Abstract
The International Peripheral T-Cell Lymphoma Project is a collaborative effort designed to gain better understanding of peripheral T-cell and natural killer (NK)/T-cell lymphomas (PTCLs). A total of 22 institutions in North America, Europe, and Asia submitted clinical and pathologic information on PTCLs diagnosed and treated at their respective centers. Of the 1314 eligible patients, 181 had anaplastic large-cell lymphoma (ALCL; 13.8%) on consensus review: One hundred fifty-nine had systemic ALCL (12.1%) and 22 had primary cutaneous ALCL (1.7%). Patients with anaplastic lymphoma kinase–positive (ALK+) ALCL had a superior outcome compared with those with ALK− ALCL (5-year failure-free survival [FFS], 60% vs 36%; P = .015; 5-year overall survival [OS], 70% vs 49%; P = .016). However, contrary to prior reports, the 5-year FFS (36% vs 20%; P = .012) and OS (49% vs 32%; P = .032) were superior for ALK− ALCL compared with PTCL, not otherwise specified (PTCL-NOS). Patients with primary cutaneous ALCL had a very favorable 5-year OS (90%), but with a propensity to relapse (5-year FFS, 55%). In summary, ALK− ALCL should continue to be separated from both ALK+ ALCL and PTCL-NOS. Although the prognosis of ALK− ALCL appears to be better than that for PTCL-NOS, it is still unsatisfactory and better therapies are needed. Primary cutaneous ALCL is associated with an indolent course.
Introduction
The definition of anaplastic large-cell lymphoma (ALCL) has evolved since its original description in 1985 by Stein and colleagues1 as a lymphoma characterized by large anaplastic lymphoid cells with uniform, strong expression of CD30 and a tendency to grow cohesively and invade lymph node sinuses. Subsequent immunophenotypic and genetic studies resulted in restriction of the diagnosis to cases of T-cell or null lineage, and recognition that primary cutaneous and systemic types were clinically and immunophenotypically distinctive. The current World Health Organization (WHO)2 classification distinguishes systemic ALCL from primary cutaneous ALCL (cut-ALCL).
The discovery of a unique chromosomal translocation revealed biologic heterogeneity within the category of systemic ALCL. The t(2;5)(p23;35) fuses the anaplastic lymphoma kinase (ALK) gene on chromosome 2 with the nucleophosmin (NPM) gene on chromosome 5, resulting in a fusion gene that encodes for an 80-kDa NPM-ALK chimeric protein with constitutive tyrosine kinase activity.3,4 Other partner chromosomes have also been identified, all resulting in ALK protein overexpression and sometimes different immunohistochemical staining patterns.5 With antibodies to the ALK protein, ALK expression can be demonstrated in 50% to 85% of all systemic ALCLs.2 Notably, ALK+ ALCL is characterized by a spectrum of cytologic features that allow the recognition of morphologic variants (common, lymphohistiocytic, small cell, and mixed).2 The frequency of ALK expression varies according to the median age of the cohort studied, with higher frequencies observed in the pediatric population, and also depends on the stringency of the pathologic criteria used for the diagnosis.6,7 ALK+ ALCL is clinically distinctive, with patients presenting at a young age and having a prognosis that is superior to those with ALK− ALCL.8-11 Recent gene expression and comparative genomic hybridization (CGH) studies have also established that ALK+ and ALK− tumors have unique gene expression signatures and genomic imbalances, further confirming that they are distinct entities at a molecular and genetic level.12-14 The fourth edition of the WHO classification (in press) distinguishes ALK+ and ALK− of systemic ALCL as separate disease entities (N.L.H., personal communication).
ALK− ALCL is defined in the WHO classification as a lymphoma that is morphologically within the spectrum of ALK+ ALCL, with strong and uniform expression of CD30, but lacking ALK protein expression.15 As morphologic features are often subjective, and there are no unique defining immunophenotypic or genetic features, ALK− ALCL can be a difficult diagnosis. In addition, at least one study reported that the prognosis of ALK− ALCL may be similar to that of peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS).16 Thus, it has been suggested that ALK− ALCL is simply a morphologic variant within the otherwise heterogeneous category of PTCL-NOS.15,17
The International Peripheral T-Cell Lymphoma Project was undertaken as a large retrospective study of patients with PTCL from North America, Europe, and Asia with the goal of better characterizing and ultimately understanding this uncommon group of non-Hodgkin lymphomas (NHLs). One objective of the study was to analyze the clinical and immunophenotypic features of the different subtypes of ALCL and compare them with each other and with PTCL-NOS.
Methods
A total of 22 institutions in North America, Europe, and Asia participated in the study (see Appendix). Approval was obtained from the institutional review board (IRB) for this study at the coordinating center (University of Nebraska Medical Center [UNMC]) and at each participating center as per the institutional standard. The patients selected were previously untreated patients aged 19 years or older with de novo PTCL or natural killer (NK)/T-cell lymphoma diagnosed between January 1, 1990, and December 31, 2002. Patients with mycosis fungoides, lymphomatoid papulosis, and Sézary syndrome were excluded. The patients were consecutive from each institution and were required to have adequate tissue biopsies for diagnosis and classification. Patients with only needle aspiration cytology specimens were excluded. At each institution, the local pathologist reviewed the diagnostic pathology slides and reports for each patient, and recorded the results of local immunophenotypic, cytogenetic, and molecular studies that had been performed in the initial diagnosis of the patient on a standard datasheet. The local pathologist also selected representative slides and a formalin-fixed tissue block from each patient to submit for regional review and more detailed immunophenotyping. Patients in which the tissue blocks were exhausted or no longer available for study were also acceptable if the slides and immunostains or flow cytometry data were available for review and adequate for diagnosis and classification. Clinical characteristics of the patients, including treatment data and follow-up information, were also required. The 22 local sites, which provided a total of 1314 patients, are shown in Table 1.
Study sites and number of cases by region
| Study sites . | Patients, no. (%) . |
|---|---|
| North America | 332 (25.3) |
| British Columbia Cancer Agency—Vancouver | |
| National Cancer Institute—Bethesda | |
| University of Nebraska Medical Center—Omaha | |
| Massachusetts General Hospital—Boston | |
| University of Southern California—Los Angeles | |
| Arizona Cancer Center—Tucson | |
| Europe | 450 (34.2) |
| University of Barcelona Hospital—Barcelona | |
| Spanish National Cancer Center—Madrid | |
| Norwegian Radium Hospital—Oslo | |
| University of Würzburg Hospital—Würzburg | |
| St. Bartholomew's Hospital—London | |
| University of Bologna Hospital—Bologna | |
| University of Modena Hospital—Modena | |
| Intergruppo Italiano Linfomi—Modena | |
| Centre Hospitalier Lyon-Sud—Lyon | |
| Asia | 532 (40.5) |
| King Chulalongkorn Hospital—Bangkok | |
| Queen Mary Hospital—Hong Kong | |
| Singapore General Hospital—Singapore | |
| National Cancer Center Hospital—Tokyo | |
| Aichi Cancer Center Hospital—Nagoya | |
| Okayama University Hospital—Okayama | |
| Fukuoka University Hospital—Fukuoka | |
| Samsung Medical Center—Seoul |
| Study sites . | Patients, no. (%) . |
|---|---|
| North America | 332 (25.3) |
| British Columbia Cancer Agency—Vancouver | |
| National Cancer Institute—Bethesda | |
| University of Nebraska Medical Center—Omaha | |
| Massachusetts General Hospital—Boston | |
| University of Southern California—Los Angeles | |
| Arizona Cancer Center—Tucson | |
| Europe | 450 (34.2) |
| University of Barcelona Hospital—Barcelona | |
| Spanish National Cancer Center—Madrid | |
| Norwegian Radium Hospital—Oslo | |
| University of Würzburg Hospital—Würzburg | |
| St. Bartholomew's Hospital—London | |
| University of Bologna Hospital—Bologna | |
| University of Modena Hospital—Modena | |
| Intergruppo Italiano Linfomi—Modena | |
| Centre Hospitalier Lyon-Sud—Lyon | |
| Asia | 532 (40.5) |
| King Chulalongkorn Hospital—Bangkok | |
| Queen Mary Hospital—Hong Kong | |
| Singapore General Hospital—Singapore | |
| National Cancer Center Hospital—Tokyo | |
| Aichi Cancer Center Hospital—Nagoya | |
| Okayama University Hospital—Okayama | |
| Fukuoka University Hospital—Fukuoka | |
| Samsung Medical Center—Seoul |
From each institution, the phenotype datasheets, diagnostic slides, and tissue blocks were sent to one of 5 regional centers for review by an expert hematopathologist. A standard panel of immunostains was performed on each patient, including CD20, CD2, CD3, CD4, CD5, CD8, CD30, CD56, TCR-β, TIA-1, Ki67, and in situ hybridization for Epstein-Barr virus–encoded RNAs (EBERs). Other immunostains, polymerase chain reaction (PCR) analyses, and fluorescence in situ hybridization (FISH) studies were performed as needed, and all patients were diagnosed according to the criteria of the WHO classification.2 The percentages of transformed tumor cells (blasts), and tumor cells expressing CD30 or Ki67, were estimated in each case, as were the percentages of tumor cells and background cells expressing either CD4 or CD8. The results of these studies and the diagnosis were recorded on standard phenotype and diagnosis datasheets, respectively, for each case.
Panels of 4 expert hematopathologists, drawn from the contributing local sites and regional centers, traveled to the regional centers to review the cases. The composition of the panels differed at the various regional centers. At each center, the diagnostic slides for each case were classified independently by each expert according to the criteria of the WHO classification.2 The initial diagnosis was based upon examination of the hematoxylin-eosin– and/or Giemsa-stained slides, the immunostains, and the phenotype datasheets, but with only limited clinical information from the time of initial diagnosis, including the anatomic biopsy site and site of the largest tumor mass (ie, diagnosis 1). After recording this diagnosis, the expert was presented with the entire clinical datasheet and a second diagnosis was rendered (ie, diagnosis 2). The previous diagnosis could not be changed based on the clinical information subsequently revealed. If a patient was considered unclassifiable, the expert was required to give a reason such as inadequate material, poor slide preparation, additional phenotyping needed, additional information needed, or other reasons. In addition to the independent diagnosis rendered by each of the 4 expert hematopathologists, a consensus diagnosis was also reached for each patient. A consensus diagnosis was considered to have been reached if at least 3 of the 4 experts on the panel agreed on the second diagnosis (diagnosis 2). All patients without a consensus diagnosis and all unclassifiable patients were jointly reviewed using a multiheaded microscope and discussed by the 4 experts in a consensus conference, and an attempt was made to reach a consensus diagnosis. If additional sections, immunostains, molecular or cytogenetic studies, or other information were required, a diagnostic algorithm was developed by the panel and the additional materials or data were obtained, if possible, and reviewed at a subsequent consensus conference at the center. If the additional materials or data could not be obtained during the site visit, the required materials and information were subsequently sent to the expert hematopathologist at the regional center who arbitrated the case based on the algorithm.
For the diagnosis of systemic ALCL, the definition included characteristic morphologic features (large malignant cells with abundant cytoplasm; large, often indented or kidney-shaped nuclei with prominent nucleoli; at least some cells with a paranuclear hof [ie, “hallmark cells”]; and a cohesive growth pattern, often with sinus involvement) together with homogeneous and strong expression of CD30 in a membrane and Golgi pattern. Morphologies other than the common variant were noted. ALK− ALCL resembled the common variant of ALK+ ALCL, except for the absence of ALK-protein expression, and was differentiated from PTCL-NOS by the lack of these features in the latter. Patients with ALK− ALCL had to lack all B-cell antigens, including PAX5, and express at least one T cell–associated antigen or demonstrate a T-cell receptor gene rearrangement. The diagnosis of primary cut-ALCL was based on a combination of typical morphology (ie, infiltrate of large lymphoid cells, strongly positive for CD30 and lack of epidermatropism), absence of ALK expression, and characteristic clinical features, including the presence of de novo cutaneous disease without systemic involvement. Patients with a history of lymphomatoid papulosis were excluded.
The clinical information for each patient was abstracted from the medical record and recorded on a standardized data form for computerized data entry. These data included coded patient and site identifiers, patient sex, ethnicity, age, date and site of the diagnostic biopsy, disease sites, and Ann Arbor stage. Additional data recorded included the site and diameter of the largest tumor, presence of B symptoms, performance status, and baseline laboratory parameters. The initial therapy, response, and details of progression or relapse, survival status, and cause of death were recorded in each case. For some patients, sufficient data were not available for inclusion in some of the clinical or survival analyses.
The International Prognostic Index (IPI)18 was used to stratify patients within the various disease entities, and a new prognostic model for PTCL-NOS (PIT: elevated LDH [lactate dehydrogenase], PS [performance status] ≥ 2, age ≥ 60 years, and bone marrow involvement) was also applied to systemic ALCL.19 Treatment outcome was determined by overall survival (OS) and failure-free survival (FFS). OS was defined as the time from diagnosis to death from any cause, with surviving patient follow-up censored at the last contact date. FFS was defined as the time from diagnosis to the first occurrence of progression, relapse after response, or death from any cause. Follow-up of patients not experiencing any of these events was censored at the date of last contact. Estimates of OS and FFS distributions were calculated using the method of Kaplan and Meier.20 Time-to-event distributions were compared using the log-rank test.21 Clinical and prognostic factor comparisons were performed using the chi-square or Fisher exact test. Multivariate analysis was performed with a Cox proportional hazards regression model using stepwise selection.
Results
Of the 1314 eligible patients submitted, a diagnosis of ALCL was made in 181 patients (13.8%) on consensus review: 159 with systemic ALCL (12.1% of all patients) and 22 with cutaneous ALCL (1.7% of all patients). Of the patients with systemic ALCL, 87 (55%) were ALK+ and 72 (45%) were ALK−. The high proportion of patients with ALK− ALCL likely reflects the exclusion of pediatric patients. All patients with cut-ALCL were ALK−. The median follow-up duration was 3.5 years for ALK+ ALCL, 1.7 years for ALK− ALCL, and 5.2 years for cut-ALCL.
Comparison of systemic ALCL, ALK+ and ALK−
Clinical features.
Patients with ALK+ ALCL were younger than those with ALK− ALCL (median age, 34 years vs 58 years), but both types had a predominance of males (Table 2). Most patients with ALCL had advanced-stage disease (ALK+, 65%; ALK−, 58%) and B symptoms. There was a similar frequency of patients with either nodal-only disease or with multiple (more than one) extranodal sites of involvement. Although not statistically significant, patients with ALK+ ALCL were more likely to have bone, bone marrow, or subcutaneous tissue involvement, and splenic disease (splenomegaly and/or splenic lesions) (Table 3). In contrast, there was a greater proportion of patients with cutaneous, hepatic, or gastrointestinal involvement in the ALK− ALCL group (Table 3). There was a similar distribution of patients across the IPI risk groups in both ALK+ and ALK− ALCL, with a large proportion having a low-risk score of 0 or 1 (ALK+, 41%; ALK−, 45%; Table 2).
Clinical features of ALK+ versus ALK− ALCL, systemic type
| Clinical feature . | ALK+ . | ALK− . | P* . | PTCL-NOS . | P† . |
|---|---|---|---|---|---|
| Total no. patients (%) | 87 (55) | 72 (45) | 331 | ||
| Median age, y | 34 | 58 | < .001 | 57 | .30 |
| Age less than 60 y, no. (%) | 74 (86) | 42 (58) | < .001 | 170 (50) | .21 |
| Male-female ratio | 1.7:1 | 1.5:1 | .74 | 1.9:1 | .41 |
| Stage, no. (%) | |||||
| II | 30 (35) | 30 (42) | .38 | 102 (31) | .18 |
| III | 25 (29) | 15 (21) | 87 (26) | ||
| IV | 31 (36) | 27 (37) | 145 (43) | ||
| Elevated LDH, no. (%) | 31 (37) | 31 (46) | .28 | 158 (49) | .62 |
| Performance status over 2, no. (%) | 30 (35) | 21 (30) | .56 | 60 (18) | .02 |
| Nodal only disease, no. (%) | 39 (54) | 38 (49) | .52 | 124 (42) | .07 |
| Extranodal sites more than 1, no. (%) | 17 (19.5) | 15 (21) | .84 | 99 (29) | .15 |
| Bulky disease more than 10 cm, no. (%) | 17 (21) | 6 (11) | .17 | 19 (7) | .25 |
| B symptoms, no. (%) | 52 (60) | 41 (57) | .72 | 118 (35) | < .001 |
| Hemoglobin less than 110 g/L, no. (%) | 17 (27) | 18 (32) | .54 | 61 (22) | .11 |
| Platelets less than 150 × 109/L, no. (%) | 6 (10) | 6 (11) | .83 | 64 (24) | .03 |
| IPI score, no. (%) | |||||
| 0,1 | 40 (49) | 27 (41) | .50 | 88 (28) | .066 |
| 2 | 18 (22) | 13 (20) | 111 (35) | ||
| 3 | 12 (15) | 16 (24) | 71 (22) | ||
| 4,5 | 12 (14) | 10 (15) | 48 (15) | ||
| 5-y FFS, % | 60 | 36 | .015 | 20 | .012 |
| 5-y OS, % | 70 | 49 | .016 | 32 | .032 |
| 5-y FFS by IPI, % | |||||
| 0,1 | 80 | 62 | 35 | ||
| 2 | 61 | 44 | .002‡ | 16 | < .001‡ |
| 3 | 23 | 16 | 13 | ||
| 4,5 | 25 | 13 | 8 | ||
| 5-y OS by IPI, % | |||||
| 0,1 | 90 | 74 | 52 | ||
| 2 | 68 (P< .001‡) | 62 | < .001‡ | 33 | < .001‡ |
| 3 | 23 | 31 | 16 | ||
| 4,5 | 33 | 13 | 13 | ||
| Clinical feature . | ALK+ . | ALK− . | P* . | PTCL-NOS . | P† . |
|---|---|---|---|---|---|
| Total no. patients (%) | 87 (55) | 72 (45) | 331 | ||
| Median age, y | 34 | 58 | < .001 | 57 | .30 |
| Age less than 60 y, no. (%) | 74 (86) | 42 (58) | < .001 | 170 (50) | .21 |
| Male-female ratio | 1.7:1 | 1.5:1 | .74 | 1.9:1 | .41 |
| Stage, no. (%) | |||||
| II | 30 (35) | 30 (42) | .38 | 102 (31) | .18 |
| III | 25 (29) | 15 (21) | 87 (26) | ||
| IV | 31 (36) | 27 (37) | 145 (43) | ||
| Elevated LDH, no. (%) | 31 (37) | 31 (46) | .28 | 158 (49) | .62 |
| Performance status over 2, no. (%) | 30 (35) | 21 (30) | .56 | 60 (18) | .02 |
| Nodal only disease, no. (%) | 39 (54) | 38 (49) | .52 | 124 (42) | .07 |
| Extranodal sites more than 1, no. (%) | 17 (19.5) | 15 (21) | .84 | 99 (29) | .15 |
| Bulky disease more than 10 cm, no. (%) | 17 (21) | 6 (11) | .17 | 19 (7) | .25 |
| B symptoms, no. (%) | 52 (60) | 41 (57) | .72 | 118 (35) | < .001 |
| Hemoglobin less than 110 g/L, no. (%) | 17 (27) | 18 (32) | .54 | 61 (22) | .11 |
| Platelets less than 150 × 109/L, no. (%) | 6 (10) | 6 (11) | .83 | 64 (24) | .03 |
| IPI score, no. (%) | |||||
| 0,1 | 40 (49) | 27 (41) | .50 | 88 (28) | .066 |
| 2 | 18 (22) | 13 (20) | 111 (35) | ||
| 3 | 12 (15) | 16 (24) | 71 (22) | ||
| 4,5 | 12 (14) | 10 (15) | 48 (15) | ||
| 5-y FFS, % | 60 | 36 | .015 | 20 | .012 |
| 5-y OS, % | 70 | 49 | .016 | 32 | .032 |
| 5-y FFS by IPI, % | |||||
| 0,1 | 80 | 62 | 35 | ||
| 2 | 61 | 44 | .002‡ | 16 | < .001‡ |
| 3 | 23 | 16 | 13 | ||
| 4,5 | 25 | 13 | 8 | ||
| 5-y OS by IPI, % | |||||
| 0,1 | 90 | 74 | 52 | ||
| 2 | 68 (P< .001‡) | 62 | < .001‡ | 33 | < .001‡ |
| 3 | 23 | 31 | 16 | ||
| 4,5 | 33 | 13 | 13 | ||
ALK+ versus ALK−.
ALK− versus PTCL-NOS.
Comparison of IPI risk groups within specified subtype.
Extranodal sites of involvement in ALK+ and ALK− ALCL and PTCL-NOS
| Extranodal site . | ALK+, no. (%) . | ALK−, no. (%) . | PTCL-NOS, no. (%) . |
|---|---|---|---|
| Bone marrow | 10 (12) | 5 (7) | 72 (21)* |
| Peripheral blood (circulating tumor cells) | 2 (4) | 2 (3) | |
| Bone | 12 (14) | 5 (7) | 7 (2)* |
| Epidural | 2 (2) | 0 | 1 (.3) |
| Subcutaneous tissue | 9 (10) | 2 (3) | 21 (6) |
| Skin | 7 (8) | 12 (17) | 55 (16) |
| Liver | 3 (3) | 7 (10) | 39 (12) |
| Lung | 7 (8) | 9 (13) | 27 (8) |
| Spleen | 9 (10) | 2 (3) | 51 (15)* |
| Central nervous system | 1 (1) | 0 | 2 (.6) |
| Gastrointestinal | |||
| Stomach | 0 | 2 (3) | 10 (3) |
| Small intestine | 2 (2) | 2 (3) | 6 (2) |
| Large intestine | 0 | 0 | 5 (2) |
| Pleural effusion | 3 (3) | 4 (6) | 7 (2) |
| Pericardial effusion | 0 | 1 (1) | 1 (.3) |
| Ovary | 1 (1) | 0 | 0 |
| Breast | 1 (1) | 1 (1) | 1 (3) |
| Paranasal | 0 | 1 (1) | 3 (1) |
| Extranodal site . | ALK+, no. (%) . | ALK−, no. (%) . | PTCL-NOS, no. (%) . |
|---|---|---|---|
| Bone marrow | 10 (12) | 5 (7) | 72 (21)* |
| Peripheral blood (circulating tumor cells) | 2 (4) | 2 (3) | |
| Bone | 12 (14) | 5 (7) | 7 (2)* |
| Epidural | 2 (2) | 0 | 1 (.3) |
| Subcutaneous tissue | 9 (10) | 2 (3) | 21 (6) |
| Skin | 7 (8) | 12 (17) | 55 (16) |
| Liver | 3 (3) | 7 (10) | 39 (12) |
| Lung | 7 (8) | 9 (13) | 27 (8) |
| Spleen | 9 (10) | 2 (3) | 51 (15)* |
| Central nervous system | 1 (1) | 0 | 2 (.6) |
| Gastrointestinal | |||
| Stomach | 0 | 2 (3) | 10 (3) |
| Small intestine | 2 (2) | 2 (3) | 6 (2) |
| Large intestine | 0 | 0 | 5 (2) |
| Pleural effusion | 3 (3) | 4 (6) | 7 (2) |
| Pericardial effusion | 0 | 1 (1) | 1 (.3) |
| Ovary | 1 (1) | 0 | 0 |
| Breast | 1 (1) | 1 (1) | 1 (3) |
| Paranasal | 0 | 1 (1) | 3 (1) |
Statistical significance versus ALK− ALCL.
Pathologic features.
By definition, patients with ALCL had large neoplastic cells with a cohesive growth pattern. The cells had abundant cytoplasm and indented, kidney-shaped, or pleomorphic nuclei, and uniform strong expression of CD30 in a membrane and Golgi pattern. Among ALK+ ALCL, 4 patients were diagnosed with the small-cell variant, and 4 patients were diagnosed with the lymphohistiocytic variant. A greater proportion of ALK− ALCL tumors were CD2+ (59% vs 23%, P < .001) and CD3+ (45% vs 12%; P < .001), and ALK+ ALCL tumors were more often EMA+ (epithelial membrane antigen; 83% vs 43%; P < .001), consistent with a prior report16 (Table 4). Cytotoxic protein expression (TIA1, granzyme B, or perforin) was more common in ALK+ ALCL, but the difference from ALK− ALCL was not statistically significant (Table 4).
Immunophenotypic features of ALK+ and ALK− ALCL, and PTCL-NOS
| Immunophenotype . | ALK+, % . | ALK−, % . | P* . | PTCL-NOS, % . | P† . | PTCL-NOS CD30+ > 80%, % . | P‡ . |
|---|---|---|---|---|---|---|---|
| CD30 | 100 | 100 | — | 32 | < .001 | 100 | — |
| CD2 | 23 | 59 | < .001 | 86 | < .001 | 73 | .38 |
| CD3 | 12 | 45 | < .001 | 93 | < .001 | 80 | .02 |
| CD4 | 40 | 35 | .44 | 56 | .002 | 27 | .70 |
| CD8 | 5 | 10 | .34 | 19 | .21 | 20 | .37 |
| TIA1, granzyme B, or perforin | 80 | 66 | .09 | 32 | < .001 | 27 | < .009 |
| EMA | 83 | 43 | < .001 | 3 | < .001 | 0 | .008 |
| CD56 | 7 | 4 | .32 | 6 | .56 | 0 | .80 |
| CD43 | 44 | 50 | 1.0 | 93 | .003 | 80 | .23 |
| Immunophenotype . | ALK+, % . | ALK−, % . | P* . | PTCL-NOS, % . | P† . | PTCL-NOS CD30+ > 80%, % . | P‡ . |
|---|---|---|---|---|---|---|---|
| CD30 | 100 | 100 | — | 32 | < .001 | 100 | — |
| CD2 | 23 | 59 | < .001 | 86 | < .001 | 73 | .38 |
| CD3 | 12 | 45 | < .001 | 93 | < .001 | 80 | .02 |
| CD4 | 40 | 35 | .44 | 56 | .002 | 27 | .70 |
| CD8 | 5 | 10 | .34 | 19 | .21 | 20 | .37 |
| TIA1, granzyme B, or perforin | 80 | 66 | .09 | 32 | < .001 | 27 | < .009 |
| EMA | 83 | 43 | < .001 | 3 | < .001 | 0 | .008 |
| CD56 | 7 | 4 | .32 | 6 | .56 | 0 | .80 |
| CD43 | 44 | 50 | 1.0 | 93 | .003 | 80 | .23 |
Information missing: granzyme B, 60% ALK−; 75% ALK+; and 80% PTCL-NOS; perforin, 72% ALK−; 95% ALK+; and 98% PTCL-NOS; CD43, 81% ALK−; 82% ALK+; and 92% PTCL-NOS; EMA, 41% ALK−; 45% ALK+; and 91% PTCL-NOS.
ALK+ versus ALK−.
ALK− versus PTCL-NOS.
ALK− versus PTCL-NOS more than 80% CD30+ cells.
Response to treatment and survival.
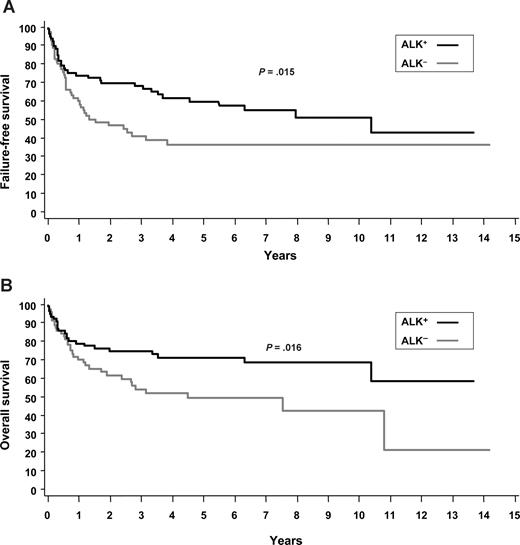
Most patients received multiagent chemotherapy (ALK+, 95%; ALK−, 93%) and, typically, an anthracycline-based regimen was used (ALK+, 95%; ALK−, 88%). A total of 5 patients with ALK− ALCL and 6 patients with ALK+ ALCL underwent high-dose chemotherapy with stem cell support as part of their primary therapy. The overall response rate to primary treatment was 76% in ALK− ALCL and 88% in ALK+ ALCL. The 5-year OS exceeded FFS in both ALK+ (70% vs 60%) and ALK− ALCL (49% vs 36%), suggesting that salvage therapies were effective in some patients (Table 2). Consistent with prior studies, the FFS (P = .015) and OS (P = .016) favored ALK+ ALCL (Figure 1; Table 2). However, patients with ALK+ ALCL were younger than those with ALK− ALCL (Table 1) and, if the comparison of ALK− and ALK+ patients is limited to those 40 years of age and older (n = 64 and n = 30, respectively) or younger than 40 years (n = 7 and n = 55, respectively), there was no difference in FFS or OS (results not shown), suggesting that age is a prominent factor driving outcome differences.
Survival systemic ALCL. (A) FFS of ALK+ and ALK− ALCL. (B) OS of ALK+ and ALK− ALCL.
Survival systemic ALCL. (A) FFS of ALK+ and ALK− ALCL. (B) OS of ALK+ and ALK− ALCL.
Prognostic factors.
Clinical and laboratory features were tested in univariate analysis for their impact on FFS and OS (Table 5). Poor performance status, high stage, and elevated LDH were poor prognostic factors in both ALK+ and ALK− ALCL. In contrast, increased age, multiple extranodal sites of involvement, and anemia (hemoglobin < 110 g/L) were poor prognostic factors only in ALK+ ALCL. Patients with ALK+ and ALK− ALCL with stage III disease had a more favorable outcome than those with stage IV disease (Table 5), suggesting that those with advanced stage still confined to nodal sites had a better prognosis. This was particularly evident for ALK+ ALCL, in which only the stage IV patients had a poor outcome (stage I/II or III vs stage IV: 5-year FFS, 70% vs 42%; P = .043; 5-year OS of 85% vs 37%; P = .003; survival figures not shown).
Prognostic factors in univariate analysis that predicted FFS or OS in ALK+ and ALK− ALCL
| Clinical features . | ALK+ . | ALK− . | ||
|---|---|---|---|---|
| OS . | FFS . | OS . | FFS . | |
| Age over 60 y | < .001 | .025 | .14 | .50 |
| Male sex | .80 | .69 | .43 | .73 |
| PS more than 2 | .01 | .003 | < .001 | < .001 |
| Stage* | .003 | .04 | .04 | .016 |
| Elevated LDH | .006 | .02 | .003 | .007 |
| Extranodal sites more than 1 | < .001 | < .001 | .06 | .096 |
| Bulky disease more than 10 cm | .72 | .30 | .33 | .73 |
| B symptoms | .18 | .47 | .14 | .04 |
| Hemoglobin less than 110 × g/L | .04 | < .001 | .50 | .60 |
| Platelets less than 150 × 109/L | .46 | .71 | .21 | .09 |
| Clinical features . | ALK+ . | ALK− . | ||
|---|---|---|---|---|
| OS . | FFS . | OS . | FFS . | |
| Age over 60 y | < .001 | .025 | .14 | .50 |
| Male sex | .80 | .69 | .43 | .73 |
| PS more than 2 | .01 | .003 | < .001 | < .001 |
| Stage* | .003 | .04 | .04 | .016 |
| Elevated LDH | .006 | .02 | .003 | .007 |
| Extranodal sites more than 1 | < .001 | < .001 | .06 | .096 |
| Bulky disease more than 10 cm | .72 | .30 | .33 | .73 |
| B symptoms | .18 | .47 | .14 | .04 |
| Hemoglobin less than 110 × g/L | .04 | < .001 | .50 | .60 |
| Platelets less than 150 × 109/L | .46 | .71 | .21 | .09 |
Stage I/II versus III versus IV.
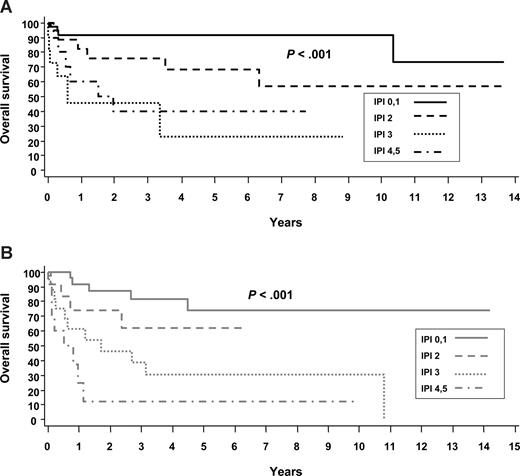
The IPI effectively identified risk groups with different prognoses within ALK+ and ALK− ALCL, although those with an IPI score of 3 or more fell into the poor-risk category regardless of ALK status (Table 2; Figure 2). The new T-cell prognostic index developed for PTCL-NOS (PIT)19 was also applied to ALK+ and ALK− ALCL and was similarly predictive of FFS and OS in both groups (results not shown). Given that the distribution of patients across the risk groups is very similar with the 2 prognostic models (eg, ALK+ ALCL: PIT, 0 risk factors [RFs], 42%; 1 RF, 2 factors, 16%; 3 or 4 RFs, 12%; ALK− ALCL: PIT, 0 RFs, 26%; 1 RF, 38%; 2 RFs, 21%; 3 or 4 RFs, 15%; Table 2) and that there is a low frequency of bone marrow involvement observed in ALCL (Table 3), the PIT mirrors the IPI in this patient population.
Overall survivals of systemic ALCL by the IPI. (A) ALK+ ALCL. (B) ALK− ALCL.
A number of biologic factors were also tested in ALK− and ALK+ ALCL for their impact on prognosis, including CD8+ background T cells of 10% and greater or 20%, and CD56 expression by the tumor cells, but none reached statistical significance (results not shown). However, tissue was not available for analysis in all patients, and the frequency of positive patients for these parameters was low.
In multivariate analysis, controlling for the IPI, anemia (hemoglobin < 110 g/L) remained a significant predictor of FFS (hazard ratio [HR], 4.03; P = .007), but not OS (P = .09), in ALK+ ALCL. In ALK− ALCL, after controlling for the IPI, no other factors remained in the model.
Comparison of ALK− ALCL and PTCL-NOS
A prior report suggested that patients with ALK− ALCL and PTCL-NOS have similar outcomes.16 Therefore, we compared the presenting clinical and pathologic features and the survival of these 2 subtypes. All morphologic variants of PTCL-NOS were included. A detailed analysis of the patients with PTCL-NOS will be reported in a separate publication.
There was a greater proportion of patients with a poor performance status (30% vs 18%; P = .02) and B symptoms (57% vs 35%; P < .001) in the ALK− ALCL group compared with the PTCL-NOS group (Table 2). Bone marrow disease (22% vs 7%; P = .004; Table 3), splenic involvement (15 vs 3%; P = .003; Table 3), and thrombocytopenia (24% vs 11%; P = .032; Table 2) were more frequent in patients with PTCL-NOS.
PTCL-NOS tumors were also different phenotypically from ALK− ALCL. They were more frequently CD2+, CD3+, CD4+, and CD43+, and less often expressed EMA or cytotoxic proteins than ALK− ALCL (Table 4). ALK− ALCL tumors were more likely to be positive for cytotoxic markers (P < .001; Table 4). One-third of patients with PTCL-NOS had some CD30+ cells (32%), and 15 (4.5%) patients had 80% or more CD30+ cells. Comparison of ALK− ALCL to patients with PTCL-NOS with strong CD30 expression (≥ 80% of cells), demonstrated findings comparable with those above, although the frequency of CD4+ patients was lower in this subgroup (Table 4).
Contrary to a prior report,16 the 5-year FFS (36% vs 20%; P = .012; Figure 3A; Table 2) and OS (49% vs 32%; P = .032; Figure 3B; Table 2) were superior in ALK− ALCL compared with PTCL-NOS. Similar results were obtained when the survival analysis was restricted to only patients who received combination chemotherapy with curative intent (5-y FFS, 39% vs 20%; P = .011; 5-year OS, 51% vs 32%; P = .028; survival figures not shown). Excluding the rare morphologic variants of PTCL-NOS, such as the lymphoepithelioid (n = 28), T-zone variant (n = 5), and parafollicular variant (n = 5), further highlighted the outcome differences between PTCL-NOS and ALK− ALCL (PTCL-NOS excluding variants: 5-year FFS, 18%; P = .003; 5-year OS, 29%; P = .009). Furthermore, confining the analysis to cases of PTCL-NOS with high CD30 expression (≥ 80% of cells), a group that can be difficult to differentiate histologically from ALK− ALCL, magnified the difference in 5-year FFS (PTCL-NOS CD30+ ≥ 80%, 9%; P < .001; Figure 3C) and 5-year OS (PTCL-NOS CD30+ ≥ 80%, 19%; P = .008; Figure 3D) compared with ALK− ALCL. A multivariate analysis including only those patients treated with combination chemotherapy, controlling for the IPI, demonstrated that histologic subtype (ie, ALK− ALCL vs PTCL-NOS) remained a significant predictor of FFS (P = .03; PTCL-NOS HR, 1.48) with a similar trend for overall survival (P = .07).
Survival of ALK− ALCL and PTCL-NOS. (A) FFS of ALK− ALCL and PTCL-NOS. (B) OS of ALK− ALCL and PTCL-NOS. (C) FFS of ALK− ALCL and PTCL-NOS (CD30+ ≥ 80% cells). (D) OS of ALK− ALCL and PTCL-NOS (CD30+ ≥ 80% cells).
Survival of ALK− ALCL and PTCL-NOS. (A) FFS of ALK− ALCL and PTCL-NOS. (B) OS of ALK− ALCL and PTCL-NOS. (C) FFS of ALK− ALCL and PTCL-NOS (CD30+ ≥ 80% cells). (D) OS of ALK− ALCL and PTCL-NOS (CD30+ ≥ 80% cells).
Cutaneous ALCL
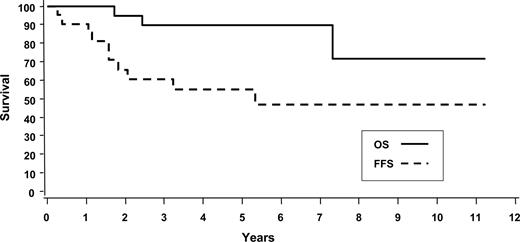
There were 22 patients with primary cutaneous ALCL, representing only 1.7% of all patients submitted for the project. This proportion likely underrepresents the true frequency of this entity since, in some centers, these patients are referred to dermatologists and dermatopathologists. As expected, our patients were typically middle-aged men (64%; median age, 55 years) with a good performance status (0,1: 95%), who had predominantly localized disease (stage IE or 2E: 86%). All patients were strongly positive for CD30. There was high expression of T-cell markers such as CD2 (76%) and CD3 (64%), and EMA expression was expectedly low (20%). The 5-year OS and FFS were 90% and 55%, respectively (Figure 4). A total of 13 patients (65%) received an anthracycline-based chemotherapy regimen as part of their primary treatment. Comparing these patients with all others (6 received radiotherapy alone; 2 had no further therapy; 1 had single-agent chemotherapy) demonstrated no difference in 5-year FFS (P = .99) or OS (P = .77; survival figures not shown).
Discussion
In the WHO classification,2 ALCL is divided into cutaneous and systemic types, with the latter further delineated by the presence or absence of ALK protein expression. The presence of ALK protein defines a group with an excellent prognosis when treated with standard chemotherapy.8-10,22 Our results confirm the distinctive clinical features of ALK+ ALCL, because our patients were younger and had a more favorable prognosis compared with ALK− ALCL or PTCL-NOS. However, this favorable prognosis may be largely dictated by the younger age at presentation, as we found no outcome differences when the analysis was limited to ALCL patients aged 40 years and older. Interestingly, patients with stage III disease had a better outcome compared with those with stage IV disease in ALK+ ALCL, a feature further highlighted by the prognostic importance of multiple extranodal sites of involvement. Prior studies have suggested that extranodal disease is more prevalent in ALK+ ALCL.8,16 However, in the present study, the frequency was similar in both types, although there were some differences in the extranodal sites involved. Bone marrow, bone, subcutaneous tissue, and splenic involvement was seen at a higher frequency in ALK+ patients, whereas skin, liver, and gastrointestinal involvement was more frequent in ALK− ALCL. This is in contrast to a prior report in which all extranodal sites were observed at a higher frequency in ALK− ALCL.8 Immunophenotypically, CD2 and CD3 expression was more common in ALK− ALCL. These clinical and pathologic differences, along with studies showing molecular and genetic differences,12-14 support the separation of ALK− ALCL and ALK+ ALCL.
The IPI is a widely used prognostic tool for diffuse large B-cell lymphoma that was developed and validated prior to the recognition of systemic ALCL as defined today. However, several small retrospective studies have suggested that the IPI may be useful for risk stratification of patients with systemic ALCL.8,16 We found that the IPI was very effective in defining different risk categories in both patients with ALK− and ALK+ ALCL. Importantly, 39% of our patients with ALK+ ALCL had an IPI score of 3 or more and a 5-year FFS of only 25% to 30%. Recently, a new prognostic model (PIT), which includes some of the IPI risk factors in addition to bone marrow involvement, was proposed for PTCL-NOS.19 Not surprisingly, it also identified different risk categories within ALCL. However, given the low frequency of bone marrow involvement in both ALK+ and ALK− ALCL, it essentially gives the same information already provided by the IPI. Similar to Hodgkin lymphoma, we also found that the presence of stage IV disease and anemia are of particular prognostic importance in patients with ALK+ ALCL.23 Thus, in systemic ALCL, both ALK expression and clinical factors, including the IPI, must be considered to estimate prognosis and guide treatment strategies.
In contrast to ALK+ ALCL, the nature of ALK− ALCL has been more difficult to delineate due, in part, to the absence of uniformly applied criteria to define this entity. It has been argued that, because ALK− ALCL lacks distinctive immunophenotypic features and appears to have a prognosis similar to PTCL-NOS, it should be considered a subtype of PTCL-NOS.15,16 However, our comparison of ALK− ALCL with PTCL-NOS revealed some important differences that justify placing it in a category separate from PTCL-NOS to permit further study. Pathologically, in addition to its distinctive morphology, ALK− ALCL was always CD30+, more frequently cytotoxic marker–positive and EMA+, and was less likely to express various T-cell markers (CD2, CD3, CD4, CD43) than PTCL-NOS. Clinically, patients with PTCL-NOS were more likely to have bone marrow or splenic involvement and thrombocytopenia, although they had a surprisingly better performance status and fewer B symptoms. Furthermore, we found that, in contrast to a prior study,16 patients with ALK− ALCL had a more favorable prognosis than those with PTCL-NOS. The 5-year FFS (36%) and OS (49%) of ALK− ALCL in our study is comparable with prior estimates (5-year FFS, 45%; 5-year OS, 40%-45%).9,16,22 Moreover, the distribution of patients across the IPI groups is similar to a prior study.16 Thus, the lack of statistical differences in prior reports may have been the result of small patient numbers. The survival of patients with PTCL-NOS who have strong expression of CD30 (≥ 80% + cells) was extremely poor (5-year FFS, 9%; 5-year OS, 19%) compared with ALK− ALCL, highlighting the importance of both morphology and immunophenotype to diagnose ALK− ALCL. Other studies have also found that differences exist between these histologic subtypes at the molecular and biologic level. ZAP70, a key molecule in T-cell receptor signaling, is almost always expressed in PTCL-NOS (92%), but expression is rare in ALK-neg ALCL (7%), consistent with the lack of T-cell receptor β expression on the cell surface in ALCL.24 Comparative genomic hybridization also supports the idea that PTCL-NOS and ALK− ALCL are genetically distinct because losses of chromosomes 5q and 9p are observed exclusively in PTCL-NOS.13,14 Given that PTCL-NOS is clearly a biologically heterogenous disease, including ALK− ALCL could be considered a step backward. Taken together, these results support the notion that ALK− ALCL should be distinguished from PTCL-NOS based on pathologic, clinical, and prognostic differences. Given the absence of unique immunophenotypic or genetic markers, the diagnosis of ALK− ALCL requires both characteristic morphology and strong, homogenous CD30 expression. Expression of cytotoxic granule proteins is an important finding and should be tested in all patients in whom this diagnosis is considered. In addition, because of the morphologic overlap between these patients and lymphocyte-depleted variants of classical Hodgkin lymphoma, absence of B-cell antigen expression (particularly Pax5) should be required for the diagnosis.
The small group of patients with cut-ALCL had the expected clinical features of predominantly male patients with localized disease. Consistent with prior studies,25 the 5-year OS was excellent (90%); however, there was a propensity for cutaneous relapse, with a 5-year FFS of only 55%. A proportion of patients with cut-ALCL received anthracycline-based combination chemotherapy but had no better FFS or OS compared with all other patients, most of whom received radiotherapy alone. Thus, aggressive multiagent chemotherapy does not appear to affect the natural history of the disease and should generally be avoided in cut-ALCL, as also suggested in other studies.25
In summary, ALK− ALCL should be distinguished from both ALK+ ALCL and PTCL-NOS. Given the absence of unique immunophenotypic or genetic markers, the diagnosis of ALK− ALCL requires both characteristic morphology and strong, homogenous CD30 expression. Although the outcome of ALK− ALCL appears to be better than PTCL-NOS, it is still poor and new strategies are needed to improve cure rates. Finally, patients with cut-ALCL have an indolent clinical course with no apparent benefit from multiagent chemotherapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.J.S. analyzed and interpreted the data and wrote the manuscript; D.D.W. designed the research, collected the data, participated in the pathology review, analyzed and interpreted the data, and wrote the manuscript; J.M.V. designed the research and collected, analyzed, and interpreted the data; J.O.A. designed the research and analyzed and interpreted the data; F.U. did the statistical analysis and analyzed the data; N.L.H., E.S.J., S.A.P., M.C., R.D.G., and L.R. participated in the pathology review and analyzed and interpreted the data; and J.M.C. analyzed and interpreted the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kerry J. Savage, British Columbia Cancer Agency, 600 West 10th Ave, Vancouver, BC, V5Z 4E6 Canada; e-mail: ksavage@bccancer.bc.ca.
Appendix
Participating sites and physicians in the International Peripheral T-Cell Lymphoma Project: British Columbia Cancer Agency, Vancouver, BC: Kerry Savage, MD; Joseph Connors, MD; Randy Gascoyne, MD; Mukesh Chhanabhai, MD; National Cancer Institute, Bethesda, MD: Wyndham Wilson, MD; Elaine Jaffe, MD; University of Nebraska Medical Center, Omaha, NE: James Armitage, MD; Julie Vose, MD; Dennis Weisenburger, MD; James Anderson, PhD; Fred Ullrich, MS; Martin Bast, BS; Massachusetts General Hospital, Boston, MA: Ephraim Hochberg, MD; Nancy Harris, MD; Los Angeles County Hospital–University of Southern California, Los Angeles, CA: Alexandra Levine, MD; Bharat Nathwani, MD; Arizona Cancer Center, Tucson, AZ: Thomas Miller, MD; Lisa Rimsza, MD; University of Barcelona Hospital, Barcelona, Spain: Emili Montserrat, MD; Armando Lopez-Guillermo, MD; Elias Campo, MD; Spanish National Cancer Center, Madrid, Spain; Marta Cuadros, MD; Javier Alvarez Ferreira, MD; Beatriz Martinez Delgado, MD; Norwegian Radium Hospital, Oslo, Norway: Harold Holte, MD; Jan Delabie, MD; University of Würzburg Hospital, Würzburg, Germany: Thomas Rüdiger, MD; Konrad Müller-Hermelink, MD; Peter Reimer, MD; Patrick Adam, MD; Nurnberg, Germany: Martin Wilhelm, MD; Hamburg, Germany: Norbert Schmitz, MD; Munich, Germany: Christoph Nerl, MD; Saint Bartholomew's Hospital, London, United Kingdom: Andrew Lister, MD; Andrew Norton, MD; St James Hospital, Leeds, United Kingdom: Kenneth A. MacLennan, MD; University of Bologna Hospital, Bologna, Italy: Pier Luigi Zinzani, MD; Stefano Pileri, MD; Intergruppo Italiano Linfomi and the University of Modena Hospital, Modena, Italy: Massimo Federico, MD; Monica Bellei, PhD; Centre Hospitalier Lyon-Sud, Lyon, France: Bertrand Coiffier, MD; Francoise Berger, MD; King Chulalongkorn Hospital, Bangkok, Thailand: Intragumtornchai Tanin, MD; Pongsak Wannakrairot, MD; Queen Mary Hospital, Hong Kong, China: Wing Au, MD; Raymond Liang, MD; Florence Loong, MD; Singapore General Hospital, Singapore: Sandeep Rajan, MD; Ivy Sng, MD; National Cancer Center Hospital of Japan, Tokyo, Japan: Kensei Tobinai, MD; Yoshihiro Matsuno, MD; Aichi Cancer Center, Nagoya, Japan: Yasuo Morishima, MD; Shigeo Nakamura, MD; Masao Seto, MD, PhD; Okayama University Hospital, Okayama, Japan: Mitsune Tanimoto, MD; Tadashi Yoshino, MD; Fukuoka University Hospital, Fukuoka, Japan: Junji Suzumiya, MD; Koichi Ohshima, MD; Samsung Medical Center, Seoul, Korea: Won-Seog Kim, MD; Young-Hyeh Ko, MD.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal