We analyzed prognostic factors of response, response duration, and possible impact on survival of epoetin α, epoetin β, or darbepoetin α (DAR) with or without granulocyte colony-stimulating factor in 403 myelodysplastic syndrome (MDS) patients. Sixty-two percent (40% major and 22% minor) and 50% erythroid responses were seen, and median response duration was 20 and 24 months according to IWG 2000 and 2006 criteria, respectively. Significantly higher response rates were observed with less than 10% blasts, low and int-1 International Prognostic Scoring System (IPSS), red blood cell transfusion independence, serum EPO level less than 200 IU/L, and, with IWG 2006 criteria only, shorter interval between diagnosis and treatment. Significantly longer response duration was associated with major response (IWG 2000 criteria), IPSS low to INT-1, blasts less than 5%, and absence of multilineage dysplasia. Minor responses according to IWG 2000 were reclassified as “nonresponders” or “responders” according to IWG 2006 criteria. However, among those IWG 2000 minor responders, response duration did not differ between IWG 2006 responders and nonresponders. Multivariate adjusted comparisons of survival between our cohort and the untreated MDS cohort used to design IPSS showed similar rate of progression to acute myeloid leukemia in both cohorts, but significantly better overall survival in our cohort, suggesting that epoetin or DAR treatment may have a favorable survival impact in MDS.
Introduction
Myelodysplastic syndromes (MDSs) are clonal disorders characterized by ineffective hematopoiesis leading to blood cytopenias, especially anemia, and by frequent evolution to acute myeloid leukemia (AML). Several classifications have been used to categorize MDS. The French-American-British (FAB) group proposed morphologic classification based on percentages of blasts in blood and bone marrow, and of ringed sideroblasts.1,2 The World Health Organization (WHO) classification refined FAB proposals by introducing in particular the notion of multilineage dysplasia (MD) in MDS with no excess of marrow blasts.3,4 The International Prognostic Scoring System (IPSS), which combines cytogenetic results, the number of cytopenias, and the percentage of marrow blasts, is the most widely used prognostic score that helps clinicians in therapeutic decisions.5,6
Treatment of MDS varies between risk groups. In the higher- risk group (including IPSS high and Int-2 risk groups, with high risk of progression to AML and short survival), treatment aiming at modifying the disease course (ie, allogeneic bone marrow transplantation whenever possible or otherwise high- or low-dose chemotherapy or hypomethylating agents) is generally proposed.7 In the lower-risk group (including IPSS low and int-1 risk groups, with lower risk of progression to AML and more prolonged survival), which represents the majority of the patients, treatment generally aims at improving cytopenias, and includes growth factors (epoetin alone or associated with granulocyte colony-stimulating factor [G-CSF]),8,9 thalidomide,10,11 lenalidomide, and less often hypomethylating agents (5-azacytidine, decitabine)12,13 or arsenic derivatives.14
Anemia remains the most frequent cytopenia in lower-risk MDS. Epoetin α, epoetin β, and darbepoetin (DAR) have been used for several years, alone or in combination with G-CSF, to treat anemia of MDS. (For the purpose of this article, epoetin α and β and DAR will be grouped under the denomination of rEPO.) Previous studies have shown that low pretreatment serum EPO levels, low transfusion requirement, and no or limited excess of marrow blasts were associated with better response rates to rEPO with or without G-CSF. Some other studies also found that MDS with multilineage dysplasia and sideroblastic anemia had lower response rates.9 In addition, the largest rEPO study reported to date (129 patients) with sufficient follow-up showed that low- and int-1 risk group patients had longer response to rEPO with or without G-CSF and that treatment with rEPO with or without G-CSF probably had no effect on progression to AML and survival in MDS.15
The objectives of the present study were (1) to confirm prognostic factors of response and duration of response to rEPO with or without G-CSF in 403 MDS patients from French and Belgian centers of the GFM, (2) to compare the response rates using recently modified response criteria (IWG 2006) with those obtained using IWG 2000 criteria,16 and (3) to compare outcome of our cohort (in terms of progression to AML and survival) with that of untreated patients included in the International MDS Risk Analysis Workshop (IMRAW) database that was used to establish IPSS.
Methods
This study was approved by the institutional review boards of all participating centers, a list of which is available on the Blood website; see the Supplemental Materials link at the top of the online article. Informed consent was obtained in accordance with the Declaration of Helsinki.
Patients
Four hundred thirty-three patients with MDS according to FAB criteria from 25 French and Belgian hematologic centers of the Groupe Francophone des Myélodysplasies (GFM) who had received rEPO (ie, epoetin α or β, or DAR) with or without G-CSF treatment at weekly doses of 60 000 U for epoetin and 300 μg for DAR during at least 12 weeks between 1998 and May 2006 were included in the study. Thirty patients were excluded (10 unclassified MDS, 16 chronic myelomonocytic leukemia [CMML], 4 refractory anemia with excess of blasts in transformation [RAEB-t]) because they did not meet WHO criteria for MDS or could not be adequately classified, leaving 403 patients in the final cohort. One hundred fifty-eight (39%) of them had been included in 3 GFM clinical trials that used rEPO17,–19 and the remaining patients had been treated according to GFM recommendations for the use of rEPO (described in http://www.gfmgroup.org, password 5q17p). Main inclusion criteria of the 3 clinical trials were (1) IPSS low and int-1 MDS, (2) Hb level less than 100 g/L (10 g/dL) or need for more than 2 red blood cell units of transfusions during the 2 months preceding the date of inclusion, (3) serum EPO level less than 500 iu/L, and (4) de novo MDS, excluding therapy-related cases. GFM recommendations for treating patients with rEPO were similar. None of the patients included in the study received, during the disease course, azacytidine, decitabine, or lenalidomide.
Morphologic analysis
Bone marrow aspirate smears stained with May-Grünwald-Giemsa were reexamined by a panel of morphologists (at least 2 morphologists) who assessed the degree of dysplasia in each lineage (dyserythropoiesis, dysgranulopoiesis, and dysmegakaryopoiesis) and reclassified patients initially classified with FAB criteria only (mainly before 2001) in WHO classification. Dysplasia, quantified with Flandrin's cytologic scoring,20 was considered positive for one lineage if there were more than 10% of dysplastic cells, and multilineage dysplasia was defined by at least 2 dysplastic lineages.
Treatment
rEPO treatment consisted of epoetin α (OrthoBiotech, Raritan, NJ) 60 000 IU/week or epoetin β (Roche, Indianapolis, IN) 60 000 IU/week, or darbepoetin α (Amgen, Thousand Oaks, CA) 300 μg/week. In some patients, G-CSF treatment (filgastrim [Amgen] or lenograstim [Chugai, Tokyo, Japan]) was associated with rEPO treatment, as recommended,14,21 the dosage being adjusted to maintain the white blood cell (WBC) count lower than 10 × 109/L.
Response to treatment was evaluated at week 12 according to IWG 2000 response criteria.22 Major response was defined by an increase in hemoglobin (Hb) level more than 20 g/L (2 g/dL) or no longer a need for transfusion, and minor response by an increase of Hb level by 10 to 20 g/L (1-2 g/dL) or transfusion requirement reduced by 50% or more. All responses were reclassified according to IWG 2006 criteria16 where erythroid response is defined by an increase of Hb level by more than 15 g/L (1.5 g/dL) (in patients with baseline Hb < 110 g/L [11 g/dL]) or a reduction of red blood cell (RBC) units transfused by an absolute number of at least 4 every 8 weeks.
Statistical methods
Prognostic factors of response.
Comparisons between erythroid responders and nonresponders at week 12 (based on IWG 2000 and IWG 2006 criteria) were tested using Pearson chi-square test or Fisher exact test for qualitative data, and Wilcoxon test for quantitative variables. Baseline characteristics at introduction of rEPO treatment were compared: sex, age, presence of multilineage versus unilineage dysplasia, marrow blasts percentage, serum EPO level, karyotype, transfusion requirement over the 2 last months, IPSS score, number of cytopenias, type of rEPO (DAR vs epoetin α and β alone or with G-CSF), and MDS duration prior to rEPO treatment. A multivariate logistic regression analysis was used to identify independent predictors of response at week 12 using IWG 2000 and IWG 2006 response criteria. Crude and adjusted odds ratio were provided together with their 95% confidence intervals. Multivariate analysis was adjusted for all variables with a P value less than .2 in univariate analysis and either FAB or WHO diagnosis. As karyotype, blasts, and cytopenias belong to the IPSS score definition, these variables were not included in the multivariate analysis along with IPSS even if their P values were less than .2 in univariate analysis. The final multivariate model for response to rEPO was adjusted for age at EPO introduction, IPSS score, EPO level, transfusion requirement, and diagnosis (WHO classification) for both IWG 2000 and IWG 2006 response criteria.
In responding patients at week 12, prognostic factors of duration of erythroid response were also analyzed using Kaplan-Meier estimates (log rank test) for univariate analysis and multivariate Cox regression analysis.
Outcomes.
We analyzed time to AML evolution, defined as the time in months between EPO introduction and documented leukemic transformation (ie, at least 30% of marrow blasts). Data were censored when patients died or were lost to follow up (5 patients).
Overall survival was defined as the time between introduction of EPO treatment and death. Data were censored at the end of follow-up (May 2006).
Comparison with the IPSS/IMRAW cohort.
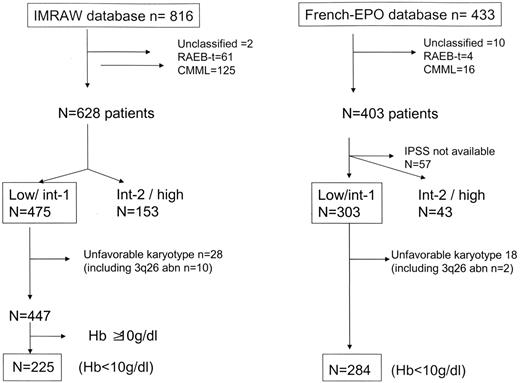
Finally, and to assess a possible influence of rEPO on disease progression and survival, we compared results obtained in our cohort with those of the IPSS/IMRAW cohort.5 This historical cohort, where MDS patients had received only supportive care and no active treatment (including rEPO), contains 816 patients. We restricted the comparison to patients with Hb level less than 100 g/L (10 g/dL), and low and int-1 IPSS, and excluded CMML, RAEB-t according to FAB classification, unclassifiable MDS, poor-risk karyotype, and 3q26 deletion, as these patients are no longer considered as having MDS in the WHO classification or are not the best candidates for rEPO treatment (high risk of AML evolution) (Figure 1). Thus, 225 patients in the IMRAW and 284 patients in the French cohort were included for outcome comparison. Overall survival (OS) and time to AML evolution from diagnosis for the IMRAW patients and since rEPO introduction for the French-EPO were analyzed using univariate and multivariate Cox proportional hazards regression. Reasons why we chose the date of diagnosis in the IMRAW cohort, and the date of introduction of rEPO in the French-EPO cohort, were that in the IMRAW cohort, patients were followed from diagnosis, and all measures used to calculate the IPSS score were collected at time of diagnosis. In the French-EPO cohort, however, baseline patient characteristics including IPSS scoring were measured at the introduction of rEPO treatment. The proportional hazards assumption was checked graphically. Multivariate analysis was adjusted on main known prognostic factors: sex, age, FAB diagnosis (as WHO diagnosis was not available for IMRAW patients), hemoglobin level, percentage of marrow blasts, karyotype, and IPSS score. Baseline hemoglobin level was chosen instead of baseline RBC transfusion requirement, a recently identified prognostic factor in MDS,23 because (1) it also constitutes an important prognostic factor in most rEPO reported series,5,24,25 and (2) RBC transfusion requirement was not known in the IMRAW cohort.
Flow chart for the comparison between the French-EPO and the IMRAW database.
Because the baseline characteristics of patients from the 2 cohorts were slightly different and because residual confounding factors can never be totally excluded even with a multivariate adjustment, we also performed a matched-pair analysis where patients from the French-EPO cohort and IPSS/IMRAW cohort were matched for age at diagnosis (< 70 years or ≥ 70 years), FAB diagnosis (refractory anemia [RA] or refractory anemia with ringed sideroblasts [RARS], RAEB with ≤ 10% of blasts), and karyotype (good, intermediate) in a ratio of 1:1. This matched-pair analysis led to a study sample of 200 patients in the French-EPO and 200 patients in the IPSS/IMRAW cohort.
All statistical analyses were 2-sided and P values less than .05 were considered to have statistical significance. Calculations were performed using the SAS package version 9.01 (SAS Institute, Cary, NC).
Results
Patient characteristics and treatment
Our study population included 403 patients with a median age of 74 years (interquartile range [IQR] 25%-75%: 66-79 years). Patients could be classified, according to FAB classification in 143 refractory anemia (RA), 142 refractory anemia with ringed sideroblasts (RARS), and 118 refractory anemia with excess of blasts (RAEB) (Table 1) and according to WHO classification in 60 RA, 68 refractory cytopenia with multilineage dysplasia (RCMD), 83 RARS, 57 refractory cytopenia with multilineage dysplasia and ringed sideroblasts (RCMD-RS), 91 RAEB-1, 26 RAEB-2, and 18 5q− syndrome. Karyotype was favorable in 261 patients, intermediate in 66 patients, unfavorable in 19 patients, and a cytogenetic failure or not done in 57 patients. IPSS score was low in 139 patients (35%), int-1 in 164 (40%), Int-2 in 37 (8%), high in 6 (2%), and not determined in 57 patients (14%). Median pretreatment EPO level was 76 IU/L (range: 6-5665 IU/L) and only 7% patients had EPO level higher than 500 IU/L. All patients had Hb level less than 100 g/L (10 g/dL) and 55% required RBC transfusions, including 36% who received more than 2 RBC units/month. Treatment consisted of epoetin alone (α or β) in 164 patients, DAR alone in 107 patients, epoetin with G-CSF in 104 patients, and DAR with G-CSF in 28 patients.
Characteristics of patients and prognostic factors of response to rEPO at week 12 according to IWG 2000 criteria (N=403)
| . | Patients no. (%) . | Overall response, % . | Major response, % . | Minor response, % . | Overall response versus no response* . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis† . | |||||||||
| OR . | 95% CI . | P . | OR . | 95% CI . | P . | |||||
| Age at diagnosis, y | ||||||||||
| Younger than 70 | 150 (37) | 54 | 36 | 18 | 1 | — | — | 1 | — | — |
| 70 or older | 253 (63) | 67 | 44 | 23 | 1.6 | 1.0-2.4 | .03 | 1.4 | 0.9-2.3 | .13 |
| Sex | ||||||||||
| Male | 224 (56) | 61 | 38 | 23 | 1 | — | — | — | — | — |
| Female | 179 (44) | 64 | 46 | 18 | 1.2 | 0.8-1.7 | .47 | — | — | — |
| FAB | ||||||||||
| RA | 143 (36) | 69 | 53 | 16 | 1 | — | — | — | — | — |
| RARS | 142 (35) | 62 | 39 | 23 | 0.8 | 0.5-1.3 | — | — | — | — |
| RAEB less than 10 | 92 (23) | 56 | 33 | 23 | 0.6 | 0.4-1.0 | — | — | — | — |
| RAEB 10 or more | 26 (6) | 34 | 15 | 19 | 0.2 | 0.1-0.6 | .009 | — | — | — |
| WHO | ||||||||||
| RA | 60 (15) | 68 | 54 | 14 | 1 | — | — | 1 | — | — |
| RCMD | 68 (17) | 72 | 54 | 18 | 1.2 | 0.6-2.6 | — | 1.2 | 0.6-2.7 | — |
| RARS | 83 (21) | 59 | 40 | 19 | 0.7 | 0.3-1.3 | — | 0.7 | 0.3-1.4 | — |
| RCMD-RS | 57 (14) | 70 | 37 | 33 | 1.2 | 0.5-2.6 | — | 1.5 | 0.7-3.6 | — |
| RAEB-1 | 91 (23) | 56 | 33 | 23 | 0.6 | 0.3-1.3 | — | 0.9 | 0.4-1.8 | — |
| RAEB-2 | 26 (6) | 34 | 15 | 19 | 0.2 | 0.1-0.6 | — | 0.8 | 0.2-2.7 | — |
| 5q− syndrome | 18 (4) | 50 | 50 | 0 | 0.5 | 0.2-1.4 | .02 | 0.6 | 0.2-1.9 | .38 |
| Karyotype | ||||||||||
| Poor | 19 (5) | 43 | 21 | 22 | 1 | — | — | — | — | — |
| Good | 261 (65) | 63 | 43 | 20 | 2.9 | 1.3-6.2 | — | — | — | — |
| Intermediate | 66 (16) | 63 | 38 | 25 | 2.1 | 0.8-5.5 | — | — | — | — |
| NA | 57 (14) | 61 | 40 | 21 | 2.3 | 1.0-5.6 | .05 | — | — | — |
| Multilineage dysplasia | ||||||||||
| No | 180 (45) | 61 | 36 | 25 | 1 | — | — | — | — | — |
| Yes | 223 (55) | 63 | 46 | 17 | 1.1 | 0.8-1.7 | .52 | — | — | — |
| BM blasts | ||||||||||
| 5 or more | 105 (26) | 53 | — | — | 1 | — | — | — | — | — |
| Less than 5 | 298 (74) | 66 | 48 | 18 | 1.7 | 1.1-2.7 | .02 | — | — | — |
| EPO level, IU/L | ||||||||||
| More than 200 | 92 (23) | 42 | 37 | 25 | 1 | — | — | 1 | — | — |
| 200 or less | 248 (62) | 69 | 52 | 17 | 2.9 | 1.8-4.7 | — | 2.0 | 1.2-3.5 | — |
| NA | 63 (15) | 63 | 33 | 30 | 2.3 | 1.2-4.4 | <.001 | 2.1 | 1.0-4.2 | .03 |
| IPSS | ||||||||||
| Int-2 and high | 43 (11) | 36 | 17 | 19 | 1 | — | — | 1 | — | — |
| Low and int-1 | 303 (75) | 65 | 47 | 18 | 3.6 | 1.8-7.0 | — | 2.5 | 1.0-6.4 | .05 |
| NA | 57 (14) | 3.5 | 1.5-7.9 | .001 | 2.2 | 0.8-6.1 | .14 | |||
| RBC transfusion before rEPO treatment | ||||||||||
| No | 182 (45) | 76 | 60 | 16 | 1 | — | — | 1 | — | — |
| Yes | 221 (55) | 51 | 27 | 24 | 0.3 | 0.2-0.5 | <.001 | 0.4 | 0.2-0.6 | <.001 |
| Addition of G-CSF | ||||||||||
| No | 271 (67) | 66 | 46 | 20 | 1 | — | — | 1 | — | — |
| Yes | 132 (33) | 58 | 34 | 24 | 0.7 | 0.5-1.1 | .17 | 0.8 | 0.5-1.3 | .46 |
| Interval from diagnosis, mo | ||||||||||
| 6 or more | 198 (49) | 59 | 35 | 24 | 1 | — | — | 1 | — | — |
| Fewer than 6 | 205 (50) | 66 | 47 | 19 | 1.4 | 0.9-2.04 | .13 | 1.4 | 0.9-2.0 | .13 |
| . | Patients no. (%) . | Overall response, % . | Major response, % . | Minor response, % . | Overall response versus no response* . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis† . | |||||||||
| OR . | 95% CI . | P . | OR . | 95% CI . | P . | |||||
| Age at diagnosis, y | ||||||||||
| Younger than 70 | 150 (37) | 54 | 36 | 18 | 1 | — | — | 1 | — | — |
| 70 or older | 253 (63) | 67 | 44 | 23 | 1.6 | 1.0-2.4 | .03 | 1.4 | 0.9-2.3 | .13 |
| Sex | ||||||||||
| Male | 224 (56) | 61 | 38 | 23 | 1 | — | — | — | — | — |
| Female | 179 (44) | 64 | 46 | 18 | 1.2 | 0.8-1.7 | .47 | — | — | — |
| FAB | ||||||||||
| RA | 143 (36) | 69 | 53 | 16 | 1 | — | — | — | — | — |
| RARS | 142 (35) | 62 | 39 | 23 | 0.8 | 0.5-1.3 | — | — | — | — |
| RAEB less than 10 | 92 (23) | 56 | 33 | 23 | 0.6 | 0.4-1.0 | — | — | — | — |
| RAEB 10 or more | 26 (6) | 34 | 15 | 19 | 0.2 | 0.1-0.6 | .009 | — | — | — |
| WHO | ||||||||||
| RA | 60 (15) | 68 | 54 | 14 | 1 | — | — | 1 | — | — |
| RCMD | 68 (17) | 72 | 54 | 18 | 1.2 | 0.6-2.6 | — | 1.2 | 0.6-2.7 | — |
| RARS | 83 (21) | 59 | 40 | 19 | 0.7 | 0.3-1.3 | — | 0.7 | 0.3-1.4 | — |
| RCMD-RS | 57 (14) | 70 | 37 | 33 | 1.2 | 0.5-2.6 | — | 1.5 | 0.7-3.6 | — |
| RAEB-1 | 91 (23) | 56 | 33 | 23 | 0.6 | 0.3-1.3 | — | 0.9 | 0.4-1.8 | — |
| RAEB-2 | 26 (6) | 34 | 15 | 19 | 0.2 | 0.1-0.6 | — | 0.8 | 0.2-2.7 | — |
| 5q− syndrome | 18 (4) | 50 | 50 | 0 | 0.5 | 0.2-1.4 | .02 | 0.6 | 0.2-1.9 | .38 |
| Karyotype | ||||||||||
| Poor | 19 (5) | 43 | 21 | 22 | 1 | — | — | — | — | — |
| Good | 261 (65) | 63 | 43 | 20 | 2.9 | 1.3-6.2 | — | — | — | — |
| Intermediate | 66 (16) | 63 | 38 | 25 | 2.1 | 0.8-5.5 | — | — | — | — |
| NA | 57 (14) | 61 | 40 | 21 | 2.3 | 1.0-5.6 | .05 | — | — | — |
| Multilineage dysplasia | ||||||||||
| No | 180 (45) | 61 | 36 | 25 | 1 | — | — | — | — | — |
| Yes | 223 (55) | 63 | 46 | 17 | 1.1 | 0.8-1.7 | .52 | — | — | — |
| BM blasts | ||||||||||
| 5 or more | 105 (26) | 53 | — | — | 1 | — | — | — | — | — |
| Less than 5 | 298 (74) | 66 | 48 | 18 | 1.7 | 1.1-2.7 | .02 | — | — | — |
| EPO level, IU/L | ||||||||||
| More than 200 | 92 (23) | 42 | 37 | 25 | 1 | — | — | 1 | — | — |
| 200 or less | 248 (62) | 69 | 52 | 17 | 2.9 | 1.8-4.7 | — | 2.0 | 1.2-3.5 | — |
| NA | 63 (15) | 63 | 33 | 30 | 2.3 | 1.2-4.4 | <.001 | 2.1 | 1.0-4.2 | .03 |
| IPSS | ||||||||||
| Int-2 and high | 43 (11) | 36 | 17 | 19 | 1 | — | — | 1 | — | — |
| Low and int-1 | 303 (75) | 65 | 47 | 18 | 3.6 | 1.8-7.0 | — | 2.5 | 1.0-6.4 | .05 |
| NA | 57 (14) | 3.5 | 1.5-7.9 | .001 | 2.2 | 0.8-6.1 | .14 | |||
| RBC transfusion before rEPO treatment | ||||||||||
| No | 182 (45) | 76 | 60 | 16 | 1 | — | — | 1 | — | — |
| Yes | 221 (55) | 51 | 27 | 24 | 0.3 | 0.2-0.5 | <.001 | 0.4 | 0.2-0.6 | <.001 |
| Addition of G-CSF | ||||||||||
| No | 271 (67) | 66 | 46 | 20 | 1 | — | — | 1 | — | — |
| Yes | 132 (33) | 58 | 34 | 24 | 0.7 | 0.5-1.1 | .17 | 0.8 | 0.5-1.3 | .46 |
| Interval from diagnosis, mo | ||||||||||
| 6 or more | 198 (49) | 59 | 35 | 24 | 1 | — | — | 1 | — | — |
| Fewer than 6 | 205 (50) | 66 | 47 | 19 | 1.4 | 0.9-2.04 | .13 | 1.4 | 0.9-2.0 | .13 |
OR indicates odds ratio; —, not applicable; and NA, not available.
Results from logistic regression.
Multivariate analysis was adjusted for IPSS, age, EPO level, transfusions, administration of G-CSF, and WHO diagnosis.
Median interval between diagnosis and treatment by rEPO with or without G-CSF was 6 months (IQR: 1-26 months).
Overall response to treatment
After 12 weeks of rEPO treatment, 251 (62%) patients met IWG 2000 criteria for erythroid response (40% major response, 22% minor response). Using IWG 2006 criteria, response was achieved in 50% of the patients. Of the 88 minor responders according to IWG 2000 criteria, 56 patients (63%) were reclassified as nonresponders and 32 (37%) as responders, according to IWG 2006 criteria.
Prognostic factors of response and response duration to rEPO according to IWG 2000 and IWG 2006 criteria
IWG 2000 response criteria Table 1.
In univariate analysis, significantly higher response rates were observed in patients older than 70 years (P = .03), marrow blasts less than 5% (P = .02), EPO level of 200 IU/L or higher (P < .001), low and int-1 IPSS score (P = .001), absence of transfusion requirement (P < .001), and favorable or intermediate karyotype (P = .05). Regarding WHO classification, only RAEB-2 had significantly lower response rates. We found no impact of unilineage versus multilineage dysplasia (63% of response in multilineage dysplasia versus 61% of nonresponders, P = .52) on response rate (Table 1). Of note was the relatively high response rate of 56% in RAEB-1. Patients with the 5q− syndrome had a response rate of 50%. Interval between diagnosis and rEPO introduction did not influence response rate (P = .13). The response rate was similar in patients treated with rEPO alone (66%), and those treated with rEPO with G-CSF (58%, P = .17). This was particularly the case for RARS and RCMD-RS (62% response with rEPO alone and 64% with rEPO + G-CSF, P = .8). In multivariate analysis, EPO level of 200 IU/L or less, absence of transfusion requirement, and low and int-1 IPSS score remained the only factors associated with better response to rEPO.
Median duration of response from the onset of rEPO was 20 months (range: 3-74 months). Fifty-four (21.4%) of the 251 responders relapsed. Relapse was associated with evidence of progression to higher-grade MDS in 13% and to AML in 15%, whereas no evident disease progression was seen in the remaining 72% relapses. In univariate analysis, shorter response duration was significantly associated with the quality of response (median: 14 months for minor response vs 24 months for major response, P = .003), with blasts more than 10% (median: 12 months vs 22 months for blasts < 10%, P = .007), with presence of multilineage dysplasia (median: 16 months vs 24 months without multilineage dysplasia, P = .02) and with IPSS Int-2/high (median: 8 months vs 21 months for low/int-1, P = .004) (Table 2). No difference in response duration was found according to age, sex, transfusion requirement, or EPO level (Table 2). In multivariate analysis, factors predictive of shorter response were minor response, presence of del 5q, and RAEB-2.
Duration of response to rEPO according to IWG 2000 response criteria
| . | Patients, no. . | Median response duration, mo . | Univariate analysis . | Multivariate analysis* . | ||||
|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |||
| Type of response | ||||||||
| Major | 168 | 24.4 | 1 | — | — | 1.00 | — | — |
| Minor | 83 | 13.8 | 1.8 | 1.3-2.6 | .003 | 1.63 | 1.1-2.5 | .02 |
| Age, y | ||||||||
| Younger than 70 | 83 | 16.8 | 1 | — | — | — | — | — |
| 70 or older | 168 | 20 | 0.9 | 0.7-1.4 | .73 | — | — | — |
| Sex | ||||||||
| Female | 115 | 20 | 1 | — | — | — | — | — |
| Male | 136 | 20 | 0.9 | 0.7-1.3 | .76 | — | — | — |
| FAB | ||||||||
| RA | 99 | 24.4 | 1 | — | — | — | — | — |
| RAEB less than 10 | 53 | 16 | 1.6 | 1.0-2.6 | — | — | — | — |
| RAEB 10 or more | 9 | 8 | 4.6 | 1.9-10.9 | — | — | — | — |
| RARS | 90 | 18.1 | 1.2 | 0.8-1.8 | <.001 | — | — | — |
| WHO | ||||||||
| RA | 41 | 30 | 1 | — | — | 1.00 | — | — |
| RCMD | 49 | 22.3 | 1.9 | 1.0-3.6 | — | 1.63 | 0.8-3.2 | .15 |
| RARS | 49 | 17.6 | 1.7 | 0.9-3.2 | — | 1.78 | 0.8-4.2 | .19 |
| RCMD-RS | 41 | 24.3 | 2 | 1.0-3.8 | — | 1.71 | 0.7-4.0 | .22 |
| RAEB-1 | 53 | 16 | 2.3 | 1.2-4.5 | — | 2.00 | 0.9-4.4 | .08 |
| RAEB-2 | 9 | 8 | 7.4 | 2.8-19.7 | — | 4.01 | 1.1-14.5 | .04 |
| 5q− | 9 | 11.2 | 3.7 | 1.5-9.0 | <.001 | 4.04 | 1.6-10.4 | .004 |
| Karyotype | ||||||||
| Good | 165 | 22.3 | 1 | — | — | — | — | — |
| Intermediate | 39 | 16.7 | 1.0 | 0.6–1.7 | — | — | — | — |
| Poor | 7 | na | 1.3 | 0.4–4.0 | — | — | — | — |
| Failure or not done | 40 | 21 | 0.9 | 0.5–1.5 | .94 | — | — | — |
| Multilineage dysplasia | ||||||||
| No | 109 | 24 | 1 | — | — | 1.00 | — | — |
| Yes | 142 | 16 | 1.5 | 1.0–2.2 | .02 | 1.13 | 0.6-2.2 | .72 |
| BM blasts | ||||||||
| More than 10% | 9 | 12 | 1 | — | — | — | — | — |
| 10% or less | 242 | 22.3 | 0.3 | 0.1–0.8 | .007 | — | — | — |
| IPSS | ||||||||
| Int-2 and high | 15 | 8 | 1 | — | — | 1.00 | — | — |
| Low and int-1 | 199 | 21 | 0.4 | 0.2-0.7 | — | 0.53 | 0.2-1.2 | .31 |
| Not available | 37 | 28.4 | 0.3 | 0.1-0.7 | .004 | 0.49 | 0.2-1.3 | .14 |
| EPO level, IU/L | ||||||||
| 200 or more | 40 | 25 | 1 | — | — | — | — | — |
| Less than 200 | 171 | 20 | 1.0 | 0.6-1.7 | .97 | — | — | — |
| Not available | 40 | 11 | 1.9 | 1.0-3.5 | .05 | — | — | — |
| Transfusion requirement | ||||||||
| No | 138 | 24 | 1 | — | — | 1.00 | — | — |
| Yes | 113 | 18 | 1.4 | 0.9-1.9 | .07 | 1.11 | 0.7-1.7 | .61 |
| . | Patients, no. . | Median response duration, mo . | Univariate analysis . | Multivariate analysis* . | ||||
|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |||
| Type of response | ||||||||
| Major | 168 | 24.4 | 1 | — | — | 1.00 | — | — |
| Minor | 83 | 13.8 | 1.8 | 1.3-2.6 | .003 | 1.63 | 1.1-2.5 | .02 |
| Age, y | ||||||||
| Younger than 70 | 83 | 16.8 | 1 | — | — | — | — | — |
| 70 or older | 168 | 20 | 0.9 | 0.7-1.4 | .73 | — | — | — |
| Sex | ||||||||
| Female | 115 | 20 | 1 | — | — | — | — | — |
| Male | 136 | 20 | 0.9 | 0.7-1.3 | .76 | — | — | — |
| FAB | ||||||||
| RA | 99 | 24.4 | 1 | — | — | — | — | — |
| RAEB less than 10 | 53 | 16 | 1.6 | 1.0-2.6 | — | — | — | — |
| RAEB 10 or more | 9 | 8 | 4.6 | 1.9-10.9 | — | — | — | — |
| RARS | 90 | 18.1 | 1.2 | 0.8-1.8 | <.001 | — | — | — |
| WHO | ||||||||
| RA | 41 | 30 | 1 | — | — | 1.00 | — | — |
| RCMD | 49 | 22.3 | 1.9 | 1.0-3.6 | — | 1.63 | 0.8-3.2 | .15 |
| RARS | 49 | 17.6 | 1.7 | 0.9-3.2 | — | 1.78 | 0.8-4.2 | .19 |
| RCMD-RS | 41 | 24.3 | 2 | 1.0-3.8 | — | 1.71 | 0.7-4.0 | .22 |
| RAEB-1 | 53 | 16 | 2.3 | 1.2-4.5 | — | 2.00 | 0.9-4.4 | .08 |
| RAEB-2 | 9 | 8 | 7.4 | 2.8-19.7 | — | 4.01 | 1.1-14.5 | .04 |
| 5q− | 9 | 11.2 | 3.7 | 1.5-9.0 | <.001 | 4.04 | 1.6-10.4 | .004 |
| Karyotype | ||||||||
| Good | 165 | 22.3 | 1 | — | — | — | — | — |
| Intermediate | 39 | 16.7 | 1.0 | 0.6–1.7 | — | — | — | — |
| Poor | 7 | na | 1.3 | 0.4–4.0 | — | — | — | — |
| Failure or not done | 40 | 21 | 0.9 | 0.5–1.5 | .94 | — | — | — |
| Multilineage dysplasia | ||||||||
| No | 109 | 24 | 1 | — | — | 1.00 | — | — |
| Yes | 142 | 16 | 1.5 | 1.0–2.2 | .02 | 1.13 | 0.6-2.2 | .72 |
| BM blasts | ||||||||
| More than 10% | 9 | 12 | 1 | — | — | — | — | — |
| 10% or less | 242 | 22.3 | 0.3 | 0.1–0.8 | .007 | — | — | — |
| IPSS | ||||||||
| Int-2 and high | 15 | 8 | 1 | — | — | 1.00 | — | — |
| Low and int-1 | 199 | 21 | 0.4 | 0.2-0.7 | — | 0.53 | 0.2-1.2 | .31 |
| Not available | 37 | 28.4 | 0.3 | 0.1-0.7 | .004 | 0.49 | 0.2-1.3 | .14 |
| EPO level, IU/L | ||||||||
| 200 or more | 40 | 25 | 1 | — | — | — | — | — |
| Less than 200 | 171 | 20 | 1.0 | 0.6-1.7 | .97 | — | — | — |
| Not available | 40 | 11 | 1.9 | 1.0-3.5 | .05 | — | — | — |
| Transfusion requirement | ||||||||
| No | 138 | 24 | 1 | — | — | 1.00 | — | — |
| Yes | 113 | 18 | 1.4 | 0.9-1.9 | .07 | 1.11 | 0.7-1.7 | .61 |
HR indicates hazard ratio; and —, not applicable.
Results from Cox multivariate analysis.
IWG 2006 response criteria.
Predictive factors of response and response duration using IWG 2006 criteria were generally similar to those found with IWG 2000 criteria, with slight differences in P value (Table 3). One exception, however, was that patients who received rEPO within 6 months of diagnosis had a 56% response rate compared with 44% in patients treated after 6 months (P = .01 using univariate analysis; P = .04 using multivariate analysis).
Characteristics of patients and prognostic factors of response to rEPO at week 12 according to IWG 2006 criteria (n= 403)
| . | Overall response, % . | Overall response versus nonresponse* . | |||||
|---|---|---|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis† . | ||||||
| OR . | 95% CI . | P . | OR . | 95% CI . | P . | ||
| Age, y | |||||||
| Younger than 70 | 44 | 1 | — | — | 1 | — | — |
| 70 or older | 54 | 1.5 | 1.0-2.2 | .06 | 1.3 | 0.8-2.2 | .22 |
| Sex | |||||||
| Male | 46 | 1 | — | — | 1 | — | — |
| Female | 55 | 1.4 | 1.0-2.1 | .08 | 1.5 | 0.9-2.3 | .08 |
| FAB | |||||||
| RA | 61 | 1 | — | — | — | — | — |
| RARS | 48 | 0.6 | 0.4--0.9 | — | — | — | — |
| RAEB less than 10 | 43 | 0.5 | 0.3-0.8 | — | — | — | — |
| RAEB 10 or more | 23 | 0.2 | 0.1-0.5 | .001 | — | — | — |
| WHO | |||||||
| RA | 63 | 1 | — | — | 1 | — | — |
| RCMD | 61 | 0.9 | 0.5-1.9 | — | 0.9 | 0.4-2.0 | — |
| RARS | 46 | 0.5 | 0.3-1.0 | — | 0.5 | 0.2-1.0 | — |
| RCMD-RS | 50 | 0.6 | 0.3-1.3 | — | 0.7 | 0.3-1.6 | — |
| RAEB-1 | 43 | 0.5 | 0.2-0.9 | — | 0.7 | 0.3-1.4 | — |
| RAEB-2 | 23 | 0.1 | 0.0-0.4 | — | 0.7 | 0.2-3.1 | — |
| 5q− syndrome | 50 | 0.6 | 0.2-1.7 | .008 | 0.7 | 0.2-2.2 | .49 |
| Karyotype | |||||||
| Poor | 18 | 1 | — | — | — | — | — |
| Good | 53 | 4 | 1.7-9.7 | — | — | — | — |
| Intermediate | 49 | 2.7 | 0.9-7.7 | — | — | — | — |
| NA | 46 | 2.9 | 1.1-7.7 | .01 | — | — | — |
| Multilineage dysplasia | |||||||
| No | 52 | 1 | — | — | — | — | — |
| Yes | 48 | 0.9 | 0.6-1.3 | .50 | — | — | — |
| BM blasts | |||||||
| 5% or more | 38 | 1 | — | — | — | — | — |
| Less than 5% | 54 | 1.9 | 1.2-3.1 | .004 | — | — | — |
| EPO level, IU/L | |||||||
| More than 200 | 29 | 1 | — | — | 1 | — | — |
| 200 or less | 60 | 3.7 | 2.2-6.2 | — | 2.7 | 1.5-4.8 | — |
| NA | 41 | 1.7 | 0.9-3.3 | <.001 | 1.6 | 0.8-3.4 | .002 |
| IPSS subgroup | |||||||
| Int-2 and high | 16 | 1 | — | — | 1 | — | — |
| Low and int-1 | 55 | 6.4 | 2.8-14.8 | — | 4.6 | 1.5-14.0 | — |
| NA | 47 | 5.0 | 1.9-13.0 | <.001 | 3.4 | 1.0-11.2 | .02 |
| RBC transfusion before rEPO treatment | |||||||
| No | 66 | 1 | — | — | 1 | — | — |
| Yes | 37 | 0.3 | 0.2-0.4 | <.001 | 0.4 | 0.2-0.6 | <.001 |
| Addition of G-CSF | |||||||
| No | 55 | 1 | — | — | 1 | — | — |
| Yes | 41 | 0.6 | 0.4-1.0 | .08 | 0.7 | 0.4-1.1 | .12 |
| Interval from diagnosis, mo | |||||||
| 6 or more | 44 | 1 | — | — | 1 | — | — |
| Fewer than 6 | 56 | 1.7 | 1.1-2.5 | .01 | 1.6 | 1.0-2.5 | .04 |
| . | Overall response, % . | Overall response versus nonresponse* . | |||||
|---|---|---|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis† . | ||||||
| OR . | 95% CI . | P . | OR . | 95% CI . | P . | ||
| Age, y | |||||||
| Younger than 70 | 44 | 1 | — | — | 1 | — | — |
| 70 or older | 54 | 1.5 | 1.0-2.2 | .06 | 1.3 | 0.8-2.2 | .22 |
| Sex | |||||||
| Male | 46 | 1 | — | — | 1 | — | — |
| Female | 55 | 1.4 | 1.0-2.1 | .08 | 1.5 | 0.9-2.3 | .08 |
| FAB | |||||||
| RA | 61 | 1 | — | — | — | — | — |
| RARS | 48 | 0.6 | 0.4--0.9 | — | — | — | — |
| RAEB less than 10 | 43 | 0.5 | 0.3-0.8 | — | — | — | — |
| RAEB 10 or more | 23 | 0.2 | 0.1-0.5 | .001 | — | — | — |
| WHO | |||||||
| RA | 63 | 1 | — | — | 1 | — | — |
| RCMD | 61 | 0.9 | 0.5-1.9 | — | 0.9 | 0.4-2.0 | — |
| RARS | 46 | 0.5 | 0.3-1.0 | — | 0.5 | 0.2-1.0 | — |
| RCMD-RS | 50 | 0.6 | 0.3-1.3 | — | 0.7 | 0.3-1.6 | — |
| RAEB-1 | 43 | 0.5 | 0.2-0.9 | — | 0.7 | 0.3-1.4 | — |
| RAEB-2 | 23 | 0.1 | 0.0-0.4 | — | 0.7 | 0.2-3.1 | — |
| 5q− syndrome | 50 | 0.6 | 0.2-1.7 | .008 | 0.7 | 0.2-2.2 | .49 |
| Karyotype | |||||||
| Poor | 18 | 1 | — | — | — | — | — |
| Good | 53 | 4 | 1.7-9.7 | — | — | — | — |
| Intermediate | 49 | 2.7 | 0.9-7.7 | — | — | — | — |
| NA | 46 | 2.9 | 1.1-7.7 | .01 | — | — | — |
| Multilineage dysplasia | |||||||
| No | 52 | 1 | — | — | — | — | — |
| Yes | 48 | 0.9 | 0.6-1.3 | .50 | — | — | — |
| BM blasts | |||||||
| 5% or more | 38 | 1 | — | — | — | — | — |
| Less than 5% | 54 | 1.9 | 1.2-3.1 | .004 | — | — | — |
| EPO level, IU/L | |||||||
| More than 200 | 29 | 1 | — | — | 1 | — | — |
| 200 or less | 60 | 3.7 | 2.2-6.2 | — | 2.7 | 1.5-4.8 | — |
| NA | 41 | 1.7 | 0.9-3.3 | <.001 | 1.6 | 0.8-3.4 | .002 |
| IPSS subgroup | |||||||
| Int-2 and high | 16 | 1 | — | — | 1 | — | — |
| Low and int-1 | 55 | 6.4 | 2.8-14.8 | — | 4.6 | 1.5-14.0 | — |
| NA | 47 | 5.0 | 1.9-13.0 | <.001 | 3.4 | 1.0-11.2 | .02 |
| RBC transfusion before rEPO treatment | |||||||
| No | 66 | 1 | — | — | 1 | — | — |
| Yes | 37 | 0.3 | 0.2-0.4 | <.001 | 0.4 | 0.2-0.6 | <.001 |
| Addition of G-CSF | |||||||
| No | 55 | 1 | — | — | 1 | — | — |
| Yes | 41 | 0.6 | 0.4-1.0 | .08 | 0.7 | 0.4-1.1 | .12 |
| Interval from diagnosis, mo | |||||||
| 6 or more | 44 | 1 | — | — | 1 | — | — |
| Fewer than 6 | 56 | 1.7 | 1.1-2.5 | .01 | 1.6 | 1.0-2.5 | .04 |
OR indicates odds ratio; —, not applicable; and NA, not available.
Results from logistic regression.
Multivariate analysis was adjusted for IPSS, age, EPO level, transfusions, and WHO diagnosis.
Clinical outcome of minor responders according to IWG 2000 criteria
Eighty-eight patients had minor response according to IWG 2000. Fifty-six and 32 of them were reclassified as “nonresponders” and “responders,” respectively, according to IWG 2006 criteria. Duration of response was 11 months in the 56 nonresponders and 12.6 months in the 32 responders according to IWG 2006 criteria (P = .65). The transfusion requirement, IPSS score, and baseline EPO level did not significantly differ between these 2 subgroups. In addition, duration of response of the 32 responders in IWG 2006 was significantly shorter than that of major responders according to IWG 2000 (median: 12.5 vs 24 months, P = .001).
Outcome comparison between the French-EPO cohort and the IMRAW cohort
Five-year OS in the French cohort was 64%. Duration of MDS (≥ or < 6 months) prior to starting rEPO therapy and addition of G-CSF to rEPO did not influence OS (Figures S1,S2).
To determine whether treatment with rEPO, with or without G-CSF, had an impact on disease progression and survival, we compared outcome of our cohort to that of the untreated cohort of MDS used to design the IPSS (IMRAW cohort). Because of the heterogeneity of MDS, the comparison was restricted to low and int-1 IPSS patients with less than 100 g/L (10 g/dL) hemoglobin, and we also excluded poor-risk karyotype and 3q26 deletion patients (Figure 1). Two hundred eighty-four patients and 225 patients in the French-EPO and in the IMRAW database, respectively, met those criteria and were compared. Median follow-up from rEPO introduction in the French-EPO cohort and from MDS diagnosis in the IMRAW cohort was 26 months (IQR: 13-53 months) and 33 months (IQR: 15-61 months), respectively. Both cohorts were significantly different for age, IPSS, percentage of blasts FAB diagnosis, and, with borderline significance (P = .05), hemoglobin level and karyotype (Table 4). Therefore, adjustment on those factors was made for subsequent comparisons using multivariate Cox proportional hazards regression. In the French-EPO cohort, 12 patients progressed to AML and 35 patients died. In the IMRAW cohort, 24 patients progressed to AML and 123 died. The 5-year incidence of progression to AML was similar in the French cohort (12.2%) and in the IMRAW cohort (13.3%, P = .21).
Univariate and multivariate Cox regression analyses of survival of the selected French-EPO (n=284) and IMRAW (n=225) cohorts
| . | French-EPO, N=284, no. (%) . | IMRAW, N=225, no. (%) . | P for French-EPO vs IMRAW characteristics . | AML, French-EPO cohort, n=12 . | AML, IMRAW cohort, n=24 . | Died, French-EPO cohort, n=35 . | Died, IMRAW cohort, n=123 . | 5-year OS, % (± SD) . | Multivariate analysis of OS . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted HR . | 95% CI . | P . | |||||||||
| Age, y | |||||||||||
| Younger than 70 | 127 (45) | 130 (58) | — | 5 | 13 | 20 | 75 | 38 (5.1) | 1.1 | 0.8-1.5 | .53 |
| 70 or older | 157 (55) | 95 (42) | .003 | 7 | 11 | 15 | 48 | 55 (5.1) | 1 | — | — |
| Sex | |||||||||||
| Male | 157 (55) | 124 (55) | — | 6 | 17 | 25 | 74 | 37 (5.0) | 1 | — | — |
| Female | 127 (45) | 101 (45) | .97 | 6 | 7 | 10 | 49 | 57 (5.3) | 0.6 | 0.5-0.9 | .004 |
| FAB | |||||||||||
| RA | 111 (39) | 121 (54) | — | 3 | 9 | 16 | 65 | 43 (5.1) | 1 | — | — |
| RARS | 110 (39) | 70 (31) | — | 6 | 8 | 9 | 33 | 61 (6.1) | 0.6 | 0.4-0.8 | — |
| RAEB | 63 (22) | 34 (15) | .003 | 3 | 7 | 10 | 25 | na | 1.9 | 1.0-3.8 | .002 |
| Karyotype | |||||||||||
| Intermediate | 28 (10) | 35 (16) | — | 0 | 5 | 3 | 18 | 51 (10.9) | 1 | — | — |
| Favorable | 256 (90) | 190 (84) | .05 | 12 | 19 | 32 | 105 | 45 (3.9) | 0.9 | 0.6-1.5 | .84 |
| BM blasts,median (IQR)* | 3 (2-5) | 2 (1-4) | <.001 | — | — | — | — | — | 1.0 | 0.9-1.1 | .89 |
| IPSS | |||||||||||
| Low | 136 (48) | 78 (35) | — | 4 | 4 | 17 | 34 | 56 (5.7) | — | — | — |
| Int-1 | 148 (52) | 147 (65) | .003 | 8 | 20 | 18 | 89 | 40 (4.7) | — | — | — |
| Hb level, g/L | |||||||||||
| Less than 8 (80 g/L) | 150 (53) | 99 (44) | 9 | 14 | 20 | 57 | — | — | — | — | |
| 8 to 10 (80 to 100 g/L | 134 (47) | 126 (56) | .05 | 3 | 10 | 15 | 66 | — | — | — | — |
| rEPO treatment | |||||||||||
| Yes | 284 (100) | 0 (0) | — | — | — | — | — | 64 (8.1) | 0.43 | 0.25-0.72 | — |
| No | 0 (0) | 225 (100) | — | — | — | — | — | 39 (3.9) | 1 | — | .005 |
| . | French-EPO, N=284, no. (%) . | IMRAW, N=225, no. (%) . | P for French-EPO vs IMRAW characteristics . | AML, French-EPO cohort, n=12 . | AML, IMRAW cohort, n=24 . | Died, French-EPO cohort, n=35 . | Died, IMRAW cohort, n=123 . | 5-year OS, % (± SD) . | Multivariate analysis of OS . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted HR . | 95% CI . | P . | |||||||||
| Age, y | |||||||||||
| Younger than 70 | 127 (45) | 130 (58) | — | 5 | 13 | 20 | 75 | 38 (5.1) | 1.1 | 0.8-1.5 | .53 |
| 70 or older | 157 (55) | 95 (42) | .003 | 7 | 11 | 15 | 48 | 55 (5.1) | 1 | — | — |
| Sex | |||||||||||
| Male | 157 (55) | 124 (55) | — | 6 | 17 | 25 | 74 | 37 (5.0) | 1 | — | — |
| Female | 127 (45) | 101 (45) | .97 | 6 | 7 | 10 | 49 | 57 (5.3) | 0.6 | 0.5-0.9 | .004 |
| FAB | |||||||||||
| RA | 111 (39) | 121 (54) | — | 3 | 9 | 16 | 65 | 43 (5.1) | 1 | — | — |
| RARS | 110 (39) | 70 (31) | — | 6 | 8 | 9 | 33 | 61 (6.1) | 0.6 | 0.4-0.8 | — |
| RAEB | 63 (22) | 34 (15) | .003 | 3 | 7 | 10 | 25 | na | 1.9 | 1.0-3.8 | .002 |
| Karyotype | |||||||||||
| Intermediate | 28 (10) | 35 (16) | — | 0 | 5 | 3 | 18 | 51 (10.9) | 1 | — | — |
| Favorable | 256 (90) | 190 (84) | .05 | 12 | 19 | 32 | 105 | 45 (3.9) | 0.9 | 0.6-1.5 | .84 |
| BM blasts,median (IQR)* | 3 (2-5) | 2 (1-4) | <.001 | — | — | — | — | — | 1.0 | 0.9-1.1 | .89 |
| IPSS | |||||||||||
| Low | 136 (48) | 78 (35) | — | 4 | 4 | 17 | 34 | 56 (5.7) | — | — | — |
| Int-1 | 148 (52) | 147 (65) | .003 | 8 | 20 | 18 | 89 | 40 (4.7) | — | — | — |
| Hb level, g/L | |||||||||||
| Less than 8 (80 g/L) | 150 (53) | 99 (44) | 9 | 14 | 20 | 57 | — | — | — | — | |
| 8 to 10 (80 to 100 g/L | 134 (47) | 126 (56) | .05 | 3 | 10 | 15 | 66 | — | — | — | — |
| rEPO treatment | |||||||||||
| Yes | 284 (100) | 0 (0) | — | — | — | — | — | 64 (8.1) | 0.43 | 0.25-0.72 | — |
| No | 0 (0) | 225 (100) | — | — | — | — | — | 39 (3.9) | 1 | — | .005 |
SD indicates standard deviation; HR, hazard ratio; and —, not applicable.
indicates interquartile range.
Five-year overall survival (OS) was better in the French-EPO cohort (64%) than in the IMRAW cohort (39%, P < .001; Figure 2A). This survival benefit, in the French cohort, was restricted to patients who responded to rEPO, as nonresponders to rEPO had the same OS as the IMRAW cohort patients (Figure 2B). In multivariate analysis, rEPO treatment (hazard ratio = 0.43, [95% CI: 0.25-0.72]) was independently associated with better survival.
Overall survival comparisons between the IMRAW and French-EPO cohorts. (A) Overall survival comparison between the IMRAW and French-EPO cohorts restricted to IPSS low or int-1 without unfavorable karyotype, since diagnosis for IMRAW and since introduction of rEPO for French-EPO cohort. (IMRAW: n = 225 patients [dotted curve]; French-EPO: n = 284 patients [plain curve].) (B) Overall survival comparison between the IMRAW and French-EPO cohorts restricted to IPSS LOW INT1 without unfavorable karyotype, according to response to rEPO: IMRAW: n = 225 (solid black curve), French-EPO (rEPO responders: n = 195 [dashed gray curve]; rEPO nonresponders: n = 99 [solid gray curve]). P was less than .001 between IMRAW and rEPO responders, and P = .17 between IMRAW and rEPO nonresponders. (C) Matched-pair analysis with 200 patients in the IMRAW database and 200 patients in the French-EPO cohort.
Overall survival comparisons between the IMRAW and French-EPO cohorts. (A) Overall survival comparison between the IMRAW and French-EPO cohorts restricted to IPSS low or int-1 without unfavorable karyotype, since diagnosis for IMRAW and since introduction of rEPO for French-EPO cohort. (IMRAW: n = 225 patients [dotted curve]; French-EPO: n = 284 patients [plain curve].) (B) Overall survival comparison between the IMRAW and French-EPO cohorts restricted to IPSS LOW INT1 without unfavorable karyotype, according to response to rEPO: IMRAW: n = 225 (solid black curve), French-EPO (rEPO responders: n = 195 [dashed gray curve]; rEPO nonresponders: n = 99 [solid gray curve]). P was less than .001 between IMRAW and rEPO responders, and P = .17 between IMRAW and rEPO nonresponders. (C) Matched-pair analysis with 200 patients in the IMRAW database and 200 patients in the French-EPO cohort.
These results were confirmed by a matched-pair analysis where we matched patients of the French-EPO and the IMRAW cohorts based on age, FAB, percentage of blasts, and karyotype with a ratio 1:1, leading to a sample of 200 patients from the French-EPO and 200 patients from the IMRAW cohort (Figure 2C). In a Cox model (with sex, age, percentage of blasts, FAB, karyotype, and rEPO treatment as covariates), rEPO treatment (HR = 0.43, [95% CI: 0.25-0.72]) was associated with significantly better overall survival.
Discussion
In our population of 403 MDS patients, the largest cohort of MDS patients treated with rEPO reported so far to our knowledge, the response rate was 62% according to IWG 2000 criteria, similar to that observed in previous series of patients with the same features (large predominance of lower-risk MDS with low baseline serum EPO level).26,–28 Prognostic factors of response were largely similar to those previously published, including serum EPO less than 200 IU/L, absence of transfusion requirement, and IPSS low and int-1.15,28
Some findings were, however, somewhat different from previously published literature. First, in RARS and RCMD-RS, response rates to rEPO alone were similar to those of rEPO with G-CSF, using both IWG 2000 and IWG 2006 criteria. This does not confirm other series where rEPO alone was less efficient than rEPO with G-CSF in those MDS subsets.29 In addition, we found a good response rate in RAEB-1 (56% vs 34% in patients with > 10% blasts). Also of note was that, at least using IWG 2006 criteria, shorter interval between MDS diagnosis and introduction of rEPO was associated with higher response rates, as previously shown by Spiriti et al.30 Finally, presence of multilineage dysplasia was not associated with significantly lower response rates in our series, contrary to the Nordic series, where RA/RARS had significantly higher response rates than RCMD and RCMD-RS.31 Dysplasia is sometimes difficult to quantify and is often hardly reproducible between observers, in part due to technical variability in preparing smears. We used here Flandrin's morphologic scoring system, which allows a somewhat better reproducibility of quantification of dysplasia.20 On the other hand, multilineage dysplasia, in addition to IPSS int-2 high and minor response, was predictive of a shorter response.
Our study was also one of the first opportunities to assess, in a large patient cohort, potential advantages of IWG 200616 response criteria in MDS, compared with IWG 2000 criteria.22 We evaluated the outcome of minor responders according to IWG 2000 criteria with the new IGW 2006 criteria, where this category is no longer taken into account. Of 88 “minor responders” (IWG 2000), 56 became nonresponders and 32 became responders (IWG 2006). These 2 groups were not different in terms of age, FAB, WHO classification, IPSS, endogenous EPO level, and transfusion rates, and we found no differences in response duration between these 2 groups. Exclusion of “minor erythroid response” from IWG 2006 criteria revision was made in particular after finding that some patients who received supportive care could be classified only as minor responders.16 Our findings suggest that this revision, on the other hand, may lead to underestimation of some relatively significant and durable responses, previously considered as minor responses using IWG 2000 criteria. Using IWG 2006 criteria, however, we also found that a shorter interval from MDS diagnosis to rEPO treatment was a favorable prognostic factor of response to rEPO, not seen with IWG 2000 criteria. Therefore, new prognostic factors of response to treatments in MDS may emerge using IWG 2006 criteria.
A major issue with rEPO treatment is whether it has an impact on disease progression and survival. The Nordic group, by comparing outcome of their patients treated with rEPO with G-CSF to the IMRAW database, found no significant difference for incidence of progression to AML and survival after adjustment on blasts, karyotype, and number of cytopenias. By contrast, we found that our treated population had better OS than the IMRAW cohort. The comparison between both populations was made by restricting them to low and int-1 risk patients, also excluding patients with CMML and with poor-risk karyotype. The significant advantage of the French-EPO cohort over the IMRAW cohort persisted after adjustment on main prognostic factors (age, sex, hemoglobin level, FAB classification, karyotype). Those results are concordant with preliminary results of a very recent study, so far presented only in abstract form, which showed a survival advantage of a Nordic MDS cohort treated with rEPO over a matched Italian cohort that received RBC transfusions only.29 One explanation for the discrepancies between our findings and the first findings of the Nordic group could have been a difference in sampling and in statistical methods. To avoid possible biases due to sampling, we used 2 different statistical analyses, with homogeneous groups: the first one with adjustment on main prognostic factors of survival (age, sex, FAB classification, percentage of blasts, hemoglobin level, and karyotype) in a Cox model and the second one by matching 1 to 1 patients of the French-EPO and the IMRAW cohorts based on age, FAB, percentage of blasts, and karyotype. Adjustment could not be made on baseline transfusion requirement, a recently described prognostic factor in MDS,23 as this parameter was not available in the IMRAW cohort. However, severity of anemia at baseline, a parameter obviously correlated to transfusion requirement, and that has also demonstrated prognostic value in MDS,5,24,25 tended to be more important in the French-EPO cohort. Indeed, 53% of patients in the French cohort selected for the comparison had baseline Hb level lower than 80 g/L (8 g/dL), compared with 44% in the IMRAW database. Still, we cannot completely exclude other confounding factors, such as differences in performance status and comorbidities between the French-EPO and the IMRAW cohorts, as those parameters were not available for the present study.
Reasons for the possibly improved survival with rEPO treatment are not clear, especially because causes of nonleukemic deaths in the IMRAW series were unknown and could not be compared with those seen in our cohort. As RBC transfusion requirement may be a poor prognostic factor, per se, avoiding transfusions could improve survival for example by reducing the risk of iron overload.23,29 In addition, patients requiring transfusions have a lower median hemoglobin level than patients responding to rEPO, and this could have an impact, not only on quality of life, but also on the risk of potentially fatal cardiovascular events. The fact that the survival benefit was restricted to patients responding to rEPO treatment in our cohort possibly goes along those lines. It could be argued that the survival improvement seen in our cohort of patients, generally diagnosed after 1996, was due to progress in the overall management of MDS patients during the last 10 years, as the IMRAW cohort was diagnosed prior to 1994. However, there was no improvement of outcome in patients diagnosed between 1999 and 2003 compared with those diagnosed between 1989 and 1993 in the Düsseldorf registry (U. Germing, Heinrich-Heine University, Dusseldorf, Germany, written personal communication, 2007). Very recently, the use of drugs such as 5-azacytidine, decitabine, and lenalidomide may have had an impact on survival in MDS.7,32,33 However, those drugs are still not approved and not widely available in Europe, and none of our patients had received them. Finally, survival in our cohort was measured from the introduction of rEPO, which occurred more than 26 months from diagnosis in 25% of the patients. Not surprisingly, therefore, our cohort was characterized by worse prognostic characteristics including higher age, higher percentage of blasts, and lower percentage of RA, than in the IMRAW cohort, which logically should have translated into lower OS in our cohort.
Further prospective studies, with precise analysis of nonleukemic causes of death in patients receiving rEPO or not, are, however, obviously required to confirm if treatment with rEPO confers a survival benefit in low-risk MDS patients.
S.P., S.G., and C.K. contributed equally to this study.
F.D. and P.F. contributed equally to this study.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Prof Ulrich Gerwing for the data about the Dusseldorf registry and all the investigators of the GFM.
Authorship
Contribution: S.P. and C.K. performed research, collected the data, and wrote the article; S.G. performed the statistical analyses, contributed to data interpretation, and participated in drafting the article. F.D. and P.F. designed the study; other authors contributed patient information.
A list of the centers and investigators of the Groupe Francophone des Myélodysplasies who contributed to this study can be found in Document S1.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sophie Park, Service d'hématologie, Hôpital Cochin, APHP, Université Paris V, 27 rue du Fb St-Jacques, 75014 Paris, France; e-mail: sophie.park@cch.aphp.fr.

![Figure 2. Overall survival comparisons between the IMRAW and French-EPO cohorts. (A) Overall survival comparison between the IMRAW and French-EPO cohorts restricted to IPSS low or int-1 without unfavorable karyotype, since diagnosis for IMRAW and since introduction of rEPO for French-EPO cohort. (IMRAW: n = 225 patients [dotted curve]; French-EPO: n = 284 patients [plain curve].) (B) Overall survival comparison between the IMRAW and French-EPO cohorts restricted to IPSS LOW INT1 without unfavorable karyotype, according to response to rEPO: IMRAW: n = 225 (solid black curve), French-EPO (rEPO responders: n = 195 [dashed gray curve]; rEPO nonresponders: n = 99 [solid gray curve]). P was less than .001 between IMRAW and rEPO responders, and P = .17 between IMRAW and rEPO nonresponders. (C) Matched-pair analysis with 200 patients in the IMRAW database and 200 patients in the French-EPO cohort.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/2/10.1182_blood-2007-06-096370/4/m_zh80040812680002.jpeg?Expires=1769087048&Signature=IUqDA3cQA81u8AVZn16ocWjiYMEv0iSrA9A4Tn5aqtSYv7OXE9-O71Gkn2iaiVAe3XqoB9AVhT~TNNfeoHdjcGbRyyzlKUHv4BKWj5Txvm4Kl7WaGjGsdjESjr7pe74vWp~VSY-7Crc4gXmLWES-S~NL2pz8EAbIcpj9-mv5hiWZmJOx4fJyOthN-q8PuzDSTVruSs7VKwqUXQYql4l9zeNGeR8OHQMewm8D~0b4oeHolDw~-UGcjo47xIWYZf2OUUBe~N5reYdPofjX7UOb98frcWPMFWrS2mzLqCfHV7NB9UWauTOQRjC6S9K2LuLM3sVFEeOid9pvxn4Gv7pfYw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal