The success of chelation therapy in controlling iron overload in patients with thalassemia major is highly variable and may partly depend on the rate of transfusional iron loading. Using data from the 1-year phase III study of deferasirox, including volumes of transfused red blood cells and changes in liver iron concentration (LIC) in 541 patients, the effect of iron loading on achieving neutral or negative iron balance was assessed in patients receiving different doses of deferasirox and the comparator deferoxamine. After dose adjustment, reductions in LIC after 1 year of deferasirox or deferoxamine therapy correlated with transfusional iron intake. At a deferasirox dose of 20 mg/kg per day, neutral or negative iron balance was achieved in 46% and 75% of patients with the highest and lowest transfusional iron intake, respectively; 30 mg/kg per day produced successful control of iron stores in 96% of patients with a low rate of transfusional iron intake. Splenectomized patients had lower transfusional iron intake and greater reductions in iron stores than patients with intact spleens. Transfusional iron intake should be monitored on an ongoing basis in thalassemia major patients, and the rate of transfusional iron loading should be considered when choosing the appropriate dose of an iron-chelating agent. This study is registered at http://clinicaltrials.gov as NCT00061750.
Introduction
Successful iron chelation therapy depends on the achievement of neutral or negative iron balance, in which the excretion of iron equals or exceeds the rate of new iron accumulation. In β-thalassemia major, regular red blood cell (RBC) transfusions are the major source of iron intake.1 In order to maintain a hemoglobin level of 9 to 10 g/dL, annual transfusion of 10 to 20 units of packed RBCs in children younger than 10 years of age, and 25 to 50 units of packed RBCs in teenagers and adults are generally administered. Transfusional iron intake varies among patients with thalassemia as a result of differences in spleen size or previous splenectomy, the underlying molecular defect, and other factors.2,–4 However, to prevent excessive iron deposition in critical organs such as the heart and liver, chelator-induced iron excretion must at least balance the rate of ongoing transfusional iron accumulation.1 Similar principles apply to sickle cell disease (SCD), myelodysplastic syndromes (MDS), and other disorders for which regular RBC transfusions are an important part of overall management.
In November 2005, the United States Food and Drug Administration approved the orally active iron chelator deferasirox (Exjade, ICL670) for the treatment of iron overload. In the pivotal study of this chelator, the reduction in liver iron concentration (LIC) at the doses subsequently recommended for clinical use was comparable to deferoxamine (Desferal, DFO).5 By design, dose allocation was based on the degree of iron burden as measured by LIC at study entry, yet the response to therapy varied substantially. The present analyses were conducted to investigate the influence of transfusional iron intake on the outcome of iron chelation therapy in thalassemia major.
Methods
Data for this analysis were collected as part of the phase III study of deferasirox.5 This trial was designed and conducted in accordance with Good Clinical Practices and the Declaration of Helsinki. Institutional Review Board or Ethics Committee approval was obtained at each participating institution, and written informed consent was obtained from all patients or their legal guardians prior to enrollment.
Patient enrollment
Patients eligible for the trial were males or females at least 2 years of age with thalassemia major and transfusional iron overload (indicated by an LIC ≥ 2 mg Fe/g dry weight [dw]). All patients were regularly transfused and received at least 8 blood transfusions in the year prior to the start of the study. Patients were enrolled irrespective of prior chelation therapy, although those with concomitant conditions preventing therapy with deferasirox or DFO, a history of ocular toxicity related to iron chelation therapy, a previous poor response to DFO, or noncompliance with prescribed therapy were excluded. More detailed inclusion and exclusion criteria for the trial have been published elsewhere.5
Study design
The study was a multicenter, randomized (1:1), open-label, 1-year phase III trial comparing deferasirox with DFO. The initial dose of deferasirox or DFO was based on a predefined algorithm according to the patient's baseline LIC.5 The algorithm was designed on the assumption that patients with low LIC would require less iron to be removed, and therefore would need a lower dose in comparison with patients with a high LIC. An important exception to this algorithm was permitted in the DFO arm. Patients with a LIC of less than 7 mg Fe/g dw who were assigned to DFO were allowed to remain on the dose of the chelator that they were using prior to study entry. Table 1 shows the mean actual dose of the chelators during the study according to the initial dose. Because changes in dose were uncommon, the mean actual doses over the 1-year study are very similar to the initial doses, and therefore initial dose is used in the analysis.
Mean dose during 1 year of treatment with deferasirox or DFO according to initial dose
| . | Doses . | |||
|---|---|---|---|---|
| Deferasirox | ||||
| Initial dose, mg/kg per day | 5 | 10 | 20 | 30 |
| Mean actual dose, mg/kg per day | 6.2 | 10.2 | 19.4 | 28.2 |
| DFO | ||||
| Initial dose, mg/kg per day | <25 | 25-35 | 35-50 | ≥50 |
| Mean actual dose, mg/kg per day | 26.1 | 30.5 | 40.2 | 52.5 |
| . | Doses . | |||
|---|---|---|---|---|
| Deferasirox | ||||
| Initial dose, mg/kg per day | 5 | 10 | 20 | 30 |
| Mean actual dose, mg/kg per day | 6.2 | 10.2 | 19.4 | 28.2 |
| DFO | ||||
| Initial dose, mg/kg per day | <25 | 25-35 | 35-50 | ≥50 |
| Mean actual dose, mg/kg per day | 26.1 | 30.5 | 40.2 | 52.5 |
Assessments
Measuring iron burden.
Liver biopsy for LIC determination was performed using standardized methodology.6 In order to minimize potential variation in LIC due to different analytical methods, iron content was measured using atomic absorption spectrometry in a single central laboratory in Rennes, France (Clinique des Maladies du Foie [Clinic for Hepatic Illnesses], Center Hospitalier Universitaire).7 In patients in whom biopsy was contraindicated or impractical, LIC was measured by superconducting quantum interference device (SQUID) at centers in Turin, Italy; Hamburg, Germany; or Oakland, CA8 ; baseline and end-of-study assessments were conducted at the same site. Paired liver biopsy results were evaluated in 454 patients, and paired SQUID results were evaluated in 87 patients.
Transfusional iron intake.
For this analysis, all patients were stratified according to their transfusional iron intake during the study. The iron intake categories of less than 0.3, 0.3 to 0.5, and more than 0.5 mg/kg per day were predetermined, the rationale being to have approximately 50% of patients in the 0.3 to 0.5 mg/kg per day category and 25% in each of the other 2 categories. For each category of iron intake, we have calculated the annual transfusional requirements, expressed as packed RBCs with a hematocrit of 65% and as pure RBCs with a hematocrit of 100% (Table 2).
Categories of transfusional iron intake
| Transfusional requirements . | Proportion of thalassemia patients (%) . | Daily iron intake, mg/kg . | Yearly . | |
|---|---|---|---|---|
| Donor red cells given (Hct 65%), mL RBCs/kg . | Pure red cells given (Hct 100%), mL RBCs/kg . | |||
| Low | 19 | < 0.3 | < 155 | < 101 |
| Intermediate | 61 | 0.3-0.5 | 155-260 | 101-169 |
| High | 20 | > 0.5 | > 260 | > 169 |
| Transfusional requirements . | Proportion of thalassemia patients (%) . | Daily iron intake, mg/kg . | Yearly . | |
|---|---|---|---|---|
| Donor red cells given (Hct 65%), mL RBCs/kg . | Pure red cells given (Hct 100%), mL RBCs/kg . | |||
| Low | 19 | < 0.3 | < 155 | < 101 |
| Intermediate | 61 | 0.3-0.5 | 155-260 | 101-169 |
| High | 20 | > 0.5 | > 260 | > 169 |
Hct indicates hematocrit.
Calculation of total iron excretion.
The removal of iron by chelation therapy (iron excretion) after 1 year of treatment was derived from 2 parameters: the amount of iron added through transfusions and the change in body iron burden (as calculated from the change in LIC during the same period). Blood transfusions continued to be given to patients during treatment based on the investigators' judgment. Iron intake during the study (Kin, expressed in mg of iron) was calculated as9 : Kin = (total amount of RBCs transfused) × 1.08. The total amount of pure RBCs transfused was calculated as the total amount of blood (mL) multiplied by the hematocrit of each unit (percentage) divided by 100. Complete datasets, including both volume and hematocrit, were available for all transfusions in three quarters of the patients. If an individual hematocrit was missing, the average hematocrit from the respective center was used, and if this was not available, the value was assumed to be 65%. If the amount of blood transfused was measured only as units, the volume of donor pure RBCs was assumed to be 185 mL and thus the transfused iron per unit was calculated to be 200 mg of iron (185 × 1.08).
Total body iron (Us(t)) was calculated from the LIC (in mg Fe/g dw) at time t (t0 = 0, ie, baseline) based on the formula published by Angelucci10 : Us(t) = 10.6 × LIC (t) × (body weight in kg) (t).
Both the iron intake during the study (Kin) and the changes in total body iron over time (Kch = Us(to) - Us(tf)) are expressed in milligrams of iron, and therefore the total body iron excretion is expressed in mg iron/d. Here, tf denotes the time of end of study.
To account for the different weights of patients included in this study, the average iron intake and the average iron excretion is reported in milligrams iron/kg per day (using weight at time of transfusion and LIC measurement, respectively).
Total body iron excretion rate was used to investigate the effect of chelation therapy during the study. Total body iron excretion is calculated based on the amount of RBCs transfused during the study (iron intake in mg = Kin) and on the changes in total body iron (Kch): Total body iron excretion = (Kin-Kch]) / (tf-t0).
For example, if the transfusional iron intake was 4.0 g and the change in total iron burden was −1.2 g, the total iron excretion attributed to the chelator would be 4.0−(−1.2) or 5.2 g.
Statistical methodology
Statistical analyses were performed using SAS 8.2 (SAS Institute, Cary, NC). A Cochran-Mantel-Haenszel test for nonzero correlation was performed to assess whether iron intake correlated with change in LIC, dichotomized into increase or decrease, when adjusting for differences in the initial chelator dose. The reason for approaching LIC as a dichotomized variable is that LIC values measured by SQUID are generally lower than those measured by biopsy.5 Moreover, some differences in SQUID scaling were seen between the 3 centers using this technique. Values for LIC using biopsy or SQUID are comparable qualitatively (direction of change), but not quantitatively (degree of change). A significant result indicates that iron intake has an effect on LIC change that is not simply due to a correlation between iron intake and chelator dose. The test was performed using the SAS procedure FREQ with the Cochran-Mantel-Haenszel option. By SAS default, equidistant ranks 1, 2, and 3 were assigned to the 3 iron intake categories, less than 0.3, 0.3 to 0.5, and more than 0.5 mg/kg per day, respectively.
For those analyses in which the change in LIC was categorized into either increase or decrease (eg, Figures 1 and 2), all patients were included, irrespective of whether LIC was measured by biopsy or SQUID. In contrast, for the reasons noted, analyses requiring quantitative interpretation of LIC changes (eg, Figure 3) were restricted to patients who underwent biopsies.
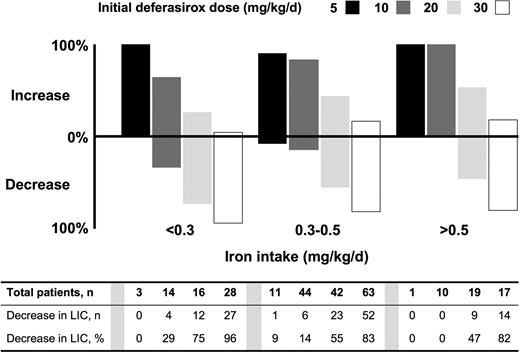
Proportion of patients with increased or decreased liver iron concentration (LIC), according to iron intake and deferasirox dose. Both dose and transfusional iron intake affected the proportion of patients achieving a reduction in LIC. A Cochran-Mantel-Haenszel test of nonzero correlation between LIC response and iron intake, adjusting for dose, demonstrated a significant correlation (P = .006).
Proportion of patients with increased or decreased liver iron concentration (LIC), according to iron intake and deferasirox dose. Both dose and transfusional iron intake affected the proportion of patients achieving a reduction in LIC. A Cochran-Mantel-Haenszel test of nonzero correlation between LIC response and iron intake, adjusting for dose, demonstrated a significant correlation (P = .006).
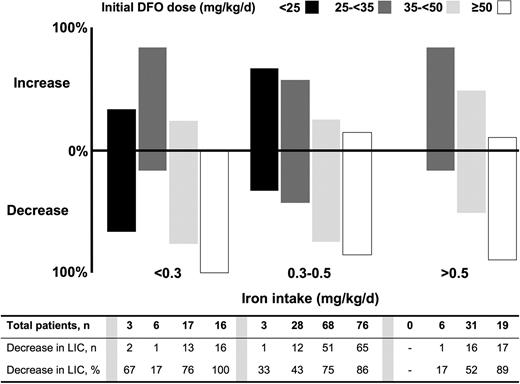
Proportion of patients with increased or decreased liver iron concentration (LIC), according to iron intake and DFO dose. As with patients who received deferasirox, both the dose of DFO therapy and transfusional iron intake affected the proportion of patients achieving a reduction in LIC. The Cochran-Mantel-Haenszel test demonstrated a significant correlation between LIC response and iron intake category (P = .032).
Proportion of patients with increased or decreased liver iron concentration (LIC), according to iron intake and DFO dose. As with patients who received deferasirox, both the dose of DFO therapy and transfusional iron intake affected the proportion of patients achieving a reduction in LIC. The Cochran-Mantel-Haenszel test demonstrated a significant correlation between LIC response and iron intake category (P = .032).
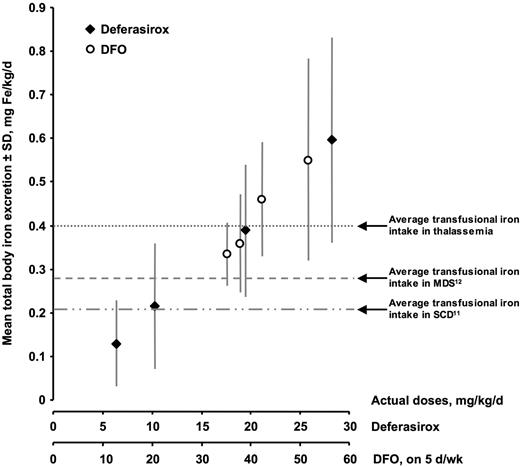
Iron excretion according to actual chelator dose. Only patients with paired liver biopsies were included in this analysis (“Results”). Both chelators produced a linear increase in iron excretion with increasing dose. Negative iron balance was achieved in most patients at a deferasirox dose of 30 mg/kg per day and a DFO dose of 40-50 mg/kg per day, 5 d/wk.
Iron excretion according to actual chelator dose. Only patients with paired liver biopsies were included in this analysis (“Results”). Both chelators produced a linear increase in iron excretion with increasing dose. Negative iron balance was achieved in most patients at a deferasirox dose of 30 mg/kg per day and a DFO dose of 40-50 mg/kg per day, 5 d/wk.
P values from a 2-sided standard t test were used to compare splenectomized and nonsplenectomized patients, with respect to mean iron intake and change in LIC and serum ferritin levels. All P values must be interpreted in an exploratory sense. These analyses were not preplanned in the study protocol, which did not specify any confirmatory analyses involving iron intake. Future clinical trials will include confirmatory analyses to verify the results observed here.
Role of the funding source
The funding source was involved in the study design, as well as the analysis and interpretation of the data, and also reviewed the paper for scientific accuracy. The corresponding author had full access to the data and had final responsibility for the decision to submit for publication.
Results
Patients and baseline characteristics
This phase III study enrolled 586 patients with thalassemia; 296 patients were randomized to receive deferasirox and 290 to receive DFO. Characteristics of the study population have been described in detail elsewhere.5 Except as noted, the analyses in the present study were limited to the 541 patients with paired results for LIC.
Chelator, transfusional iron intake, and iron balance
On average, patients received blood transfusions once every 3 weeks. The mean number of transfusion events, the mean amount of RBCs given (mL/kg), and mean transfusional iron intake were similar in both treatment groups (Table 3) and among all dose categories for each chelator (data not shown). The iron excretion exceeded iron intake in both treatment groups, suggesting that, on average, negative iron balance was achieved.
Blood transfusions and iron intake/excretion during treatment
| . | Deferasirox (n = 296) . | DFO (n = 290) . | All patients (n = 586) . |
|---|---|---|---|
| Iron intake, per year | |||
| Mean total pure RBCs, mL/kg | 129.9 ± 41.6 | 139.0 ± 42.3 | 134.4 ± 42.2 |
| Mean number of transfusion events per patient ± SD | 17.4 ± 5.9 | 18.2 ± 6.1 | 17.8 ± 6.0 |
| Total amount of iron, mg/kg | 140.3 ± 45.0 | 150.1 ± 45.7 | 145.2 ± 45.6 |
| Mean iron intake ± SD, mg/kg per day | 0.38 ± 0.11 | 0.41 ± 0.11 | 0.40 ± 0.11 |
| Mean total body iron excretion ± SD, mg/kg per day* | 0.43 ± 0.24 | 0.47 ± 0.18 | 0.45 ± 0.21 |
| . | Deferasirox (n = 296) . | DFO (n = 290) . | All patients (n = 586) . |
|---|---|---|---|
| Iron intake, per year | |||
| Mean total pure RBCs, mL/kg | 129.9 ± 41.6 | 139.0 ± 42.3 | 134.4 ± 42.2 |
| Mean number of transfusion events per patient ± SD | 17.4 ± 5.9 | 18.2 ± 6.1 | 17.8 ± 6.0 |
| Total amount of iron, mg/kg | 140.3 ± 45.0 | 150.1 ± 45.7 | 145.2 ± 45.6 |
| Mean iron intake ± SD, mg/kg per day | 0.38 ± 0.11 | 0.41 ± 0.11 | 0.40 ± 0.11 |
| Mean total body iron excretion ± SD, mg/kg per day* | 0.43 ± 0.24 | 0.47 ± 0.18 | 0.45 ± 0.21 |
SD indicates standard deviation.
Includes only those patients (n = 541) with paired measurements of LIC.
Chelator dose, transfusion rate, and liver iron concentration.
In patients receiving deferasirox, both dose and transfusional iron intake affected the proportion of patients achieving a reduction in LIC (Figure 1). At a dose of 5 or 10 mg/kg per day, LIC increased in the majority of patients at each level of transfusional iron intake. At 20 mg/kg per day, 47% and 55% of the patients in the high and intermediate iron intake categories, respectively, and 75% of patients in the low iron intake category achieved a reduction in LIC. At a dose of 30 mg/kg per day, 96% of the patients in the lowest category of iron intake achieved a reduction in LIC. A Cochran-Mantel-Haenszel test of nonzero correlation between LIC response and iron intake, adjusting for dose, demonstrated a significant correlation (P = .0055).
A similar pattern of response was observed with DFO therapy (Figure 2), with the Cochran-Mantel-Haenszel test again demonstrating a significant correlation between LIC response and iron intake category (P = .0322).
Effect of chelator dose on change in total body iron excretion.
Overall, with both chelators, iron excretion increased linearly with increasing dose (Figure 3). As shown earlier, the average transfusional iron intake in this population of patients with thalassemia major was approximately 0.4 mg/kg per day (Table 3). At this rate of iron intake, negative iron balance was achieved in approximately 50% of patients at a deferasirox dose of 20 mg/kg per day and a DFO dose of 40 mg/kg per day, 5 d/wk. Negative iron balance was achieved in most patients at a deferasirox dose of 30 mg/kg per day and a DFO dose of 40 to 50 mg/kg per day, 5 d/wk. For purposes of comparison, Figure 3 also shows the measured transfusional iron intake in patients with MDS and SCD as determined in 2 recent studies of deferasirox.11,12
Iron intake and splenectomy
In the overall study population, 33% of patients had undergone splenectomy, and the proportion of patients who had been splenectomized increased with age (9%, 13%, 30%, 41%, and 65% in age groups less than 6, 6-11, 12-15, 16-29, and 30-49 years, respectively). Overall, the mean iron intake rate was 23% lower in patients who had been splenectomized compared with those who had not (0.33 ± 0.08 vs 0.43 ± 0.11 mg/kg per day). Probably as a result of this difference in transfusional iron intake, patients who had been splenectomized had a significantly greater reduction in LIC and serum ferritin level than those who had not been splenectomized, despite the fact that the mean doses of deferasirox and DFO were similar in both groups (Table 4).
Overall change in liver iron concentration and serum ferritin levels according to splenectomy status, irrespective of type of chelation therapy received*
| . | Mean iron intake, mg/kg per day . | Mean change in LIC ± SD, mg Fe/g dw . | Mean change in serum ferritin ± SD, μg/L . | |||
|---|---|---|---|---|---|---|
| Not splenectomized | 0.43 ± 0.11 | (n = 395) | –2.2 ± 6.7 | (n = 364) | –168.1 ± 1131.8 | (n = 379) |
| Splenectomized | 0.33 ± 0.09 | (n = 191) | –3.7 ± 7.3 | (n = 177) | –519.3 ± 1343.3 | (n = 184) |
| P value† | < .0001 | .0163 | .0013 | |||
| . | Mean iron intake, mg/kg per day . | Mean change in LIC ± SD, mg Fe/g dw . | Mean change in serum ferritin ± SD, μg/L . | |||
|---|---|---|---|---|---|---|
| Not splenectomized | 0.43 ± 0.11 | (n = 395) | –2.2 ± 6.7 | (n = 364) | –168.1 ± 1131.8 | (n = 379) |
| Splenectomized | 0.33 ± 0.09 | (n = 191) | –3.7 ± 7.3 | (n = 177) | –519.3 ± 1343.3 | (n = 184) |
| P value† | < .0001 | .0163 | .0013 | |||
In the non-splenectomized and splenectomized patients, mean doses of deferasirox (20.9 and 20.2 mg/kg/d, respectively) and DFO (44.2 and 43.8 mg/kg, respectively) were similar.
Splenectomized vs non-splenectomized patients.
Discussion
To prevent the accumulation of iron in organs such as the liver and heart, chelation therapy in regularly transfused patients must remove iron at a rate that is at least equal to the intake rate from transfusions. A phase 3 study of deferasirox demonstrated that iron excretion is related to the dose of this chelator and its comparator DFO.5 The present analysis is the first to evaluate the additional impact of transfusional iron intake on response to chelation therapy.
In this analysis, data on iron burden as measured by LIC were analyzed by stratifying the patients according to the rate of transfusional iron intake while on study: less than 0.3 (low), 0.3 to 0.5 (intermediate) or more than 0.5 (high) mg/kg per day. In general, deferasirox at a dose of 30 mg/kg per day maintained a neutral or negative iron balance at each level of transfusional iron intake, while at a dose of 20 mg/kg per day the success in lowering iron burden was most pronounced in the patients at the lowest level of transfusional iron intake. At any level of transfusional iron intake, achievement of iron balance was uncommon at lower doses of deferasirox (5 and 10 mg/kg per day). Both the impact of transfusional iron intake on efficacy of chelation therapy and the frequent failure to achieve negative iron balance at lower doses of the chelator were similar for patients receiving DFO. Separate analyses for both chelators demonstrated a statistically significant association between iron intake and change in LIC, after adjusting for dose.
The rate of transfusional iron loading is relatively well defined in thalassemia major. With the recommended transfusion scheme, patients usually receive between 100 and 200 mL of pure RBCs/kg per year (ie, 154-308 mL/kg per year of donor blood with a hematocrit of 65%).1 This rate of transfusion is equivalent to 108 to 216 mg of iron/kg per year or 0.30 to 0.59 mg/kg per day. The mean transfusional iron intake in thalassemia patients in the phase III study was 0.40 mg/kg per day (intermediate range). At this level of iron intake, iron balance can be achieved in approximately 50% of patients receiving deferasirox at a dose of 20 mg/kg per day. Neutral or negative iron balance is more consistently achieved with a deferasirox dose of 30 mg/kg per day or a DFO dose of 40 to 50 mg/kg per day.
Other recent studies support the association between transfusional iron intake and iron balance.10,–12 In studies of deferasirox in various transfusion-dependent anemias and in SCD, the mean rate of transfusional iron intake was 0.28 mg/kg per day in patients with MDS and 0.21 mg/kg per day in patients with SCD.11,12 These rates of transfusional iron intake are substantially less than in thalassemia major, and in both MDS and SCD a larger percentage of patients achieved negative iron balance consistently across all dose groups. These studies, combined with the analysis in the present study (Figure 3), suggest that iron balance can be achieved at a dose of 20 mg/kg per day in patients with lower transfusional iron intake, such as those with MDS and SCD. However, the rates of transfusional iron loading in MDS and SCD may vary more widely than in thalassemia major and, therefore, transfusional iron intake should be measured when selecting the starting dose of either chelator for these conditions. For example, the annual transfusional iron intake for patients with SCD who receive regular simple transfusions to maintain hemoglobin S levels below 30% for the prevention of stroke are comparable to those of patients with thalassemia major.13 On the other hand, a similar program using erythrocytapheresis has a markedly reduced rate of transfusional iron accumulation, as do the irregular and short-term transfusion programs that are frequently used in the management of other complications of SCD.13,14
These data support a starting dose of deferasirox of 20 to 30 mg/kg per day in most patients with thalassemia major, and they highlight the importance of monitoring transfusional iron intake and response to treatment during chelation therapy. Some patients will continue to accumulate iron even at this dose, and higher doses of deferasirox or alternative chelation strategies should be considered in such circumstances. Chelation therapy should be regularly adjusted to meet a patient's specific needs, based on transfusion requirement (ie, decreased or increased for patients with low or high iron intake, respectively), severity of iron overload, and treatment goal (ie, to maintain or reduce body iron levels).15 This dosing strategy is supported by a previous analysis showing that deferasirox has high and stable efficiency across doses,16 which suggests that dose escalation or reduction may result in predictable changes in iron excretion. Future long-term trials with repeated measurements of LIC will be able to address the impact of dose adjustments on LIC. However, it is important to note that the selection of higher doses of deferasirox also should take into account possible dose-related adverse effects such as increased levels of serum creatinine.
This analysis also evaluated the impact of splenectomy on transfusional iron intake and response to chelation therapy. Patients who had previously been splenectomized did not require as many transfusions during the study as those whose spleens were intact, confirming the findings of previous studies.3,4,17 In the current study, this lower rate of iron intake is associated with a greater overall decrease in LIC and serum ferritin in both the deferasirox and DFO treatment arms. The early introduction and consistent use of a modern hypertransfusion regimen may reduce the need for splenectomy to control the rate of iron intake.2,18 However, for patients with continued iron accumulation despite hypertransfusion and regular chelation therapy, splenectomy may be useful after appropriate consideration of other strategies to reduce transfusional iron intake and with awareness of the important risks of splenectomy such as sepsis and thrombosis.
In summary, selection of a dose for iron chelation therapy has historically been based primarily on efficacy data and safety parameters. The results of this study demonstrate that it is important to consider a patient's transfusional iron intake when choosing the initial dose and making appropriate changes in dose during the course of iron chelation therapy.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs M.D. Cappellini, C. Kattamis, and A. Piga, who were members of the Study Monitoring Committee. We thank Alexander Chesi, Novartis Pharma AG, for his contribution to the development of this manuscript.
Funding for this study was provided by Novartis Pharma AG, Basel, Switzerland, who also funded Andrew Jones, PhD, to provide editorial support.
Authorship
Contribution: A.C. and J.P. participated in the design of the study, the analysis and interpretation of the data, and the writing of the manuscript; E.G. participated in statistical analysis of data from this study and related studies on deferasirox sponsored by Novartis and contributed to writing the manuscript, in particular the statistical methodology and parts of “Results.” All authors have seen and approved the final version.
The investigator list for the described study has already been published in full (Cappellini et al5 ).
Conflict-of-interest disclosure: A.C. is the principal investigator at the Children's Hospital of Philadelphia site of 2 ongoing clinical trials of deferasirox (107E and 108E) sponsored by Novartis, serves on the Study Monitoring Committee for these trials, and serves on the Data and Safety Monitoring Board for clinical trials of the iron chelator deferiprone sponsored by Apotex; E.G. is an employee of the study sponsor, Novartis Pharma, and holds shares in Novartis Pharma; J.P. is the principal investigator for deferasirox studies 107, 108, and 109 in the United Kingdom, sponsored by Novartis, has served on advisory boards for deferasirox, and has received research funding for work on deferasirox from Novartis.
Correspondence: Alan Cohen, Division of Hematology, 34th St and Civic Center Blvd, Philadelphia, PA 19104-4399; e-mail: cohen@email.chop.edu.