown), we reasoned that the ectopic promoter of the BCL-2 transgene would require a different set of transcription factors compared with the endogenous mouse gene and, in turn, be less dependent on the presence of CaMKIV. On the other hand, CaMKIV was still required in BCL-2tg/tg hybrid mice to link the LPS–mediated pathway with Bcl-xL expression (Figure 7D). This finding provides an additional evidence for a role for CaMKIV in the pathway responsible for Bcl-xL expression and also documents the dominant role played by Bcl-2 in modulating the lifespan of LPS-activated DC in a manner that involves CaMKIV signaling.
Discussion
Stimulation of TLR4 has been associated with the initiation of both apoptotic and antiapoptotic pathways, the balance of which determines the outcome of innate and adaptive immune responses.4,–6 Here we describe a novel CaMK cascade-dependent antiapoptotic pathway responsible for the survival of LPS-activated DCs. The results obtained, using pharmacologic inhibition of CaMKs, ectopic expression of CaMKIV, and a CaMKIV kinase-inactive mutant as well as mice null for CaMKIV, demonstrate that a CaMKIV signaling cascade controls the phosphorylation of CREB and accumulation of Bcl-2 necessary to support the antiapoptotic branch of the TLR4 pathway.
The multifunctional CaMK family proteins are involved in the control of differentiation and survival of several cell types, including neurons and hematopoietic stem cells.26,27 Analysis of mouse embryos revealed expression of CaMKIV mRNA in the developing nervous system as well as in the hematopoietic-related tissues.25,26 These developmental patterns coincide temporally with periods of significant cellular differentiation in the nervous system, axonal migration and neuron survival. Correlation of CaMKIV expression with differentiation is also evident in adult animals as Camk4−/− mice show major defects in maintenance of hematopoietic stem cells, postnatal maturation of Purkinje cells, thymopoiesis, ovulation, and terminal differentiation of spermatozoa.26,33,,,,,–39 Here we show that CaMKIV expression is tightly regulated during the developmental program of human monocyte–derived DCs, a well-characterized model of myeloid cell differentiation, and is also expressed in murine mature DCs isolated from secondary lymphoid tissues.
Extensive gene expression analyses performed using microarray or SAGE technologies failed to identify Camk4 among the mRNAs that were altered during the monocyte-derived DC differentiation process.40,–42 In agreement with these findings, we show comparable Camk4 mRNA levels in monocyte and monocyte-derived DCs. However, we provide evidence for a cytokine-dependent, rapid accumulation of CaMKIV in differentiating DCs that is overcome by a calpain-dependent mechanism that keeps CaMKIV levels low in the absence of stimulation. Calpain is a cysteine protease activated by an increase in intracellular Ca2+43,44 that influences normal signal transduction pathways by cleaving cytoskeletal proteins, membrane proteins, and enzymes normally involved in cell survival.45 The susceptibility of CaMKIV to calpain has been documented in cerebellar granule cell neurons.31 More recently, a role for calpain has been proposed in the mechanism regulating podosome turnover and composition in murine DCs.46 Here, we show that inhibition of calpain activity leads to accumulation of CaMKIV in the perinuclear region of monocytes cultured in regular medium. However, our data also reveal that stabilization of CaMKIV by calpain inhibition is not sufficient to promote the nuclear translocation of CaMKIV that occurs in response to GM-CSF and IL-4. Our findings provide novel evidence to suggest that differentiating cytokines may inhibit the degradation of CaMKIV and stimulate the entry of this enzyme into the nucleus where it participates in the regulation of genes, such as Bcl-2, that are necessary to support the survival of DCs.
Recently, it has been reported that the selective inhibition of another multifunctional CaMK, CaMKII, interferes with terminal differentiation of monocyte-derived DCs by preventing up-regulation of costimulatory and MHC II molecules as well as secretion of cytokines induced by TLR4 agonists.47 The findings described in the present study indicate that CaMKIV selectively regulates survival of stimulated DCs without interfering with their differentiation. Thus, in DC, as in neuronal cells, the coordinated activation of CaMKII and CaMKIV seems to be required to orchestrate the differentiation and survival programs.33
Isolated DCs are prone to apoptosis that can be modulated by a variety of bioactive molecules, including cytokines, CD40 agonists, and TLR ligands, which share the ability to regulate the levels of Bcl-2 family proteins.8 TLR agonists seem to promote DC survival mainly by controlling the timing of the accumulation of Bcl-2 family proteins,8 leading to the idea that Bcl-2 acts as a “molecular timer” to set the lifespan of DCs and the magnitude of the adaptive immune response.8 The crucial role of Bcl-2 in the regulation of DC lifespan has been confirmed in vivo using transgenic mice expressing the human BCL-2 gene under the control of the murine CD11c promoter as well as by testing the ability of Bcl-2 null DCs to survive.8,46 In agreement with these findings, we show here that the number of viable CD11c+ cells progressively decreases during culture, a phenomenon that is associated with a parallel decline in the level of Bcl-2. Stimulation of TLR4 triggers accumulation of proapoptotic Bcl-2 family proteins and induces the progressive temporal decline in the level of Bcl-2. The timing of these events sets the lifespan of DCs and our results support this contention. That is, freshly isolated DCs (time 0) contain higher amounts of Bcl-2 relative to DC cultured for 24 hours in the presence of LPS. However, a different conclusion can be drawn when taking into account the spontaneous loss of Bcl-2 expression observed in DCs cultured in absence of any stimuli coupled with the net accumulation of Bcl-2 that occurs in cells cultured with LPS for 24 hours. Therefore, while isolated DCs activate the “Bcl-2 molecular timer” and undergo spontaneous apoptosis, signals transduced by TLR4 modify the loss of Bcl-2 and thus prolong the lifespan of activated DCs.
Our results reveal that genetic ablation of CaMKIV results in a decrease in the number of mature DC present in lymphoid tissues of adult mice. Moreover, isolated DCs derived from Camk4−/− genotype show a marked defect in their capability to prolong lifespan in response to LPS, a phenomenon that is associated with the failure of TLR4 signaling to prevent the temporal decline in Bcl-2 and accumulation of Bcl-xL. However, the analysis of CaMKIV-null DC overexpressing transgenic Bcl-2 provide support for a dominant role Bcl-2 in regulating the lifespan of LPS-activated DCs and demonstrate that one of the crucial roles of the CaMKIV cascade in activated DC might be the regulation of the temporal accumulation of Bcl-2.
CaMKIV regulates survival and differentiation of several cell types, including hematopoietic progenitors, neurons, thymocytes, and osteoblasts; one common mechanism is by activating transcription by stimulating pCREB.26,27,34,48 We show that pharmacologic inhibition of CaMKs as well as the genetic ablation of the CaMKIV gene affects the early events triggered by TLR4 stimulation by preventing accumulation of pCREB. Recently, it has been shown that a CREB-dependent pathway inhibits the pathogen-induced apoptosis of bone marrow–derived macrophages.12 Our data confirm the relevance of pCREB in the survival program and document its involvement in the molecular mechanisms regulating the lifespan of LPS-activated DCs. Furthermore, they identify the CaMKIV/CREB signaling cascade as a novel pathway that is essential in the antiapoptotic branch of the TLR4 signaling pathway.
Genetic or microenvironmental factors may control the lifespan of activated DCs and in turn regulate the adaptive immune response by promoting the eradication of pathogens or the development of immune-mediated diseases. In this context, our results may contribute to a better understanding of the mechanisms used by pathogens to control the lifespan of antigen-presenting cells, and may inform novel perspectives to manipulate the immune response by targeting components of the CaMKIV cascade in DCs.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof Salvatore Formisano for providing human buffy-coats, Dr Jiro Kasahara for IC anti-CaMKIV antibody, Dr Alessio Cardinale for offering helpful discussion and technical assistance on calpain experiments, and Dr Antonio Staiano and Salvatore Sequino for advising us on animal care and procedures in Italy.
This work was supported by Italian National Program for AIDS research grant 40F.66 and 40G.49 (L.R.), PRIN 2004-2004055579 and 2006-051402 (L.R.), NIH grant DK074701 (A.R.M.), ACS fellowship F-05-171-01-LIB (U.S.), and PRIN 2004-2004069479 and Telethon GGP04039 (L.P.).
National Institutes of Health
Authorship
Contribution: M.I. designed and performed research and drafted the manuscript; M.G., L.V., A.M.M., F.B., E.C., D.A., A.C., V.C., and G.M. performed research on human monocyte–derived DCs; L.M.G.-T., U.S., and T.J.R. performed research on murine models; L.P. designed research using lentivaral vectors; A.R.M. interpreted data and drafted the manuscript; G.R. and M.V. interpreted data and drafted the manuscript; and L.R. designed and performed research, analyzed and interpreted data, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Luigi Racioppi, Departmnent of Cellular and Molecular Biology and Pathology, Via S Pansini 5, 80131 Naples, Italy; e-mail: racioppi@unina.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal