Abstract
Nonmyeloablative stem cell transplantation in patients with follicular lymphoma has been designed to exploit the graft-versus-lymphoma immunity. The long-term effectiveness and toxicity of this strategy, however, is unknown. In this prospective study, we analyzed our 8-year experience. Patients received a conditioning regimen of fludarabine (30 mg/m2 daily for 3 days), cyclophosphamide (750 mg/m2 daily for 3 days), and rituximab (375 mg/m2 for 1 day plus 1000 mg/m2 for 3 days). They were then given an infusion of human leukocyte antigen-matched hematopoietic cells from related (n = 45) or unrelated donors (n = 2). Tacrolimus and methotrexate were used for graft-versus-host disease (GVHD) prophylaxis. Forty-seven patients were included. All patients experienced complete remission, with only 2 relapses. With a median follow-up time of 60 months (range, 19-94), the estimated survival and progression-free survival rates were 85% and 83%, respectively. All 18 patients who were tested and had evidence of JH/bcl-2 fusion transcripts in the bone marrow at study entry experienced continuous molecular remission. The incidence of grade 2-IV acute GVHD was 11%. Only 5 patients were still undergoing immunosuppressive therapy at the time of last follow-up. We believe that the described results are a step forward toward developing a curative strategy for recurrent follicular lymphoma.
Introduction
Allogeneic stem cell transplantation can result in long-term disease control in patients with follicular lymphoma, in part through an immune-mediated graft-versus-lymphoma effect.1,2 A comparative retrospective study showed a significantly lower relapse rate in patients with follicular lymphoma who underwent allogeneic transplantation compared with those who underwent autologous transplantation.3 However, the high treatment-related mortality rate of allogeneic transplantation (30%-40%), caused primarily by direct regimen toxicity and a high incidence of severe graft-versus-host disease (GVHD), often offsets any potential survival benefits
Nonmyeloablative stem cell transplantation is designed to exploit the graft-versus-lymphoma effect without the attendant toxicity of myeloablative conditioning.4 However, the risk of other toxicities and GVHD cannot be underestimated, and long-term effectiveness is not known because long-term follow-up is lacking in earlier studies.
The purpose of this study was to address this gap of knowledge. We exploited the graft-versus-lymphoma effect by administering a nonmyeloablative conditioning regimen of fludarabine, cyclophosphamide, and rituximab to 47 patients with relapsed follicular lymphoma. Patients were followed for a median of 60 months after transplantation. We found a long-term survival rate of 85%, with low incidences of toxicity and severe GVHD. All patients experienced complete remission, with only 2 relapses.
Methods
In this phase 2 trial, we included patients treated at M. D. Anderson Cancer Center for relapsed follicular lymphoma between March 1999 and April 2005. Eligibility criteria included patients aged 19 to 70 years who had chemosensitive relapsed disease, with partial response or better to salvage chemotherapy. Patients were required to have a Zubrod performance status score of 2 or lower, serum bilirubin lower than 2 mg/dL, serum creatinine lower than 1.6 mg/dL, no symptomatic cardiac or pulmonary disease, and no active infection; female patients were to be nonpregnant. In addition, patients were required to have a human leukocyte antigen (HLA)–compatible sibling donor. An HLA phenotypically identical unrelated donor was chosen only if patients had no sibling donors and were not candidates for autologous transplantation. Patients were treated on a single protocol, and all patients enrolled in the trial were included in the analysis. Written informed consent was obtained from all patients in accordance with the Declaration of Helsinki. The study was reviewed and approved by The University of Texas M. D. Anderson Cancer Center institutional review board.
Clinical evaluation
All lymph node biopsies were reviewed by an experienced hematopathologist to confirm the diagnosis. Patients underwent a physical examination, a complete blood count with differential count, a serum chemistry panel, serum β-2 microglobulin measurement, chest radiography, computed tomography of the abdomen and pelvis, functional imaging with positron emission tomography with 18F-fluoro-deoxyglucose or Gallium67 scans, and bilateral bone marrow aspiration and biopsies with flow cytometric immunophenotypic analysis. Disease stage was evaluated using Ann Arbor criteria and assigned an International Prognostic Index score.5 Patients were evaluated 1, 3, 6, and 12 months after transplantation and every 6 months thereafter. Responses were scored using the standard criteria for patients with lymphoma as described by Cheson et al.6 Peripheral blood CD19+ lymphocyte levels were generally measured at the same time intervals. Patients were assessed more frequently at the discretion of their primary physician.
Preparative regimen for transplantation and GVHD
All patients received fludarabine (30 mg/m2) and cyclophosphamide (750 mg/m2), each given intravenously for 3 days (−5 to −3 before transplantation).4 They were also given 375 mg/m2 of rituximab on day −13, and 1000 mg/m2 on days −6, +1, and +8. The scheduling of rituximab was designed to benefit from its known properties of chemosensitization7 (hence a dose on day −13), its synergy with fludarabine8 (hence a dose on day −6), and to maximize its benefit when given in the setting of minimal residual disease after cytoreductive chemotherapy (hence the doses on days +1 and +8). Rituximab was also given to enhance the graft-versus-lymphoma effect,9 and for its potential role in decreasing the risk of GVHD.10 Allogeneic hematopoietic transplantation was performed on day 0. Patients received unfractionated peripheral blood progenitor cells (if the donor was a matched sibling) or marrow (if the donor was unrelated).
Patients who underwent transplantations from a matched unrelated donor received 15 mg/kg equine antithymocyte globulin intravenously on days −5 to −3 before transplantation to reduce the risk of rejection. Supportive care was provided as described previously.4
Prophylaxis for GVHD consisted of tacrolimus and methotrexate, 5 mg/m2 intravenously, on days 1, 3, and 6 after transplantation. The same dosage was also given on day 11 to patients with an unrelated donor. Tacrolimus levels were adjusted to be 5-10 ng/mL and administered for 6 months to patients who experienced a remission. Acute and chronic GVHD was graded according to consensus criteria.11,12 GVHD postdonor lymphocyte infusions (DLIs) was graded according to the National Institutes of Health consensus criteria.12
Assessment of donor chimerism and engraftment
A chimerism analysis was performed on whole blood and on blood mononuclear cells separated into T and B cells, by polymerase chain reaction (PCR), with primer sets flanking microsatellite repeats as described previously in detail.9 Neutrophil count recovery was defined as having occurred on the first of 3 consecutive days that the absolute neutrophil count exceeded 0.5 × 109/L of blood. Platelet count recovery was defined as having occurred on the day that the platelet count exceeded 20 × 109/L of blood, independent of platelet transfusions.
Immunomanipulation after transplantation
DLIs were administered in combination with rituximab treatment for patients with persistent or progressive disease after transplantation if GVHD was not present.9 Tacrolimus doses were rapidly tapered. Rituximab was then given at a dose of 375 mg/m2 intravenously followed by 3 weekly doses of 1000 mg/m2. A DLI of 1 × 107 CD3-positive T cells/kg was given after the first 2 doses of rituximab if no GVHD occurred. An escalated DLI dose was given at 6-week intervals if there was persistent active disease and no GVHD. DLIs were not routinely administered in patients with stable mixed chimerism if they remained in remission. Patients experiencing a rapid decrease of donor cells received DLIs with the goal of achieving complete donor chimerism.
PCR methods for detecting JH/bcl-2 fusion transcripts
High molecular weight DNAs were isolated from the patients' peripheral blood samples. One microgram of DNA was subjected to real-time PCR amplification using primers, reagents, and PCR conditions as described previously.13,14 In short, the crossover site sequences of the t(14;18) at the major breakpoint region (MBR) or its minor cluster region (MCR) were coamplified with a reference target, cyclophilin, using forward primers derived from the MBR region or the MCR region of the bcl-2 gene and the reverse primer derived from the JH region of the immunoglobulin heavy chain (IgH) gene, and primers for the cyclophilin reference target in an ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA) for 40 cycles of the following conditions: 94°C for 15 seconds, 58°C for 15 seconds, and 72°C for 30 seconds. Also added to the PCR reaction were double-labeled fluorogenic probes specific for the MBR region or the MCR region and the cyclophilin reference target. The MBR and the MCR probe are labeled with 6-carboxyfluorescein and carboxytetramethyl rhodamine (TAMRA) at the 5′ and 3′ ends, respectively. The cyclophilin probe is labeled with Vic and TAMRA at the 5′ and 3′ ends, respectively. Comparing with the standard curve plots constructed from serial dilutions of a positive control cell line, the amounts of the JH/bcl-2 fusion transcripts in the samples were quantified using cycle threshold methods as recommended by the manufacturer. The quantification was then normalized with the levels of the cyclophilin reference target by taking the ratio of the JH/bcl-2 fusion transcript positives versus the cyclophilin reference target. PCR for each sample was performed in duplicates and the average of the 2 quantifications was recorded. Molecular response was defined as the disappearance of JH/bcl-2 amplicons in bone marrow after therapy in patients with known t(14;18) at baseline.
Statistical methods
Patients and transplantation characteristics were compared using the χ2 test for categoric variables and the Mann-Whitney test for continuous variables. Actuarial estimates of overall survival (OS) and progression-free survival (PFS) rates were calculated according to the Kaplan and Meier method.15 OS was estimated from the time of transplantation to time of death or last follow-up. The incidence of disease progression and GVHD were estimated by the cumulative incidence method16 considering death with disease and death without GVHD as competing risks, respectively. Cox's regression analysis was used to evaluate the affect of chimerism on day 90 on the incidence of chronic GVHD. P values less than .05 were considered statistically significant. The statistical analysis was performed using Stata 7.0 software (Stata Corp, College Station, TX).
Results
Patient characteristics
Between March 1999 and April 2005, 47 consecutive patients were enrolled; these patients ranged in age from 33 to 68 years (median, 53 years; Table 1), and 9 of them have been reported previously.4 The time from diagnosis to transplantation ranged from 7 months to 24 years (median, 3 years). All patients had relapsed chemosensitive follicular lymphoma, achieving a partial or complete response to salvage chemotherapy. Patients had been treated with a median of 3 (range, 2-7) chemotherapy regimens. Thirty-nine patients had received treatments with rituximab before transplantation, either combined with chemotherapy (35 patients) or as a single agent (4 patients). Nine of the 35 patients who had chemoantibody combinations also received single-agent rituximab either as maintenance (2 patients) or as treatment for recurrent disease (7 patients). Of the 11 patients who received rituximab alone as treatment for disease, 5 responded, and 6 were refractory. Sixteen patients had no response to their initial chemotherapy or responded but relapsed within 6 months. Seven of these 16 patients had bulky disease at their diagnosis (range, 15-121 cm2). In 9 patients (19%), the disease had recurred after prior autologous stem cell transplantation. The median time to recurrence was 16 months, and 4 patients had recurrent disease within 9 months of the autologous transplantation.
Patient characteristics
| Characteristic . | Result . |
|---|---|
| No. of patients | 47 |
| Median age, y (range) | 53 (33-68) |
| Sex, no. (%) | |
| Male | 25 (53) |
| Female | 22 (47) |
| Chemotherapies before transplantation (%) | |
| Rituximab-chemo combinations | 35 (74) |
| 3 regimens | 7 (15) |
| 4 regimens | 12 (25.5) |
| >5 regimens | 5 (10.5) |
| No. of patients with prior autologous transplantation (%) | 9 (19) |
| Additional therapies before transplantation (%) | |
| Radio-immunotherapy | 1 |
| Rituximab, single agent | 13 (28) |
| Total lymphoid irradiation | 1 |
| Localized radiation | 11 (23) |
| Interferon | 7 (15) |
| Other investigational agents | 4 (8) |
| International Prognostic Index score at study entry, median (range) | 1 (0-3) |
| Grade, no. (%) | |
| 1 or 2 | 37 (79) |
| 3 | 10 (21) |
| Median time from diagnosis to transplantation, y (range) | 3 (0.7-24) |
| Disease status at transplantation, no (%) | |
| Complete remission | 18 (38) |
| Partial remission | 29 (62) |
| Stem cell source, no (%) | |
| Marrow/blood | 2 (4)/45 (96) |
| Related/unrelated donor | 45 (96)/2 (4) |
| CD34-positive cell infused, ×106/kg, median (range) | 4.5 (3-7) |
| Characteristic . | Result . |
|---|---|
| No. of patients | 47 |
| Median age, y (range) | 53 (33-68) |
| Sex, no. (%) | |
| Male | 25 (53) |
| Female | 22 (47) |
| Chemotherapies before transplantation (%) | |
| Rituximab-chemo combinations | 35 (74) |
| 3 regimens | 7 (15) |
| 4 regimens | 12 (25.5) |
| >5 regimens | 5 (10.5) |
| No. of patients with prior autologous transplantation (%) | 9 (19) |
| Additional therapies before transplantation (%) | |
| Radio-immunotherapy | 1 |
| Rituximab, single agent | 13 (28) |
| Total lymphoid irradiation | 1 |
| Localized radiation | 11 (23) |
| Interferon | 7 (15) |
| Other investigational agents | 4 (8) |
| International Prognostic Index score at study entry, median (range) | 1 (0-3) |
| Grade, no. (%) | |
| 1 or 2 | 37 (79) |
| 3 | 10 (21) |
| Median time from diagnosis to transplantation, y (range) | 3 (0.7-24) |
| Disease status at transplantation, no (%) | |
| Complete remission | 18 (38) |
| Partial remission | 29 (62) |
| Stem cell source, no (%) | |
| Marrow/blood | 2 (4)/45 (96) |
| Related/unrelated donor | 45 (96)/2 (4) |
| CD34-positive cell infused, ×106/kg, median (range) | 4.5 (3-7) |
At the time of enrollment for nonmyeloablative transplantation, 29 patients (62%) were in partial remission (PR), and 18 (38%) were in complete remission (CR). Although patients were responding to salvage chemotherapy in preparation for transplantation, the duration of that potential response is unknown, because patients started their transplant procedure as soon as response was observed after 2 to 3 cycles of chemotherapy and as soon as they had their donor identified and the stem cells collected. The median time between the patients' last relapse to the initiation of the conditioning regimen for transplantation was 3 months (range, 1.5-8 months). In only 3 patients, this elapsed time was longer than 5 months. This was because of viral meningitis (1 case), facial nerve palsy (1 case), and treatment for leptomeningeal disease (1 case).
Transplantation and engraftment
Forty-five patients (96%) underwent allogeneic transplantation from a human leukocyte antigen-identical sibling donor; the remaining 2 underwent transplantation from a matched unrelated donor. All patients received an unmanipulated graft, from the peripheral blood (45 patients), or bone marrow (2 patients).
A median number of 4.5 × 106/kg CD34-positive cells were infused (range, 3-7 × 106/kg). Neutrophil counts recovered to more than 0.5 × 109/L a median of 11 days after transplantation (range, 8-17 days). Twenty-eight patients (60%) required no platelet transfusions; in the remaining patients, platelet counts recovered to more than 20 × 109/L a median of 10 days after transplantation (range, 6-17 days).
Donor chimerism and response
Forty-six patients experienced donor cell engraftment. The median values of donor T-cells and myeloid cells by day 30 after transplantation were 89% and 83% (range, 0%-100%), respectively. The median level of T-cells has increased to 99% (range, 27%-100%) by day 90, in 33 patients who were tested. The median level of myeloid cells increased to 99% (range, 16%-100%) by day 90 in 39 patients who were tested. One patient developed primary graft failure after receiving a donor transplant containing 3.9 × 106/kg CD34+. Two other patients who received a donor transplant containing 4.5 and 6 × 106/kg CD34+, experienced a secondary graft failure, presumably related to rejection of unknown causes, at 6 and 20 months, respectively, after transplantation. These 3 patients who experienced primary or secondary graft failure, had autologous hematopoietic recovery.
All patients achieved CR after transplantation. The patient who had a primary graft failure experienced CR after a second allogeneic transplantation. The median time to CR in patients who had evidence of active disease at study entry was 5.5 months. Two relapses occurred. One was observed at 18 months; this patient underwent DLI with rituximab and experienced a continuous CR at 24+ months. The other patient had experienced secondary graft failure 20 months after transplantation and was treated with rituximab, which led to CR. The other patient with secondary experienced graft failure at 6 months did not relapse and remains in CR, 30 months after transplantation.
Three patients received DLI in this study. One was related to progression as described above. Two other patients had a rapid decrease in donor chimerism without lymphoma relapse; they achieved complete donor chimerism after DLI of 107/kg CD3+ cells. These 2 patients subsequently developed manifestations of chronic GVHD at 3 and 4 months, respectively, after their DLI infusion.
Eighteen patients were tested by PCR and had evidence of t(14;18) in the bone marrow at study entry. After treatment, 100 bone marrow PCR samples were available for analysis. Ninety-eight of these, obtained at a median of 45 months after transplantation (range, 4-72 months) and were negative for detectable disease. Two samples from 2 different patients were positive early after transplantation but became negative 3 months later.
We evaluated the relationship between disease response, risk of relapse, the incidence of chronic GVHD and donor T-cell chimerism by day 90. T-cell chimerism was evaluated in 33 patients, and 17 (52%) had mixed chimerism in this compartment. Twelve (71%) of these 17 patients were in PR at transplantation. All achieved CR without DLI. There was no difference in the rate of chronic GVHD (P = .5) and risk of relapse in patients with mixed chimerism compared with the patients who had 100% donor cells by day 90.
B-cell immune reconstitution
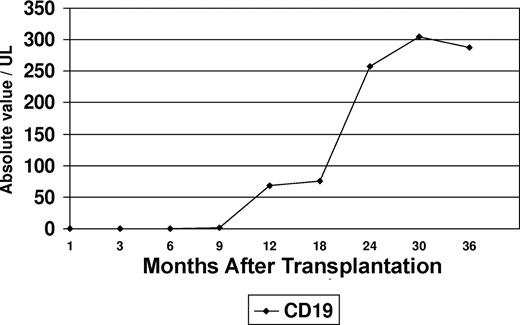
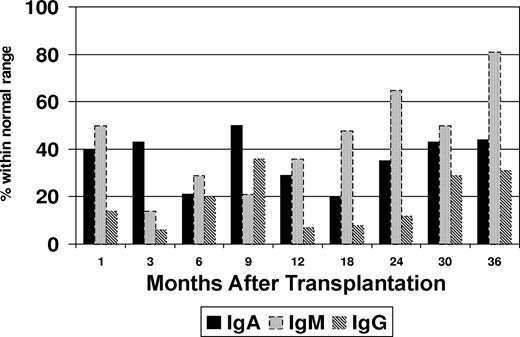
Flow cytometric immunophenotyping was used to assess B-cell recovery in the peripheral blood after transplantation. Peripheral blood CD19+ cells were undetectable in 30 samples tested within 9 months after transplantation (Figure 1). Increasing levels of CD19+ cells were noted at 12 months (median, 68 cells/μL; range, 6-202 cells/μL), and appreciable levels did not occur until 24 months (median, 257 cells/μL; range, 84-698 cells/μL) after transplantation. It was also noted that a proportion of patients maintained normal serum immunoglobulin (Ig) levels at time intervals at which CD19+ cells were undetectable (Figure 2). This proportion varied in 13 to 15 patients tested at different intervals. At 3 months after transplantation, the fraction of patients who had normal IgA levels was higher than those who had normal IgG (P = .02) or IgM levels (P = .2). An increase in the serum levels of IgM levels was noted to coincide with the initial modest rise in peripheral blood CD19+ cells at 12 months, whereas IgA and IgG levels lagged behind. At 24 months after transplantation, the proportion of patients who were found to have normal levels of IgM was significantly higher than those who had normal IgG (P = .001) or IgA (P = .08).
Proportion of patients with normal immunoglobulin (Ig) levels after transplantation.
Proportion of patients with normal immunoglobulin (Ig) levels after transplantation.
Survival and toxicity
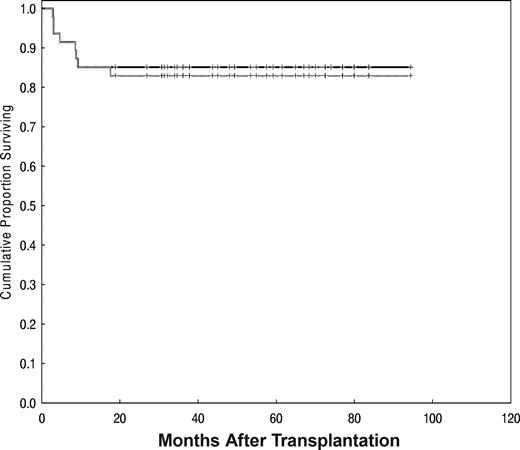
With a median follow-up time of 60 months (range, 19-94 months), the estimated OS and PFS rates were 85% (95% confidence interval [CI], 71%-93%) and 83% (95% CI, 69%-91%), respectively (Figure 3).
OS (solid line) and PFS were 85% and 83%, respectively, with a median follow-up of 60 months (range, 19-94 months).
OS (solid line) and PFS were 85% and 83%, respectively, with a median follow-up of 60 months (range, 19-94 months).
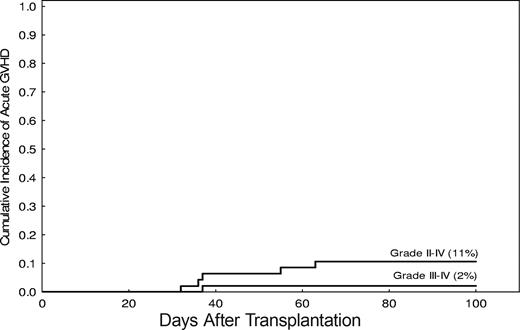
The incidence of grade 2-IV acute GVHD was 11% (95% CI, 31%-66%; Figure 4). Grade 2 acute GVHD occurred in 5 patients. Grade 3 acute GVHD was observed in one patient only, and none had grade 4. The median time to onset of acute GVHD was 35 days after transplantation (range, 19-63 days).
Incidence of severe acute GVHD after nonmyeloablative stem cell transplantation.
Incidence of severe acute GVHD after nonmyeloablative stem cell transplantation.
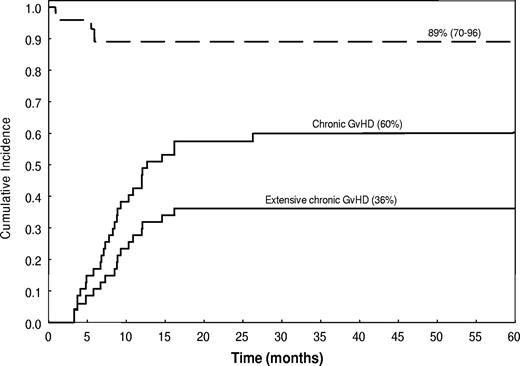
The incidence of chronic GVHD was 60% (95% CI, 47%-76%) and the incidence of its extensive form was 36% (95% CI, 25%-53%). Of the 28 patients who developed chronic GVHD, 20 (71%) had a de novo onset. Nineteen patients developed chronic extensive GVHD: this involved the skin (14 patients), gastrointestinal tract (10 patients), mouth (3 patients), liver (3 patients), and lung (1 patient). Liver GVHD was manifested by isolated elevations of liver enzymes, whereas serum bilirubin remained normal. The median time to onset of chronic GVHD was 255 days after transplantation, and the OS rate was 89%, with a median follow-up time of 57 months (range, 19-94 months; Figure 5). Only 5 patients in the study group were still undergoing immunosuppressive therapy at last follow-up.
Incidence (—) and survival duration (----) of patients with chronic extensive and limited GVHD.
Incidence (—) and survival duration (----) of patients with chronic extensive and limited GVHD.
Grade 3 toxicity was observed in 12 patients. This included hepatotoxicity in 4 patients, with isolated elevations of alanine aminotransferase and/or alkaline phosphatase levels, possibly secondary to the prophylactic antifungal medications used, nervous system in 2 patients (headache in one patient with a known history of migraines, subdural hematoma after a fall that did not require surgery), cardiovascular system in 3 patients (hypertension, deep-vein thrombosis, atypical chest pain), muscular skeletal in one patient (bone pain with filgrastim [Neupogen]), gastrointestinal in one patient (diarrhea), and infection in one patient. One patient had grade 4 toxicity and it was related to pneumonia.
Seven patients died. Infections were the major cause of death. The infections were bacterial (2 patients), fungal (2 patients), or viral (2 patients; one of these 2 patients had herpes zoster infection 4 months before initiating his transplantation procedure; this recurred in a disseminated form, 5 months after his transplantation). The immediate cause of death was not identified in one patient. None of the patients died with recurrent lymphoma.
Discussion
These results indicate that a nonmyeloablative conditioning regimen of fludarabine, cyclophosphamide, and rituximab with allogeneic stem cell transplantation can induce a continuous clinical and molecular remission in patients with relapsed, chemosensitive follicular lymphoma. Eighty-five percent of patients achieved long-term survival rate. These results compare favorably to prior studies using myeloablative conditioning, which were associated with treatment-related mortality up to 40%.17 This fulfills the ultimate objective of nonmyeloablative transplantation of long-term disease control without undue transplantation-related mortality.
The optimal dose of rituximab is unknown in the context of allogeneic stem cell transplantation, and a relatively high dose was used in this study. The standard dosage of rituximab is 375 mg/m2 once a week for 4 weeks. However, in the pivotal trial of rituximab in patients with follicular lymphoma, median serum antibody levels were found to be higher in patients who experienced a response than in those who did not at multiple time points after treatment (25.4 vs 5.9 μg/mL at 3 months, respectively; P = .001).18 Other researchers have also found a correlation between the serum concentration of a therapeutic monoclonal antibody and the clinical response.19,20 Extrapolation of these results suggests that higher doses of rituximab may result in even higher serum levels of rituximab. In the setting of allogeneic stem cell transplantation, rituximab could augment the graft-versus-lymphoma effect through antibody-dependent cellular toxicity.21 Further studies are needed to define the optimal dose of rituximab with nonmyeloablative stem cell transplantation.
Rituximab maintenance therapy after conventional chemotherapy for follicular lymphoma is the latest permutation in the application of anti-CD20 monoclonal antibody immunotherapy. Two trials in relapsed follicular lymphoma patients showed that even the excellent responses achieved with a rituximab-containing immunochemotherapy induction regimen could be optimized with subsequent rituximab maintenance dosages of one dose of 375 mg/m2 every 3 months for up to 2 years or 4 weekly doses of 375 mg/m2 every 6 months for up to 2 years.22,23 However, despite the compelling findings of these reports, relapses continue to be observed in all studies, with no prospect of a cured fraction. In our study, high doses of rituximab were given as part of the conditioning regimen. Nevertheless, the use of high-dose rituximab alone does not explain the duration of response found in our study, with only 2 relapses after a follow-up period of 60 months or the durable molecular responses found in often heavily pretreated patients whose disease had failed a prior autologous transplantation, including the spontaneous (without further use of DLI) conversion of PCR positivity for JH/bcl-2 fusion transcripts early after transplantation to PCR negativity several months later: these findings underscore instead the robustness of the graft-versus-lymphoma effect and its capacity to maintain long-term disease control. The dosage of rituximab needs to be determined in controlled trials.
The source of normal peripheral blood immunoglobulin levels in some patients during the early periods after allogeneic transplantation with rituximab remains uncertain, especially in the setting of undetectable peripheral blood CD19+ cells lymphocytes. Plasma cell aggregates that do not express CD20 and those that may have survived the nonmyeloablative conditioning regimen could be one potential source of these circulating immunoglobulins.
The precise criteria for DLI in patients undergoing nonmyeloablative stem cell transplantation for follicular lymphoma are not clear. DLI is often used to augment disease control in patients with progressive or resistant lymphoma but may also be given to patients with mixed chimerism in an effort to achieve full donor chimerism, even in the absence of measurable disease. Administration of DLIs is associated with a risk for severe GVHD, which may be life-threatening.24,25 We evaluated the relationship between disease response and donor chimerism by day 90 after transplantation. Seventeen of 33 patients had mixed chimerism by day 90, yet all experienced complete remission, and there was no additional risk of relapse compared with those patients who had full donor chimerism. This observation suggests that experiencing an early full donor chimerism is not a requirement for disease control in follicular lymphoma after T cell–replete transplantation and that the use of DLI for treatment of mixed chimerism should be avoided.
In our study, grade 2-IV acute GVHD was found in 11% of patients, a rate that is substantially lower than the 25% to 50% reported in registry studies26-28 and similar to that reported in alemtuzumab studies.29 This low rate of acute GVHD did not compromise the graft-versus-lymphoma effect in our patients. Because the median age of patients in registry studies was younger than that in our cohort and unrelated donors constituted less than 20% of the stem cell source in all of these reports, the different rates of acute GVHD rate are likely to be due to differences in conditioning regimens with rituximab and GVHD prophylaxis methods. In addition to 3 doses of methotrexate (5 mg/m2) on days +1, +3 and +6, patients in our study underwent a full 6 months of treatment with tacrolimus unless disease resistance or progression occurred.
The mechanism underlying this low incidence of GVHD is unknown but may possibly be due to the inclusion of rituximab in the nonmyeloablative regimen. Using a B cell–deficient mouse model in which mice received either control rabbit immunoglobulin or rabbit anti-IgM from birth, Schultz et al10 reported a lower incidence of GVHD in B cell–deficient animals, and the rate of GVHD was even lower if the grafts were depleted of B cells. Thus it is possible that B cells function as antigen-presenting cells and may have an important role in the pathogenesis of GVHD.
Although the incidence of chronic GVHD was 60%, this included both extensive and limited forms, a detail frequently omitted in other reports in the field. In addition, a unique finding in our report is that 20 of the 28 (71%) patients who experienced chronic GVHD had a de novo onset, which had no major negative effect on survival. The OS rate of patients with chronic GVHD was 89%, with a median follow-up time of 60 months. Only 5 patients in the study group were still receiving immunosuppressive therapy at last follow-up.
Reported outcomes of nonmyeloablative stem cell transplantation in follicular lymphoma vary widely. Some researchers have confirmed our experience of excellent medium-term disease control, reporting relapse rates of 10% or less at 2 to 3 years,26,27 whereas others have reported relapse rates of 20% to 45% over a similar time span.28-30 An inherent difficulty in interpreting the disparate results between individual reports lies with the heterogeneity of the studies (single-center vs multicenter and cooperative group vs registry studies), the heterogeneity of the patients studied (in particular, whether only patients with follicular lymphoma were included), the variation in the nonmyeloablative transplant procedure itself (eg, the conditioning regimen, GVHD prophylaxis, and stem cell source), and the duration of follow-up. There is an almost universal consensus, however, that chemosensitivity at the time of study entry is the most important determinant of response to nonmyeloablative stem cell transplantation for follicular lymphoma, with chemorefractory patients experiencing significantly inferior survival rates because of poor disease control and an increased treatment-related mortality. Innovative approaches are needed to treat these patients.
Nonmyeloablative allogeneic transplantation is an effective treatment for patients with recurrent follicular lymphoma and compares favorably to alternative approaches in advanced patients with chemosensitive disease. In the prerituximab era, there was little evidence that autologous transplantation conferred any survival advantage compared with conventional treatment for follicular lymphoma. In a recent update by Rohatinar,31 55% of patients were free of disease at 5 years. Their median age was 43 years, a decade younger than the median of patients reported in our study. Although the incorporation of rituximab within the strategy of autologous transplantation may improve the duration of remission, the addition of monoclonal antibodies or other agents would not alter the risk of death related to secondary myelodysplasia and other malignancies, which is estimated to be approximately 15%31 ; this remains of major concern particularly for patients with indolent lymphoma, whose major benefit from autologous transplantation is likely the prolongation of remission, not a cure.
In conclusion, the longer follow-up period in our study provides further insight into the long-term activity and the toxicity of nonmyeloablative transplantation regimens for follicular lymphoma, where 83% of patients experience long-term survival with remission and none have died from recurrent lymphoma. We believe that the described results are a step forward toward developing a curative strategy for follicular lymphoma and are laying the groundwork for prospective comparative trials addressing the outstanding issues in nonmyeloablative stem cell transplantation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: I.F.K. conceived and designed the study, cared for patients, collected and assembled data, analyzed and interpreted data, and wrote the manuscript; A.A., C.H., P.A., D.C., M.L., S.G., and N.T.U. were involved in patient accrual, caring for patients, and reviewing the data and manuscript; M.K. performed the stem cell collection from donors and reviewed the manuscript; P.M., L.F., F.S., S.S.N., F.H., and L.W.K. were involved in patient accrual and reviewed the data and manuscript; M.L. and L.J.M. were involved in pathology review and molecular testing; B.I.S reviewed the radiologic imaging; R.M.S. performed the statistical analysis; R.E.C. conceived and designed the study, cared for patients, analyzed and interpreted data, and reviewed the manuscript. All authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Issa F. Khouri, Department of Stem Cell Transplantation and Cellular Therapy, Unit 423, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: ikhouri@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal