Abstract
Ofatumumab is a unique monoclonal antibody that targets a distinct small loop epitope on the CD20 molecule. Preclinical data show that ofatumumab is active against B-cell lymphoma/chronic lymphocytic leukemia cells with low CD20-antigen density and high expression of complement inhibitory molecules. In a phase 1/2 trial evaluating safety and efficacy of ofatumumab in relapsed or refractory follicular non-Hodgkin lymphoma (FL) grade 1 or 2, 4 dose groups of 10 patients received 4 weekly infusions of 300, 500, 700, or 1000 mg. Patients had a median of 2 prior FL therapies and 13% had elevated lactate dehydrogenase. No safety concerns or maximum tolerated dose was identified. A total of 274 adverse events were reported; 190 were judged related to ofatumumab, most occurring on the first infusion day with Common Terminology Criteria grade 1 or 2. Eight related events were grade 3. Treatment caused immediate and profound B-cell depletion, and 65% of patients reverted to negative BCL2 status. Clinical response rates ranged from 20% to 63%. Median time to progression for all patients/responders was 8.8/32.6 months, and median duration of response was 29.9 months at a median/maximum follow-up of 9.2/38.6 months. Ofatumumab is currently being evaluated in patients with rituximab-refractory FL. This trial was registered at www.clinicaltrials.gov as #NCT00092274.
Introduction
The successful use of monoclonal antibodies (mAb's) in the treatment of human disease has steadily been growing the last decade. Rituximab, a human-mouse chimeric anti-CD20 antibody, was approved in 1997 as the first mAb for antilymphoma therapy. It was introduced as monotherapy and is now most commonly used in combination with chemotherapy for first and subsequent lines of therapy in follicular non-Hodgkin lymphoma (FL) followed by maintenance antibody treatment.1-10 However, a subgroup of patients does not respond, and in patients with initial response early relapses occur, indicating rituximab resistance. This indicates a clear unmet need to explore alternative antibodies non-cross resistant to rituximab, such as ofatumumab (HuMax-CD20; Genmab, Copenhagen, Denmark), which is a fully human CD20 mAb with a potential low incidence of human-antihuman antibody (HAHA) formation.
Ofatumumab targets a novel epitope of the CD20 molecule on B cells and releases only very slowly from the target compared with rituximab. The antibody is generated via transgenic mouse and hybridoma technology and produced in a recombinant murine cell line (NS0) using standard mammalian cell cultivation and purification technologies.11 Compared with rituximab, ofatumumab has similar antibody-dependent cellular cytotoxocity but delivers stronger complement-dependent cytotoxicity even to lymphoma cells with a low CD20 antigen density and a high number of CD55 and CD59 complement inhibitory molecules present in the cell membrane. In laboratory tests, ofatumumab was found to be superior to rituximab with respect to its ability to induce lysis in different B-cell lines (eg, SU-DHL-4, Daudi, and Raji) and to kill fresh B-chronic lymphocytic leukemia (B-CLL) cells resistant to rituximab.11,12 In animal studies, ofatumumab has been shown to deplete B cells effectively. The depletion of B cells from peripheral blood and lymph nodes of cynomologus monkeys lasted longer than the depletion induced by rituximab.13 Ofatumumab has also proved therapeutic against Daudi cell growth in an in vivo mouse xenograft model with maximum effect at 0.5 mg/kg intraperitoneally.14 These data project that ofatumumab has the potential to treat B-cell malignancies with low CD20 and high CD55 and CD59 expression, such as B-CLL and rituximab-refractory FL.
This article presents results from a phase 1/2 clinical trial with single-agent ofatumumab in relapsed or refractory FL in a dose-escalating manner, evaluating safety, efficacy, and pharmacokinetics (PK).
Methods
Objectives
The purpose of this open-label, multicenter, phase 1/2 trial was to assess the safety, tolerability, and efficacy of escalating doses of ofatumumab in patients with relapsed or refractory FL grade 1 or 2. PK evaluation was included as a secondary objective.
Patient population
The patients enrolled in the trial were 18 years of age or older with relapsed or treatment-refractory FL grade 1 or 2, defined according to the World Health Organization lymphoma classification. Patients should have progressed after completion of the previous FL treatment, which had induced complete response (CR), complete response unconfirmed (CRu), or partial response (PR) for at least 3 months, or be refractory to any FL treatment for at least 6 months. Prestudy eligibility criteria also included verification of an excisional lymph node biopsy (original or new) to be CD20+ FL by central pathology review (BARC, Ghent, Belgium) and with computerized tomography (CT) showing 2 or more clearly demarcated lesions with a largest diameter more than or equal to 1.5 cm, or one clearly demarcated lesion with a largest diameter more than or equal to 2.0 cm. No lymphoma treatment was permitted up to 4 weeks before recruitment. The exclusion criteria included suspicion of transformation to aggressive lymphoma, unless a new biopsy confirmed FL without transformation, more than 10 × 109/L circulating CD20+ lymphoma cells, platelets < 75 × 109/L, neutrophils less than 1.5 × 109/L, serum creatinine more than 1.5 times upper normal limit (unless normal creatinine clearance), total bilirubin more than 1.5 times upper normal limit (unless resulting from liver involvement of lymphoma), alanine aminotransferase or alkaline phosphatase more than 2.5 times upper normal limit (unless resulting from liver involvement of lymphoma), known or suspected central nervous system lymphoma, past or current malignant disease (except for cervical carcinoma stage 1B or less, noninvasive basal cell and squamous cell skin carcinoma, breast cancer with CR > 10 years, malignant melanoma with CR > 10 years, other cancer diagnosis with CR for > 5 years), severe uncontrolled, nonmalignant disease (eg, cardiac, pulmonary, neurologic disease), previous treatment with rituximab resulting in less than partial response or duration of response less than 6 months, treatment with rituximab within 6 months before inclusion, more than 2 previous treatment cycles with rituximab regardless of response, prior autologous or allogeneic stem cell transplantation, and previous radioimmunotherapy.
Ethical approval was obtained from the independent Ethics Committees and Institutional Review Boards of each site before trial initiation. All patients gave their written informed consent before enrollment.
The trial was performed in accordance with the Declaration of Helsinki and good clinical practice and was monitored by an independent Data Monitoring Committee.
Ofatumumab antibody
Ofatumumab is a fully human IgG1 kappa anti-CD20 mAb produced with a recombinant murine cell line (NS0) using standard mammalian cell cultivation and purification technologies. The NS0 cell line has been transfected with a gene search vector carrying the antibody genes derived from the human anti-CD20 hybridoma cell line (2F2) generated via transgenic mouse technology.11 The molecular weight of the antibody is approximately 149 kDa as determined by mass spectrometry. Ofatumumab, formulated at 20 mg/mL in an isotonic buffer of 30 mM of sodium citrate and 100 mM sodium chloride adjusted to a pH 6.5 with citric acid, was administered intravenously after dilution in a sterile and pyrogen-free saline solution. Ofatumumab was supplied by Genmab.
Treatment plan
Patients were divided into 4 dose groups receiving 4 weekly intravenous infusions of 300 mg, 500 mg, 700 mg, or 1000 mg of ofatumumab in 500 mL normal sterile saline. The 700-mg dose reflects the rituximab dose of 375 mg/m2. In addition, 2 lower doses and 1 higher dose were included. A fixed-dose regimen was chosen as a great variability of serum concentration was seen in the pivotal study for rituximab dosed per square meter.15 Ten patients in succession were enrolled at each dose level.
Ofatumumab was administered as 4 weekly intravenous infusions by an infusion pump with an in-line filter. Premedication with oral acetaminophen and intravenous antihistamine was given to all patients before each infusion. The use of concomitant glucocorticosteroids is described in the following paragraph.
Patients were assigned in consecutive order starting at the lowest dose level (300 mg). Dose escalation took place after the 10th patient had been allocated, the first 3 patients had received all 4 infusions and 1 week of follow-up, the sixth patient had received the first infusion and 1 week of follow-up, and less than 2 cases of dose-limiting toxicity (DLT) were observed in the first 6 patients. DLT was defined as: occurrence of a treatment-related adverse events (AEs) with intensity more than or equal to grade 3 at the day of infusion despite treatment with glucocorticosteroids, or any treatment-related nonhematologic AE with intensity more than or equal to grade 3 on noninfusion days, or any treatment-related hematologic AE with intensity more than or equal to grade 4 on noninfusion days. At the initiation of the trial, the infusion was started at 25 mL/hour during the first hour and increased to a maximum rate of 400 mL/hour if well tolerated. Subsequent infusions were started at 50 mL/hour if no infusion-related AEs were recorded at the previous infusion. Concomitant intravenous glucocorticosteroids (equivalent to 50 mg of prednisolone) were used only when infusion-related AEs with intensity more than or equal to grade 3 had been reported at the previous infusion or if infusions took more than 6 hours because of infusion-related AEs.
Evaluation and trial endpoints
Baseline evaluation included disease history and previous medication, current stage of disease including signs and symptoms, physical examination, laboratory assessments, CT scans with contrast of the neck, thorax, abdomen, and pelvis, bone marrow biopsy, and diagnostic FL verification (CD20+) from the original or a new excisional lymph node biopsy. Imaging modality and parameters were to remain consistent across all visits for any given patient. Baseline CT scans were initially evaluated by the site to ensure that the needed quality of the images was attained and to verify compliance with the inclusion criteria. Subsequently, a central reevaluation of all CT scans was conducted (Perceptive Informatics, Berlin, Germany).
Safety evaluations, including AEs, vital signs, laboratory safety assessments (hematology, clinical chemistry), and complement (CH50) measurements by liposome immunoassay were performed weekly throughout the treatment period. CD19+ and CD20+ B cells, T-cell subsets (CD3+, CD4+, and CD8+), and natural killer (NK) cells (CD16/56+ cells) were recorded using flow cytometry after 1, 4, 11, 19, and 26 weeks and after 9 and 12 months. Titers of HAHA in serum were quantified by an enzyme-linked immunosorbent assay at week 19 and month 12. AEs were categorized and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.16 AEs, DLT, and the percentage of patients developing HAHA were safety endpoints together with laboratory biochemistry and hematology data. AEs were used to identify the maximum tolerated dose (MTD) defined as the dose level below the dose level in which any of the following occurred: one treatment-related, fatal, life-threatening or permanently disabling serious AE (SAE), 2 cases of DLT in the first 6 patients at one dose level within 1 week of follow-up of the sixth patient after first dose, or 3 cases of DLT. A definition of DLT is given in “Treatment plan.”
Efficacy
The primary efficacy endpoint of the study was clinical response over the period from screening to week 19. The overall tumor responses from screening to week 11, 19, and 26 were secondary efficacy endpoints together with B-cell depletion in peripheral blood as percentage change in CD19+ and CD20+ cells, percentage of patients converting from BCL2-positive to BCL2-negative in the peripheral blood, time to progression, duration of response, and time to next anti-FL therapy.
Evaluation of disease was performed by physical examination (enlarged lymph nodes, hepatomegaly, and splenomegaly) and screening for B symptoms (night sweats, weight loss, or fever) at weeks 4, 7, 11, 19, and 26, and months 9 and 12, CT scans with contrast of the neck, thorax, abdomen, and pelvis at weeks 11, 19, and 26, and a bone marrow biopsy at weeks 19 and 26, if initially positive. Response to treatment was analyzed using standard outcome measures for clinical trials (CR, CRu, PR, stable disease [SD], and progressive disease [PD]) as defined by the International Workshop Response Criteria for non-Hodgkin lymphoma.17 After month 12, unvalidated progression data were collected directly from sites in periodically faxed forms. Clinical response was defined as best overall tumor response of CR, Cru, or PR. Lymphoma burden was estimated by the sum of the product of the largest perpendicular diameters (SPD) of the (maximally) 6 largest lesions (indicator lesions) based on CT scans. Changes in SPD from baseline to weeks 11, 19, and 26 were followed.
Quantification of CD19+ and CD20+ cells in the peripheral blood was performed on ethylenediaminetetraacetic acid blood samples using flow cytometry at screening, weeks 1, 4, 11, 19, and 26, and months 9 and 12. BCL2 conversion at week 19 was assessed in the peripheral blood by the detection of t(14;18) translocation using a polymerase chain reaction (PCR) detection strategy.18
PK sampling was performed on the days of treatment (days 0, 7, 14, and 21), and on days 1, 3, 5, 22, 25, 28, and 49. The concentration of ofatumumab was determined in serum using a sandwich immunoassay. Ninety-six-well enzyme-linked immunosorbent assay plates were coated with idiotype specific mouse monoclonal antiofatumumab antibody and subsequently incubated with the patient's serum and Fc specific antihuman IgG1 antibodies. The latter are enzyme-labeled antibodies (horseradish peroxidase), which function as detection probes. Subsequently, detection was performed by adding azino benzothiazoline sulphonic acid substrate resulting in a color development, which can be measured at 405 nm. The concentration of ofatumumab can be read from a standard curve by plotting the optical density as a function of the ofatumumab concentration. A 4-parameter log-logistic curve-fitting model was used for this purpose.
Statistical methods
No formal sample size calculation was performed. The planned number of patients was considered adequate to meet the primary objective of the study and is in agreement with regulatory guidance for this type of trial. The efficacy analysis was performed for the full analysis population (ie, all patients treated). Two-sided 95% confidence intervals (CIs) for clinical response were calculated using exact binomial distribution methods. Relationships between clinical response and other parameters were estimated using the Spearman's nonparametric rank correlation coefficient and tested by using 2-sided Wilcoxon nonparametric tests. The influence of possible explanatory variables on clinical response was investigated using logistic regressions. Kaplan-Meier analyses, including estimations of medians, were performed for time to progression, duration of response, and time to next anti-FL therapy, which was at the discretion of each individual clinical investigator. All tests were 2-sided and performed on the 5% significance level. No multiplicity adjustments were made.
Results
Patients
Between April 2004 and November 2005, 40 patients with relapsed or refractory FL were enrolled into the trial at the 14 trial sites. There were 20 male and 20 female, all white. Median age was 58.5 years (range, 34-75 years). Median time from original FL diagnosis to entry into the trial was 4.5 years (range, 0.7-17.1 years). The median number of all prior treatment regimens was 2 (range, 1-8). Thirteen percent (5 of 40) of patients had elevated lactic dehydrogenase at baseline, and the majority of patients were Ann Arbor stage IIIA (30%) or IVA (35%). Previous rituximab treatment (monotherapy or in combination with chemotherapy) was received by 15 patients. Four of these patients were refractory to rituximab treatment but were not thought to impact the efficacy assessment and were therefore not excluded from the trial population. Baseline characteristics of the patient populations at each dose level are presented in Table 1. All 40 patients were evaluated for safety. Of the 40 patients who received ofatumumab treatment, 37 were considered assessable for efficacy. One patient was excluded because of incomplete treatment. Two patients were not evaluable for clinical response as review of the CT scans showed absence of measurable indicator lesions at screening. Glucocorticosteroids were administered to 9 of 40 patients during the trial.
Patient demographics and pretreatment characteristics
| Dose . | 300 mg . | 500 mg . | 700 mg . | 1000 mg . |
|---|---|---|---|---|
| No. of patients | 10 | 10 | 10 | 10 |
| Median age, y (range) | 58 (47-75) | 64 (36-66) | 56 (34-73) | 56 (39-73) |
| Sex, male/female | 5/5 | 5/5 | 5/5 | 5/5 |
| Median body surface area, m2 (range) | 1.9 (1.8-2.1) | 2.0 (1.7-2.1) | 2.0 (1.5-2.5) | 1.8 (1.5-2.5) |
| No. of prior treatment regimens, median (range) | 2 (1-5) | 1.5 (1-4) | 2 (1-4) | 3 (1-8) |
| No. of patients with complete response to prior treatment regimens | 7 | 8 | 2 | 6 |
| No. of patients previously treated with rituximab | 5 | 2 | 3 | 5 |
| Ann Arbor stage | ||||
| IA | 0 | 0 | 1 | 1 |
| IIA | 3 | 1 | 2 | 1 |
| IIIA | 2 | 4 | 2 | 4 |
| IVA | 5 | 4 | 2 | 3 |
| IVB | 0 | 1 | 3 | 1 |
| No. of lesions, median (range) | 2.5 (0-4) | 3 (1-5) | 2 (1-3) | 2 (1-4) |
| Sum of the products of the largest perpendicular diameters, mm2, indicator lesions (range) | 1275 (0*-13 764) | 2568 (510-6723) | 1767 (817-6135) | 2042 (288-8134) |
| Dose . | 300 mg . | 500 mg . | 700 mg . | 1000 mg . |
|---|---|---|---|---|
| No. of patients | 10 | 10 | 10 | 10 |
| Median age, y (range) | 58 (47-75) | 64 (36-66) | 56 (34-73) | 56 (39-73) |
| Sex, male/female | 5/5 | 5/5 | 5/5 | 5/5 |
| Median body surface area, m2 (range) | 1.9 (1.8-2.1) | 2.0 (1.7-2.1) | 2.0 (1.5-2.5) | 1.8 (1.5-2.5) |
| No. of prior treatment regimens, median (range) | 2 (1-5) | 1.5 (1-4) | 2 (1-4) | 3 (1-8) |
| No. of patients with complete response to prior treatment regimens | 7 | 8 | 2 | 6 |
| No. of patients previously treated with rituximab | 5 | 2 | 3 | 5 |
| Ann Arbor stage | ||||
| IA | 0 | 0 | 1 | 1 |
| IIA | 3 | 1 | 2 | 1 |
| IIIA | 2 | 4 | 2 | 4 |
| IVA | 5 | 4 | 2 | 3 |
| IVB | 0 | 1 | 3 | 1 |
| No. of lesions, median (range) | 2.5 (0-4) | 3 (1-5) | 2 (1-3) | 2 (1-4) |
| Sum of the products of the largest perpendicular diameters, mm2, indicator lesions (range) | 1275 (0*-13 764) | 2568 (510-6723) | 1767 (817-6135) | 2042 (288-8134) |
Two patients had no indicator lesions.
Treatment
Thirty-nine of 40 patients received 4 weekly courses of ofatumumab, whereas one patient was withdrawn after only one infusion because of an SAE. Glucocorticosteroids were only administered before 36 of 157 (23%) of the infusions.
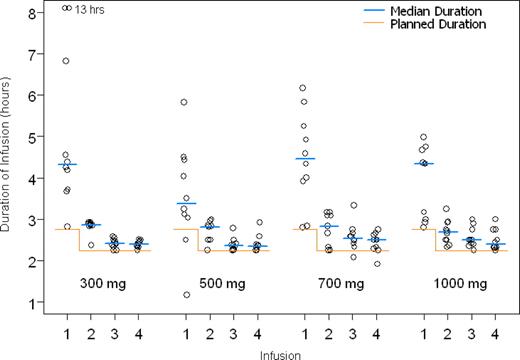
The median duration of the first infusion was 4.20 hours, ranging from 2.30 to 13 hours, and the median duration of the subsequent 3 infusions was 2.30 to 3.10 hours. The duration of the first infusion was, in all dose groups, longer than the duration of succeeding infusions (Figure 1), partly because of the higher incidence of infusion-related reactions with the first dose (Figure 2).
Safety
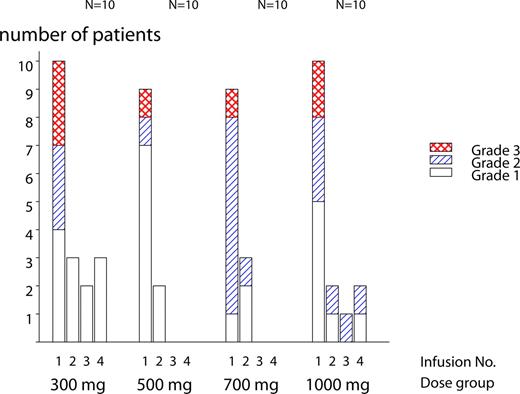
All 40 enrolled patients were evaluated for safety. One patient in the 500-mg group experienced an SAE (laryngeal edema, grade 3) during the first infusion. The patient recovered and was withdrawn from further treatment and from the trial. The event was judged to be related to ofatumumab treatment and was recorded as a DLT evaluated by the Data Monitoring Committee. Twenty patients withdrew from the study because of deterioration of study disease. Two patients withdrew because of stem cell harvesting. One patient's data were censored at the time of stem cell transplantation, whereas the other patient was censored at the last visit in the trial. The 23 withdrawn patients were distributed among dose groups as follows: 3, 7, 8, and 5 in the 300-mg, 500-mg, 700-mg, and 1000-mg dose groups, respectively. A total of 274 AEs were reported in 40 patients and 190 (70%) were judged to be related to ofatumumab (Table 2). Ninety-five percent were of grade 1 or 2 intensity. The most frequent AEs were within the system organ class “general disorders and administration site conditions” (pyrexia, chills, and fatigue), “respiratory, thoracic, and mediastinal disorders” (dyspnea, pharyngolaryngeal pain, and cough), and “skin and subcutaneous tissue disorders” (pruritus, rash, and urticaria). Other frequent AEs were headache, hypotension, and nausea. The majority of patients (38 of 40) experienced AEs on an infusion day, and more than half (55%) of the reported AEs had onset before second infusion. As shown in Figure 2, 90% to 100% of patients reported AEs on the day of first infusion, whereas only 30% or fewer patients reported AEs on the second, third, and fourth infusions. Usually, the infusion-related AEs occurred within the first few hours of starting the ofatumumab infusion, with the most common symptoms being transient chills rash, pruritus, pyrexia, and urticaria. Infusion-related AEs prolonged especially the duration of the first infusion (Figure 1). Of the 274 AEs, 13 events were CTC grade 3 (Table 2). One case of ovarian epithelial cancer (cystadenocarcinoma mucinosum grade 1) was assessed as unrelated to ofatumumab, and the patient fully recovered after surgery. Eight grade 3 events in 7 patients were judged to be related to ofatumumab, which was 2 events of dyspnea and 1 event of hypoxia, laryngeal edema, throat tightness, pruritus, rash, and abdominal pain, respectively. All occurred on the first day of infusion and all fully recovered. No relationship between dose and number or severity of AEs was observed (Table 2). After the end of the trial, 2 SAEs with fatal outcome were reported; one patient died of disease progression 5 months after final treatment, and one patient died of sepsis resulting from peritonitis 17 months after the final treatment, which was after subsequent treatment for FL. Two grade 4 SAEs were also reported after the end of the trial, one leucocytopenia secondary to treatment with chlorambucil resulting from FL progression and one additional case of mucinous cystadenocarcinoma (endometrioid adenocarcinoma grade 1) with no signs of relapse 6 months after the completion of chemotherapy with taxol and carboplatin. The patient had undergone repeated ultrasound examinations and in retrospect the tumor could have been identified before the treatment with ofatumumab. One case of ductal carcinoma grade 2 with high-grade ductal carcinoma in situ was also reported after the end of the trial. Mastectomy with axillary clearance was performed. Final outcome is pending. The investigator assessed the event to be related to ofatumumab but also to hormone replacement therapy.
Adverse events
| . | 300 mg (N = 10) . | 500 mg (N = 10) . | 700 mg (N = 10) . | 1000 mg (N = 10) . | Total (N = 40) . | Related . |
|---|---|---|---|---|---|---|
| Total no. | ||||||
| AEs | 80 | 44 | 72 | 78 | 274 | 190 |
| AEs reported on any infusion day | 49 | 30 | 37 | 43 | 159 | 152 |
| AEs on first infusion day | 41 | 24 | 34 | 35 | 134 | 132 |
| SAEs | 3 | 1 | 1 | 2 | 7 | 1 |
| Total no. of AEs ≥ grade 3 (no. of patients)* | 6 (4) | 1 (1) | 3 (3) | 3 (2) | 13 (10) | 8 (7) |
| Dyspnea | 2 | 1 | 3 | 2 | ||
| Hypoxia | 1 | 1 | 1 | |||
| Laryngeal edema | 1 | 1 | 1 | |||
| Throat tightness | 1 | 1 | 1 | |||
| Neutropenic sepsis | 1 | 1 | ||||
| Urinary tract infection | 1 | 1 | ||||
| Pruritus | 1 | 1 | 1 | |||
| Rash | 1 | 1 | 1 | |||
| Abdominal pain | 1 | 1 | 1 | |||
| Ovarian epithelial cancer | 1 | 1 | ||||
| Hydronephrosis | 1 | 1 |
| . | 300 mg (N = 10) . | 500 mg (N = 10) . | 700 mg (N = 10) . | 1000 mg (N = 10) . | Total (N = 40) . | Related . |
|---|---|---|---|---|---|---|
| Total no. | ||||||
| AEs | 80 | 44 | 72 | 78 | 274 | 190 |
| AEs reported on any infusion day | 49 | 30 | 37 | 43 | 159 | 152 |
| AEs on first infusion day | 41 | 24 | 34 | 35 | 134 | 132 |
| SAEs | 3 | 1 | 1 | 2 | 7 | 1 |
| Total no. of AEs ≥ grade 3 (no. of patients)* | 6 (4) | 1 (1) | 3 (3) | 3 (2) | 13 (10) | 8 (7) |
| Dyspnea | 2 | 1 | 3 | 2 | ||
| Hypoxia | 1 | 1 | 1 | |||
| Laryngeal edema | 1 | 1 | 1 | |||
| Throat tightness | 1 | 1 | 1 | |||
| Neutropenic sepsis | 1 | 1 | ||||
| Urinary tract infection | 1 | 1 | ||||
| Pruritus | 1 | 1 | 1 | |||
| Rash | 1 | 1 | 1 | |||
| Abdominal pain | 1 | 1 | 1 | |||
| Ovarian epithelial cancer | 1 | 1 | ||||
| Hydronephrosis | 1 | 1 |
AE indicates adverse event; N, number of subjects exposed to trial drug.
Only treatment-emergent AEs are presented.
Hematologic toxicity consisted of one episode of grade 2 thrombocytopenia, which was considered clinically relevant and was consequently reported as an AE. In addition, 7 events of neutropenia (6 grade 1 or 2 and 1 grade 3) and 6 grade 1 events of thrombocytopenia were recorded, which did not require clinical intervention. The majority of the grade 1 or 2 neutropenias (1.0-1.6 × 109/L) occurred at one visit in weeks 11 to 26 and were resolved at the following visit. The grade 3 neutropenia (0.8 × 109/L) occurred in week 19 and was resolved the following visit (week 26). The sporadic thrombocytopenias occurred from screening to week 26 and were in most cases resolved before or at week 26.
Based on AEs, the MTD was not identified for any patient.
Infections
Twenty infectious AEs were reported in 13 patients with 2 events of grade 3 infections reported as SAEs (one urinary tract infection and one neutropenic sepsis occurring 3 months after cessation of trial drug; Table 2). The 2 events were assessed as unrelated to ofatumumab by the investigators, and the patients fully recovered after treatment with ciproxin and flucloxacillin/tazocin, respectively. Eighteen infections were grade 1 or 2: upper respiratory tract infection (11), urinary tract infection (2), herpes simplex (1), candidiasis (2), and pneumonia (2) and were all assessed as unrelated to ofatumumab by the investigators. Distribution of infections among dose groups was as follows: 300 mg (5 events), 500 mg (0 events), 700 mg (3 events), and 1000 mg (5 events).
Serum chemistry
No significant changes in serum chemistry were observed.
Immunology
Mean serum IgA and IgG levels remained stable within the normal range. Mean serum IgM level was below normal at screening and tended to decrease throughout the study. Thus, the median IgM concentrations across dose groups decreased from 0.52 g/L, at baseline to 0.41 g/L at week 26. No significant changes in serum complement levels (CH50) were observed. No significant changes in T-cell counts (CD3+, CD4+, and CD8+) and NK-cell counts (CD16/56+ cells) were observed. One patient had a borderline (8-fold) increase in HAHA at week 12. At month 12, the patient was tested negative for HAHA. No safety concerns were raised from any additional safety parameters monitored.
Efficacy
Clinical responses over the period from screening to week 19 were obtained in all dose groups with response rates of 63%, 33%, 20%, and 50% with 300 mg, 500 mg, 700 mg, and 1000 mg, respectively. From week 19 to week 26, one additional responder, in the 1000-mg dose group, was seen. Best clinical responses from screening to week 26 for evaluable and intention to treat patients are shown in Table 3. In a subgroup of 14 evaluable patients previously treated with rituximab, best clinical response was 64% across dose groups (Table 3). Of the 4 patients refractory to rituximab, 3 actually responded to ofatumumab treatment (one CR, one CRu, and one PR).
Best objective clinical responses from screening to week 26
| . | 300 mg . | 500 mg . | 700 mg . | 1000 mg . | Total . |
|---|---|---|---|---|---|
| No. of patients (all/previously treated with rituximab) | 8*/4* | 10†/2 | 10/3 | 10/5 | 38†/14 |
| CR | 4/3 | 1/0 | 5/3 | ||
| Cru | 1/1 | 1/0 | 2/1 | ||
| PR | 2/0 | 2/1 | 5/4 | 9/5 | |
| SD | 3/0 | 6/2 | 6/1 | 3/1 | 18/4 |
| PD | 2/1 | 1/0 | 3/1 | ||
| Clinical response (all/previously treated with rituximab) | 5/4 | 3/0 | 2/1 | 6/4 | 16/9 |
| Evaluable patients | 63% | 33% | 20% | 60% | 43% |
| 95% CI | (25%-92%) | (8%-70%) | (3%-56%) | (26%-88%) | (27%-61%) |
| Intention to treat (ITT) | 63% | 30% | 20% | 60% | 42% |
| 95% CI | (25%-92%) | (7%-65%) | (3%-56%) | (26%-88%) | (26%-59%) |
| Patients previously treated with rituximab | 100% | 0% | 33% | 80% | 64% |
| 95% CI | (40%-100%) | (1%-91%) | (28%-100%) | (43%-95%) |
| . | 300 mg . | 500 mg . | 700 mg . | 1000 mg . | Total . |
|---|---|---|---|---|---|
| No. of patients (all/previously treated with rituximab) | 8*/4* | 10†/2 | 10/3 | 10/5 | 38†/14 |
| CR | 4/3 | 1/0 | 5/3 | ||
| Cru | 1/1 | 1/0 | 2/1 | ||
| PR | 2/0 | 2/1 | 5/4 | 9/5 | |
| SD | 3/0 | 6/2 | 6/1 | 3/1 | 18/4 |
| PD | 2/1 | 1/0 | 3/1 | ||
| Clinical response (all/previously treated with rituximab) | 5/4 | 3/0 | 2/1 | 6/4 | 16/9 |
| Evaluable patients | 63% | 33% | 20% | 60% | 43% |
| 95% CI | (25%-92%) | (8%-70%) | (3%-56%) | (26%-88%) | (27%-61%) |
| Intention to treat (ITT) | 63% | 30% | 20% | 60% | 42% |
| 95% CI | (25%-92%) | (7%-65%) | (3%-56%) | (26%-88%) | (26%-59%) |
| Patients previously treated with rituximab | 100% | 0% | 33% | 80% | 64% |
| 95% CI | (40%-100%) | (1%-91%) | (28%-100%) | (43%-95%) |
CR indicates complete response; Cru, complete response unconfirmed; PR, partial response; SD, stable disease; PD, progressive disease; and CI, confidence interval.
Two patients had no indicator lesions.
One patient was withdrawn before assessment (considered as a nonresponder in ITT).
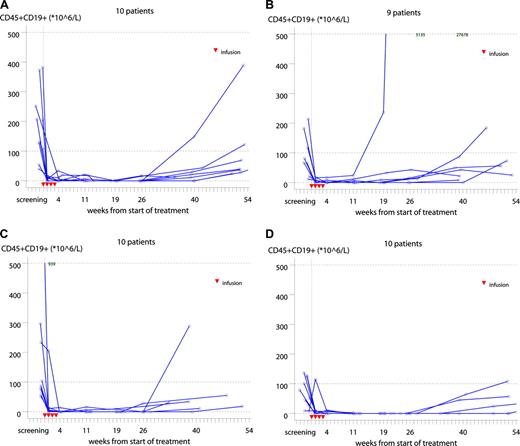
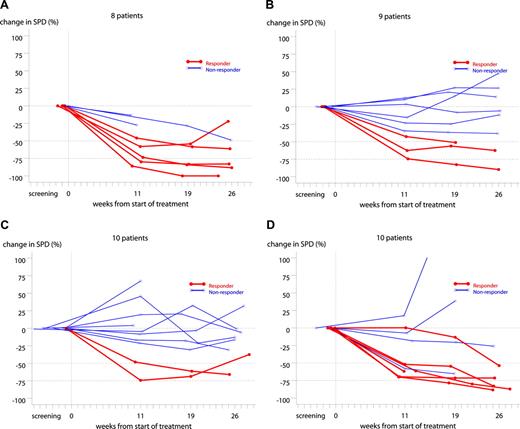
Immediate, profound, and long lasting B-cell depletion (both CD19+ and CD20+) was observed in all dose groups. The level remained nearly undetectable until approximately 6 to 10 months after treatment, followed by a slow recovery (Figures 3). Changes in SPD from baseline are shown in Figure 4. Clinical responses were seen at week 11 for 81% (13 of 16) of responders.
Individual CD19+ B cells in the 300-mg (A), 500-mg (B), 700-mg (C), and 1000-mg (D) dose groups. Red triangles represent days of ofatumumab infusions. Written values in the top of panels B and C are outliers. One patient in the 500-mg group (B) was withdrawn at visit 2; hence, data from 9 patients only are presented. The median CD19+ count at baseline was 85 × 106/L (range, 7-939). Most patients had a profound depletion of CD19+ cells after the first infusion lasting 6 to 10 months followed by a slow gradual recovery.
Individual CD19+ B cells in the 300-mg (A), 500-mg (B), 700-mg (C), and 1000-mg (D) dose groups. Red triangles represent days of ofatumumab infusions. Written values in the top of panels B and C are outliers. One patient in the 500-mg group (B) was withdrawn at visit 2; hence, data from 9 patients only are presented. The median CD19+ count at baseline was 85 × 106/L (range, 7-939). Most patients had a profound depletion of CD19+ cells after the first infusion lasting 6 to 10 months followed by a slow gradual recovery.
Individual change in SPD from baseline in the 300-mg (A), 500-mg (B), 700-mg (C), and 1000-mg (D) dose groups. Two patients in the 300-mg group (A) were without indicator lesions; hence, data from 8 patients only are presented. One patient in the 500-mg group (B) was withdrawn at visit 2; hence, data from 9 patients only are presented. Bold lines represent clinical responders. One patient in the 1000-mg group (D) showed less than 50% reduction in the SPD at week 11 and 19 but was considered as a nonresponder as a confirmational lymph node biopsy was positive.
Individual change in SPD from baseline in the 300-mg (A), 500-mg (B), 700-mg (C), and 1000-mg (D) dose groups. Two patients in the 300-mg group (A) were without indicator lesions; hence, data from 8 patients only are presented. One patient in the 500-mg group (B) was withdrawn at visit 2; hence, data from 9 patients only are presented. Bold lines represent clinical responders. One patient in the 1000-mg group (D) showed less than 50% reduction in the SPD at week 11 and 19 but was considered as a nonresponder as a confirmational lymph node biopsy was positive.
BCL2 conversion in the blood from positive to negative was recorded in all dose groups, with a 65% conversion rate in evaluable patients across dose groups. Only 4 of the reverted patients responded to treatment.
The relationship between clinical response over the period from screening to week 19 and demographic, baseline characteristics, drug administration, and PK parameters was investigated using univariate logistic regressions. No statistically significant relationships on the 5% level were found for the following parameters: age, sex, body weight, body surface area, body mass index, Ann Arbor stage, duration of FL, time from latest diagnostic biopsy, number of prior anti-FL treatments, time from latest anti-FL treatment, relapse to previous anti-FL treatment, best response to prior anti-FL treatment, previously treated with rituximab, number of indicator lesions at screening, SPD at screening, lactic dehydrogenase, hemoglobin, BCL2, BCL2 conversion, total amount of dose administered, glucocorticoids during trial, area under the curve (AUC), maximum concentration (Cmax), volume of distribution (Vz), terminal half-life (T1/2), clearance (CL), and ofatumumab concentration at week 7.
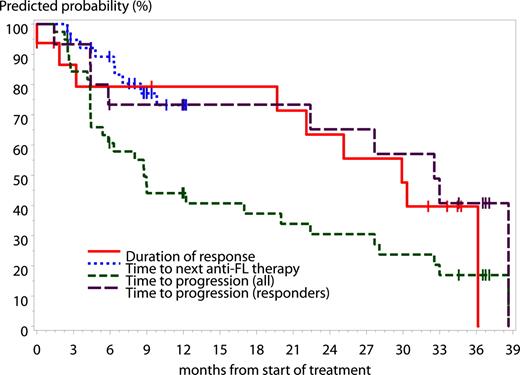
Based on Kaplan-Meier estimates at a median/maximum follow-up of 9.2/38.6 months, the median time to progression (TTP) for all patients was 8.8 months (95% CI, 5.4-20.0 months), the median TTP for responders was 32.6 months (95% CI, 22.4-38.6 months), and the median duration of response was 29.9 months (95% CI, 19.7-36.1 months). Four patients who have not progressed yet are still in follow-up. Median time to next anti-FL therapy was not reached during the study period (Figure 5).
Kaplan-Meier estimates across dose groups of time to progression (all patients), time to progression (responders), duration of response, and time to next anti-FL therapy. Based on Kaplan-Meier estimates, at a median/maximum follow-up of 9.2/38.6 months, the median TTP for all patients was 8.8 months (95% CI, 5.4-20.0 months), the median TTP for responders was 32.6 months (95% CI, 22.4-38.6 months), and the median duration of response was 29.9 months (95% CI, 19.7-36.1 months). The median time to next anti-FL therapy was not reached during the study period, and data were not collected after month 12. Given for a time point where estimates could be calculated, the proportion of patients who had time to next FL therapy more than 8.5 months was 80% (67%; 100%). All indicates 40 patients.
Kaplan-Meier estimates across dose groups of time to progression (all patients), time to progression (responders), duration of response, and time to next anti-FL therapy. Based on Kaplan-Meier estimates, at a median/maximum follow-up of 9.2/38.6 months, the median TTP for all patients was 8.8 months (95% CI, 5.4-20.0 months), the median TTP for responders was 32.6 months (95% CI, 22.4-38.6 months), and the median duration of response was 29.9 months (95% CI, 19.7-36.1 months). The median time to next anti-FL therapy was not reached during the study period, and data were not collected after month 12. Given for a time point where estimates could be calculated, the proportion of patients who had time to next FL therapy more than 8.5 months was 80% (67%; 100%). All indicates 40 patients.
Pharmacokinetics
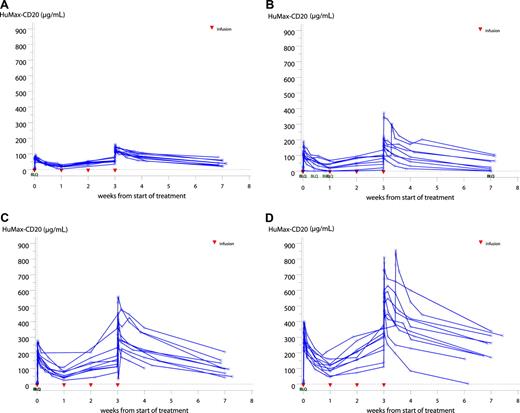
Serum concentrations of ofatumumab increased throughout the treatment course. T1/2, AUC, and Cmax of the antibody significantly increased from the first to the fourth dose simultaneously with a significantly decreased CL. At the fourth infusion, a statistically significant difference between genders was observed in time to maximum concentration, and only men showed a statistically significant change in Cmax between the first and the fourth infusion. The reason for this gender difference is not known. Values for CL and T1/2 did not indicate dose-dependent PK for either of the 2 periods. A more than proportional increase in AUC with dose was observed, suggesting nonlinear PK. Serum concentrations in the individual dose groups are shown in Figure 6. A summary of PK after the fourth dose is presented in Table 4. The median T1/2 ranged from 303 to 567 hours after the fourth dose. A correlation between exposure (AUC and Cmax) and clinical response to treatment at weeks 19 and 26 was not found. For other PK parameters (T1/2, Vz, CL), correlation to responses was only found for clinical response at week 26 and T1/2 and CL (P < .05, 2-sided Wilcoxon nonparametric test).
Ofatumumab serum concentrations in the 300-mg (A), 500-mg (B), 700-mg (C), and 1000-mg (D) dose groups. BLQ indicates below level of quantification. The limit of quantification was 0.1 μg/mL.
Ofatumumab serum concentrations in the 300-mg (A), 500-mg (B), 700-mg (C), and 1000-mg (D) dose groups. BLQ indicates below level of quantification. The limit of quantification was 0.1 μg/mL.
Pharmacokinetics after the fourth dose
| Dose, mg . | No. . | Cmax, mg/mL . | T1/2, h . | CL, mL/h per kg . | AUC0-inf, h*mg/mL . |
|---|---|---|---|---|---|
| 300 | 10 | 129 (112-161) | 447 (293-711) | 9 (1-17) | 74 616 (41 286-282 593) |
| 500 | 9 | 185 (84-373) | 303 (57-632) | 16 (4-78) | 53 261 (6957-203 068) |
| 700 | 9 | 355 (263-560) | 322 (189-625) | 10 (6-23) | 185 251 (51 925-380 185) |
| 1000 | 10 | 610 (362-857) | 567 (77-720) | 3 (1-29) | 644 080 (47 188-1 107 841) |
| Dose, mg . | No. . | Cmax, mg/mL . | T1/2, h . | CL, mL/h per kg . | AUC0-inf, h*mg/mL . |
|---|---|---|---|---|---|
| 300 | 10 | 129 (112-161) | 447 (293-711) | 9 (1-17) | 74 616 (41 286-282 593) |
| 500 | 9 | 185 (84-373) | 303 (57-632) | 16 (4-78) | 53 261 (6957-203 068) |
| 700 | 9 | 355 (263-560) | 322 (189-625) | 10 (6-23) | 185 251 (51 925-380 185) |
| 1000 | 10 | 610 (362-857) | 567 (77-720) | 3 (1-29) | 644 080 (47 188-1 107 841) |
Results are shown as median values (range).
Discussion
Although new therapies alone and in combination with old therapies have demonstrated an improvement in treatment outcome in FL, new treatment modalities with improved toxicity profile and even better responses are needed. Ofatumumab has demonstrated a higher potency and a longer duration of efficacy than rituximab in several preclinical model studies and even kills rituximab refractory B cells, indicating a different mechanism of action that could offer an advantage in the clinic.
Results from this first in human trial of ofatumumab in patients with relapsed or refractory FL have revealed a favorable safety profile of the agent. One patient experienced a related SAE (laryngeal edema) during the first infusion of 500 mg of ofatumumab and was withdrawn from the study. No stopping rules were met, and the MTD was not reached. Forty patients reported a total of 274 AEs; 95% were of grade 1 or 2 intensity, the most frequent being pyrexia, chills, pruritus, and fatigue. Thirteen (5%) AEs were grade 3 or above. Ofatumumab was judged to be related to 190 (70%) of the AEs, 8 of grade 3. The majority of patients (37) showed AEs in connection with the first infusion, whereas only 30% of patients reported events during the second, third, or fourth infusion. Usually, the infusion-related events occurred within the first few hours of the ofatumumab infusion, with the most common symptoms being transient chills, rash, pruritus, pyrexia, and urticaria, probably reflecting signs of cytokine release. In the present trial, premedication did not a priori include glucocorticosteroids, which probably could have prevented some of the infusion-related AEs. Glucocorticosteroids were only used in connection with 23% of infusions. Only one hematologic nonrelated event of grade 2 thrombocytopenia was considered clinically relevant and reported as an AE. Infectious AEs occurred in 33% of patients, including 2 patients having a grade 3 infection assessed as unrelated to ofatumumab treatment by the investigators. Evaluation of vital sign measurements, serum chemistry, and whole blood counts revealed no safety issues of clinical concern with ofatumumab infused at 4 doses up to 1000 mg. The nature and timing of the AEs are expected in patients with large tumor masses exposed to mAb's. The MTD of ofatumumab is still unknown, as is the optimal dose.
As expected, rapid, efficient, and sustained (6-10 months) B-cell depletion was observed in the blood in all dose groups, reflecting the potent cytolytic activity of the antibody. Despite this, minimal changes in serum IgM concentrations, and no clinically significant changes in serum complement, T cells, T-cell subsets, and NK cells were observed.
Objective responses were achieved in all dose groups, with response rates ranging from 20% to 63%. The response rate was not dose-dependent (dose as mg/patient, dose adjusted for body weight (mg/kg) or dose adjusted for body surface area (mg/m2). Best response rate across dose groups was 43% for evaluable patients. Previous rituximab-treated patients had a response rate across dose groups of 64%, and 3 of the responders were previously refractory to rituximab. This might indicate a different mode of action for ofatumumab. Sixty-five percent of evaluable patients converted to negative BCL2 status (no detectable cells with BCL2 rearrangements) in the peripheral blood after ofatumumb treatment. No correlation with objective overall response was found.
Values for CL and T1/2 did not indicate dose-dependent PK after either the first or the fourth dosing period. This was expected as mAb's, unlike small molecules, most often are eliminated in a nonlinear fashion. Between the 2 periods, a statistically significant change occurred for T1/2 and CL. Consequently, the exposure to ofatumumab increased during the 4 weekly dosages beyond what could be expected from normal accumulation as judged from the initial T1/2. After the fourth dose, the median T1/2 across dose groups was 410 hours and CL was 9.5 mL/hour for ofatumumab. For comparison, the mean T1/2 of rituximab is 209 hours and CL is 9.2 mL/hour.15,19 As the elimination process of mAb's takes place once the antibody is bound to the target molecule, the observed difference might be caused by the profound B-cell depletion, which occurs already after the initial infusion, leaving a minimal number of circulating B cells available for binding to ofatumumab. A correlation between exposure (AUC) and response to treatment was not found.
Because ofatumumab is a fully human antibody, immunogenicity was not expected to pose a problem. In this first in human trial, only one patient had a transient borderline (8-fold) titer increase at week 12.
In conclusion, treatment with ofatumumab was found to be well tolerated in patients with relapsed or refractory FL grade 1 or 2 in doses up to 1000 mg. In addition, preliminary data on lymphoma response are encouraging. These results warrant further exploration of ofatumumab's potential in the treatment of FL as well as other B-cell malignancies with low CD20 and high CD55 and CD59 expression, such as B-CLL. Indeed, ofatumumab has been shown to induce significant clinical responses in relapsed CLL.20 For the subsequent trial in rituximab-refractory FL patients, a low (500-mg) versus a high (1000-mg) dose of ofatumumab was selected to be explored (www.clinicaltrials.gov; #NCT00394836).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Genmab A/S for supplying study material, including ofatumumab; contributing to the design and conduct of the trial; collecting, managing, analyzing, and interpreting the data; and contributing to the preparation of the manuscript. The authors also thank Helle Aaes and Ulla Jessen, Genmab A/S, for editorial assistance.
This work was supported by clinical research funding from Genmab A/S (A. Hagenbeek, O.G., P.J., L.M.P., J.W., A. Hellmann, B.K.L., T.R., M.W., M.P., M.K., A.E., P.S., J.R.).
Authorship
Contribution: A. Hagenbeek, in collaboration with Genmab A/S, designed the research; all the authors performed the research and collected the data; N.L. analyzed the data; A. Hagenbeek, in collaboration with Genmab A/S, interpreted the data; and A. Hagenbeek and Helle Aaes prepared the manuscript.
Conflict-of-interest disclosure: M.F.F., J.P., and N.L. are employed by Genmab A/S, whose potential product was studied in the present work. The remaining authors delcare no competing financial interests.
Correspondence: Anton Hagenbeek, University Medical Center Utrecht, Department of Hematology, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands; e-mail: a.hagenbeek@umcutrecht.nl.