Intravenous immunoglobulin (IVIg) is an accepted treatment for several autoimmune diseases, including relapsing-remitting multiple sclerosis (MS). While its beneficial effects are well documented, the mechanisms leading to them are incompletely understood. IVIg is an extremely complex preparation that may contain various biological activities, mostly due to antibodies both natural and induced, but also traces of nonantibody molecules and soluble receptors found in serum.1,2 There is strong evidence for effects mediated through Fcγ receptors (FcγR) present on immune cells.3 However, it has been difficult to explain all of the beneficial effects of IVIg, especially in T cell–mediated autoimmunity, solely by FcγR- mediated interactions.
Ephrem and colleagues show that IVIg protects from autoimmune encephalomyelitis (EAE), an accepted model for MS, and demonstrate the critical involvement of T regulatory cells. First, protection was associated with sustained expansion of CD4+CD25+FoxP3+ Tregs in peripheral organs of IVIg-treated mice. Interestingly, expansion was only observed in MOG (myelin oligodendrocyte glycoprotein)–immunized mice, suggesting TCR or other (adjuvant related?) signals. Second, compared with Tregs from untreated mice, Tregs from IVIg protected animals were more potent on a per-cell basis in transferring protection to adoptive recipients. Finally, depletion of Tregs prior to IVIg treatment prevented protection. Because the central nervous system infiltration typical of EAE was entirely prevented, it appears that the Tregs inhibit effector T-cell development in the periphery, rather than their function in the target organ.
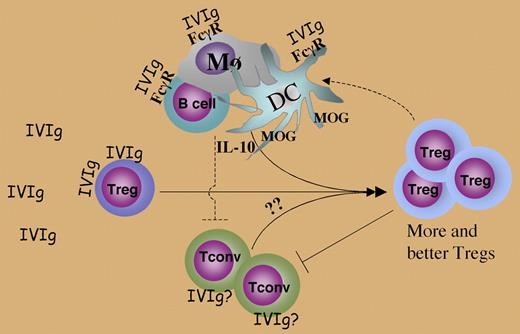
The mechanism of action of IVIg on the Tregs was partly elucidated by a series of in vivo and in vitro studies. Elegant experiments involving adoptive transfers of influenza hemagglutinin (HA)–specific, CFSE-labeled CD25+ Tregs and CD25− conventional T cells (Tconv), followed by analysis of their in vivo proliferation and FoxP3 expression, led to the conclusion that IVIg causes proliferation of existing natural Tregs, rather than de novo induction of “adaptive” Tregs from CD25− precursors. In vitro experiments revealed that IVIg enhanced proliferation of Tregs to anti-CD3 in culture without inducing IL-10 and TGF-β, and that it bound to Tregs, supporting the possibility of a direct effect on the Treg cells. The molecular interactions behind these effects remain to be elucidated, and the in vivo mechanisms are likely to be more complex, as FcγR-mediated effects on dendritic cells, monocytes, and B cells could also play a role. The authors argue against the latter possibility by citing data (not shown) that IVIg F(Ab′)2, without the Fc portion, was also protective. Nevertheless, some of the reported in vivo findings are difficult to explain as direct effects of IVIg on Tregs alone. Thus, an increase in Treg proliferation was observed only when Tregs were coinjected with Tconv, which should not have been the case if the effects of IVIg in vivo had been direct. There was also a significant enhancement of IL-10 production coming from non–T cells, and thus likely to involve FcγR related effects, that may well have contributed to protection (see figure).
The relevance of the current observations to human Tregs is supported by a recent report by Kessel et al,4 who demonstrated an in vitro enhancement by IVIg of human CD4+CD25hi Tregs obtained from peripheral blood in terms of FoxP3 expression, anti-inflammatory cytokine production, and ability to inhibit CD25− target cells. The nature of these Tregs is not clear, since in adult humans the CD4+CD25hi population is expected to contain a mixture of natural and induced Tregs, and antiinflammatory cytokine production has been associated primarily with induced Tregs. Thus, in humans IVIg could potentially also affect induced Tregs that might be more potent in established disease.
Mechanisms involved in IVIg effects on Tregs. Dashed lines depict possible in vivo interactions that may occur but have not been specifically explored in the present study.
Mechanisms involved in IVIg effects on Tregs. Dashed lines depict possible in vivo interactions that may occur but have not been specifically explored in the present study.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal