In this issue of Blood, Feldman and colleagues provide convincing evidence of a clonal relationship between follicular lymphoma and H/DC sarcoma, suggesting plasticity between these apparently disparate lineages with implications for transformation events in malignant lymphomas.
The rarity of dendritic-cell tumors and the little that is known about their pathogenesis makes them a fascinating subject. The availability of new methodologies to confirm dendritic-cell lineage and awareness of the broadening morphologic spectrum have resulted in wider recognition of this tumor. There are no known etiologic factors, although cases with features of inflammatory pseudotumor have been associated with the Epstein-Barr virus, and there is an association with Castleman disease of the hyaline vascular type.1 In the cases associated with Castleman disease, dendritic cells proliferate outside of the follicles and form clusters and sheets, suggesting that the neoplasm has evolved from the follicles.
In an earlier study, Nayer et al showed clonal cytogenetic abnormalities and Bcl2 rearrangement with t(14;18) in a case of interdigitating dendritic-cell sarcoma. 2 Now, Feldman and colleagues report on a group of patients with both follicular lymphoma and histiocytic sarcoma, including 5 derived from follicular dendritic cells and 1 interdigitating dendritic-cell sarcoma. The sarcomas were synchronous in all but 3 of the 8 tumors, which developed up to 12 years after the follicular lymphomas. In no case did the histiocytic/dendritic-cell (H/DC) tumor precede the onset of lymphoma. Molecular studies, which were performed on all 8 of the tumor pairs, provide convincing evidence of a clonal relationship between these apparently disparate neoplasms. All of the H/DC neoplasms showed t(14;18) by fluorescent in situ hybridization. Polymerase chain reaction sequencing identified identical IgH rearrangements and Bcl2 gene breakpoints in all patient tumor pairs that could be tested.
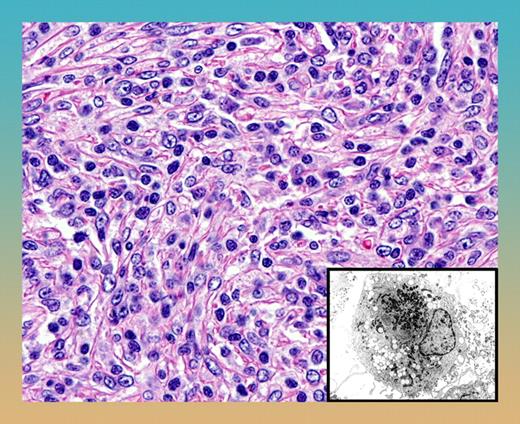
Follicular dendritic-cell sarcoma with syncytial proliferation of spindle cells. Note the characteristic sprinkling of lymphocytes within the tumor. The inset shows the ultrastructural appearance of dendritic cells with villous cell processes and prominent lysosomal granules.
Follicular dendritic-cell sarcoma with syncytial proliferation of spindle cells. Note the characteristic sprinkling of lymphocytes within the tumor. The inset shows the ultrastructural appearance of dendritic cells with villous cell processes and prominent lysosomal granules.
Plasticity between myeloid cells and dendritic cells is well known, and Paquette et al have shown that myeloid cells can be induced to differentiate into dendritic cells with interferon alpha.3 It now appears that a similar lack of lineage fidelity can occur between neoplastic lymphoid cells and H/DCs. Although this is a small group of patients, these findings have obvious implications for transforming events including clonal evolution and development of secondary neoplasms in patients with common types of low-grade lymphoma.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal