In this issue of Blood, Ashley-Koch and coworkers report that, in sickle cell disease–related pulmonary hypertension, SNPs in several genes of the TGF-β/BMP superfamily are associated with this complication.
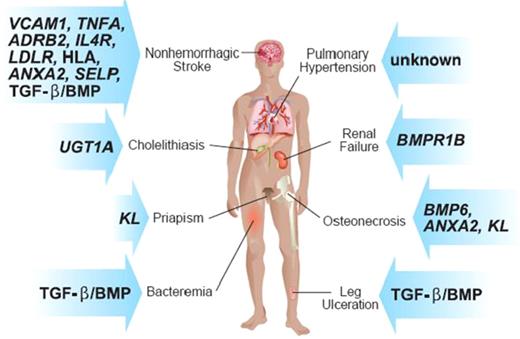
Sickle cell anemia, a common hemolytic anemia caused by homozygosity for the sickle hemoglobin mutation (glu6val) in the β-globin gene (HBB), is a characteristic Mendelian monogenic disease. Nevertheless, it is clinically heterogenous, resembling a multigenic trait. Although environment must account for part of this variability—in developing countries with little access to health care, death in childhood is frequent—even in countries with reasonable health care systems, clinical variability is striking. During the past 5 years, genetic association studies have focused on understanding the basis for this variability by linking single nucleotide polymorphisms (SNPs) in genes that might affect the pathophysiology of disease with disease subphenotypes. As shown in the figure, these subphenotypes include: stroke, priapism, leg ulcers, osteonecrosis, renal failure, bacteremia, and cholelithiasis; many associations, especially with SNPs in genes of the TGF-β/BMP pathway, have been found. The association of these polymorphisms with disease phenotypes is likely to reflect the modulation of sickle vasculopathy.
Some of the subphenotypes of sickle cell anemia and the genes in which SNPs have been associated with these phenotypes.
Some of the subphenotypes of sickle cell anemia and the genes in which SNPs have been associated with these phenotypes.
Now, the first reported study of its kind in sickle cell pulmonary hypertension, an abnormality found in about a third of adults with sickle cell anemia, and one that portends a high risk of very near term mortality,1 adds some SNPs in the TGF-β/BMP pathway to this list. Although the number of patients examined was small, and although one might quibble about the selection of cases and controls and certain analytical issues, it is satisfying that some results recapitulate observations made in other disease subphenotypes like stroke,2 and further implicate the TGF-β/BMP superfamily in the pathophysiology of disease. Other teams are studying larger groups of patients, and we should soon see if the results of these studies are confirmed. The mechanisms by which perturbation of this pathway might modulate the disease remain obscure.
Genetic association studies to date are based on searching for SNPs in candidate genes. That is, each gene was chosen because of its potential importance in this disease. As a result, the positive results are gratifying, but represent a self-fulfilling prophecy. Unraveling the genetic basis for the clinical heterogeneity of sickle cell anemia will require genome-wide association studies in which upwards of a half million SNPs—279 were tested in this study—are examined, providing an unbiased assessment of associations with disease phenotypes. This approach relieves the investigator of having to conjure up candidate genes, and will lead to unexpected and often inexplicable findings, as have been reported for diabetes, a prime example of a multigenic trait.3 To query such a large number of SNPs, patient samples must be substantial and the analytical approach must be rigorous. Nevertheless, many smaller studies could be aggregated to provide a patient base that is sufficiently large and diverse to validate the findings.
The near-term results of these studies will be an additional means of predicting outcomes and tailoring treatment. A longer-term goal is the identification of hitherto unsuspected disease modulators, like the TGF-β/BMP pathway, that might be amenable to modulation and therefore suggest new avenues of drug development.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal