Abstract
We describe a mutation in human erythrocyte band 3 (anion exchanger 1; SLC4A1) causing both hereditary spherocytosis and distal renal tubular acidosis. The proband developed a transfusion-dependent, hemolytic anemia following birth. Immunoblotting showed band 3 was reduced to approximately 35% of wildtype; other proteins of the band 3/Rh macrocomplex were also reduced. DNA sequence analysis revealed a novel homozygous mutation, c.2000C>T, leading to the amino acid substitution Ser667Phe. The parents were heterozygous for the same mutation. Sulfate influx in the patient's erythrocytes was approximately 40% wild type. The mutant band 3 produced very little chloride influx when expressed in Xenopus oocytes. Influx was partially rescued by coexpression of glycophorin A and also rescued by coexpression of wild-type band 3. At 2 years of age, an ammonium chloride challenge showed the child has incomplete distal renal tubular acidosis (dRTA). Stable expression of mutant kidney band 3 in both nonpolarized and polarized Madin-Darby canine kidney cells showed that most of the mutant protein was retained in the endoplasmic reticulum. Overall our results suggest that the Ser667Phe does not affect the anion transport function of band 3, but causes a trafficking defect in both erythrocytes and kidney cells.
Introduction
Band 3 (anion exchanger 1; SLC4A1) carries out chloride/bicarbonate exchange transport in both erythrocytes and the acid-secreting cells in the kidney.1 In erythrocytes, band 3 is intimately involved in gas transport and also in the maintenance of erythrocyte stability and morphology. In kidney cells, the action of band 3 allows the excretion of acid into the urine. The kidney isoform of band 3 is truncated and lacks the first 65 amino acids of erythroid band 3. Heterozygous mutations in the SLC4A1 gene are one of the most frequent causes of hereditary spherocytosis (HS).2 These mutations generally affect the stability of band 3; erythrocyte band 3 is reduced by 20% to 30%, giving a mild clinical presentation. Not uncommonly, the condition is discovered in adulthood. Normally these heterozygous SLC4A1 HS mutations are not associated with kidney disease, presumably because sufficient wild-type band 3 is expressed in the kidney to allow acid secretion. There have been a number of SLC4A1 mutations reported that result in a defect in the ability of the kidney to secrete acid, and cause distal renal tubular acidosis (dRTA).3 These mutations generally affect the trafficking of band 3 in kidney cells,4 but do not often affect erythrocyte band 3 expression. Trafficking of erythroid band 3 differs from kidney band 3, and depends on glycophorin A (GPA), which is not expressed in kidney cells
Homozygous SLC4A1 mutations, associated with HS, are normally lethal in humans. This is because the lack of band 3 causes the erythrocytes to be extremely unstable, leading to uncontrolled hemolytic anemia, but also because kidney function is severely impaired. Mice with targeted disruption of the slc4a1 gene have been obtained and show these defects.5,6 In humans, homozygosity for a band 3 mutation causing HS was first described through band 3 Coimbra (Val488Met).7,8 This child was born severely anemic and hydropic, had severe HS and dRTA with nephrocalcinosis, and had to be maintained on regular blood transfusions and daily bicarbonate. The mechanism by which the mutant band 3 failed to access or remain in the membrane, either in the erythrocytes or in the α-intercalated cells, was not elucidated, although a recent report shows that the Val488Met band 3 mutant is retained intracellularly when expressed in Madin-Darby canine kidney (MDCK) cells.9 The second case, known as band 3 Neapolis, resulted from the homozygous inheritance of a T-to-C substitution at the +2 position in the donor splice site of intron 2.10 The child had severe HS but reportedly no dRTA because the mutation affects exon 2, which is not expressed in kidney band 3.
In this paper, we describe the second patient with a homozygous SLC4A1 mutation (Ser667Phe) causing both HS and dRTA. This band 3 variant differs from the previous variant7 in that a significant amount of band 3 reached the erythrocyte membrane and the dRTA was incomplete.
Methods
Case reports
The male proband was born in May 2004. He is the firstborn of an Algerian couple originating from Kabylia. The parents are first cousins. The pregnancy was uneventful. The neonate had normal weight (3.05 Kg), size (47 cm), and skull perimeter (34 cm), and an Agpar of 8 and 10 at 1 and 5 minutes, respectively. At birth, the erythrocyte hemoglobin (Hb) was 139 g/L (13.9 g/dL). The baby was discharged with an iron and folic acid treatment. At day 11, the baby showed a pronounced pallor. Red cell indices were as follows: Hb, 38 g/L (3.8 g/dL); red blood cells (RBCs), 0.94 × 1012/L; reticulocytes, 162 × 109/L, 17.2%; mean corpuscular volume (MCV), 100.6 fL; and mean cell hemoglobin concentration (MCHC), 359 g/L (35.9 g/dL). A first transfusion was given. Subtotal splenectomy was carried out at 9 months of age after 11 transfusions and cancelled the need for transfusions. In addition to spherocytosis, an acidosis was noticed, which partially receded after a few months. Bicarbonate was prescribed for a month but was discontinued. Urinary pH was not measured on a regular basis, but proved to be usually below 7.00. Renal sonographies performed at 10 and 22 months of age showed no signs of nephrocalcinosis. At 2 years of age, an ammonium chloride challenge suggested that the child has incomplete dRTA; in the 7 hours of the test, the blood bicarbonates decreased to 15.6 mM, but urinary pH remained above 5.90.
The parents were unaware of carrying hereditary spherocytosis. They showed a compensated hemolysis, increased percentage of hyperdense cells, spherocytosis on blood smears, and a typical curve of HS upon osmotic gradient ektacytometry11 (Table 1; Figure 1).
Erythrocyte indices and ektacytometric parameters
| . | Proband . | Mother . | Father . |
|---|---|---|---|
| RBC, ×1012/L | 0.94 | 3.55 | 4.85 |
| Hb, g/L | 38 | 116 | 156 |
| Hematocrit, % | 9.4 | 32.6 | 43 |
| MCV, fL (86-98) | 100.6 | 91.8 | 88.6 |
| MCH, pg (27-32) | 41 | 32.5 | 32.1 |
| MCHC, g/L (measured; 320-360) | 359 | 374 | 376 |
| Hyperdense cells, %* | 13.7 | 12.5 | 8.7 |
| Reticulocytes, 109/L (%) | 162 (17.2) | 120.7 (3.4) | 101.9 (2.1) |
| MCVr, fL† | 110.5 | 101.8 | 100.2 |
| Omin‡ | 240 | 169 | 164 |
| DImax‡ | 0.05 | 0.35 | 0.34 |
| O′‡ | 361 | 350 | 351 |
| . | Proband . | Mother . | Father . |
|---|---|---|---|
| RBC, ×1012/L | 0.94 | 3.55 | 4.85 |
| Hb, g/L | 38 | 116 | 156 |
| Hematocrit, % | 9.4 | 32.6 | 43 |
| MCV, fL (86-98) | 100.6 | 91.8 | 88.6 |
| MCH, pg (27-32) | 41 | 32.5 | 32.1 |
| MCHC, g/L (measured; 320-360) | 359 | 374 | 376 |
| Hyperdense cells, %* | 13.7 | 12.5 | 8.7 |
| Reticulocytes, 109/L (%) | 162 (17.2) | 120.7 (3.4) | 101.9 (2.1) |
| MCVr, fL† | 110.5 | 101.8 | 100.2 |
| Omin‡ | 240 | 169 | 164 |
| DImax‡ | 0.05 | 0.35 | 0.34 |
| O′‡ | 361 | 350 | 351 |
Red cell indices were determined using the Advia 120 automat (Bayer Diagnostic France, Puteaux, France). Normal values for MCV, MCH, and MCHC are shown in parentheses.
MCH indicates mean corpuscular hemoglobin; MCHC, MCH concentration; and MCVr, mean cell volume of reticulocytes.
Normal values, lower than 4%; hemoglobin concentration, greater than 410 g/L (41.0 g/dL).
Normal values, 109.8 ± 6.1 fL (n = 37). In the proband, high MCV was in keeping with age (11 days), and MCH was increased accordingly.
Ektacytometric parameters Omin, DImax, and O′ are defined in the legend for Figure 1.
Blood smears and osmotic gradient ektacytometry. Blood smears (A) and osmotic gradient ektacytometry (B). (A) P indicates proband. Many red blood cells were spherocytes (➡). The remaining showed further changes, as if some fragmentation had occurred. There was a pronounced anisocytosis. A few red cells verged on stomatocytes (→). Erythroblasts were present (17%). F indicates father. Presence of many spherocytes without anisocytosis. M indicates mother. Presence of many spherocytes without anisocytosis. (B) In both parents (M and F), there was an increased osmotic fragility, a reduced maximum deformability index, and a decreased dehydration, a situation typical of HS.11 These features were dramatically enhanced in the proband (P). C indicates control. The maximum deformability index (DImax; normal values, 0.41-0.53 AU) is the maximum value of the deformability index. The “hypo-osmotic point” (Omin; normal values, 143-163 mOsm/L) is the osmolality at which the deformability index reaches a minimum in the hypotonic region; it is the same as the osmolality at which 50% of the erythrocytes hemolyze in a standard osmotic resistance test. This index thus provides a measure of the average surface area–to-volume ratio of erythrocytes. The “hyper-osmotic point” (O′; normal values, 325-375 mOsm/L) is the osmolality in the hypertonic region (right leg of the curve) at which the deformability index reaches half its maximum value. It provides information on the erythrocyte hydration.
Blood smears and osmotic gradient ektacytometry. Blood smears (A) and osmotic gradient ektacytometry (B). (A) P indicates proband. Many red blood cells were spherocytes (➡). The remaining showed further changes, as if some fragmentation had occurred. There was a pronounced anisocytosis. A few red cells verged on stomatocytes (→). Erythroblasts were present (17%). F indicates father. Presence of many spherocytes without anisocytosis. M indicates mother. Presence of many spherocytes without anisocytosis. (B) In both parents (M and F), there was an increased osmotic fragility, a reduced maximum deformability index, and a decreased dehydration, a situation typical of HS.11 These features were dramatically enhanced in the proband (P). C indicates control. The maximum deformability index (DImax; normal values, 0.41-0.53 AU) is the maximum value of the deformability index. The “hypo-osmotic point” (Omin; normal values, 143-163 mOsm/L) is the osmolality at which the deformability index reaches a minimum in the hypotonic region; it is the same as the osmolality at which 50% of the erythrocytes hemolyze in a standard osmotic resistance test. This index thus provides a measure of the average surface area–to-volume ratio of erythrocytes. The “hyper-osmotic point” (O′; normal values, 325-375 mOsm/L) is the osmolality in the hypertonic region (right leg of the curve) at which the deformability index reaches half its maximum value. It provides information on the erythrocyte hydration.
Erythrocyte membrane protein analysis
Preparation of erythrocyte membranes, sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), Coomassie blue staining, and Western blotting of membrane proteins were carried out as previously described.8 Protein concentration was estimated using the Bradford assay and equal amounts (typically 10 μg) of ghosts were loaded per track of each gel. Quantitation of proteins was done by scanning densitometry. Monoclonal antibodies were BRIC170 (anti–band 3), BRIC274 (antiankyrin), and antiactin (Abcam, Cambridge, United Kingdom), and were used as previously described.8 Rabbit antipeptide antibodies anti-RhAG, anti-Rh, anti-p55 and anti-4.1, anti-4.2, and anti-CD47 were used as described.8 Rabbit antipeptide AQP1 was raised against the synthetic peptide to C-terminal AQP1 (C-VEEYDLDADDINSRVEMKPK). Rabbit antipeptide GPC was raised against the synthetic peptide to C-terminal GPC (CDPALQDAGDSSRKEYFI). Rabbit antipeptide GPA was raised against the synthetic peptide to C-terminal GPA (C-DVPLSSVEIENPETSDQ). All 3 peptides and antibodies were produced at the University of Bristol Peptide Synthesis Facility.
Analysis of SLC4A1 by SSCPs and DNA sequencing
Anion transport studies
The number of DIDS (4,4′-di-isothiocyanato-stilbene-2,2′-disulfonic acid) binding sites was determined by titration of [35S]-sulfate influx into erythrocytes, using equal numbers of patient and control cells (determined using a cell counter) at 10% hematocrit, as described previously.13
Preparation of mutant constructs and expression in Xenopus oocytes
The cDNA clones in Xenopus expression vector BSXG1 encoding human band 3 (BSXG1.B3), the kidney isoform, kidney band 3 (BSXG1.kB3), and GPA (BSXG.GPA) have been described.14 The amino acid substitution Ser667Phe was made using the Quikchange mutagenesis kit (Strategene, Amsterdam Zuidoost, the Netherlands), BSXG1.B3, and BSXG1.kB3 as templates, and the primers TGGATGATGTTTGCCTTCGCCCTGCCTGCTCTG and CAGAGCAGGCAGGGCGAAGGCAAACATCATCCA. The sequences of the final constructs were confirmed by automated DNA sequencing (Department of Biochemistry, Geneservice, Oxford, United Kingdom). The methods used for the preparation of band 3 cRNA, expression in oocytes, and assay of 36Cl− influx have been described.14
Cell-surface protease assay and biotinylation of oocytes
The chymotrypsin surface assay was conducted after incubation for 24 and 48 hours at 18°C as previously described.15 The cell-surface biotinylation method was adapted from a mammalian cell biotinylation assay.16 Oocytes were injected with cRNAs and incubated for 24 hours at 18°C. The oocytes were washed in precooled borate buffer (10 mM boric acid, 154 mM NaCl, 7.2 mM KCl, and 1.8 mM CaCl2 [pH 9.0]) and then treated with 2 mg/mL nonpenetrating biotinylation reagent EZ-link Sulfo-NHS-biotin in borate buffer (Pierce, Rockford, IL) at 4°C for 30 minutes. The reaction was quenched using several washes of 0.192 M glycine/25 mM Tris (pH 8.3) buffer. Oocytes were then homogenized in immunoprecipitation (IP) buffer (10 mM Tris/HCl [pH 7.4], 150 mM NaCl and 1 mM EDTA, 1% (vol/vol) Triton X-100, 0.02% SDS, and antiproteases), and AE1 protein was immunoprecipitated using αrbB3Ct bound to protein A beads (30 oocytes for each cRNA injection, 10 oocytes per IP). The immunoprecipitated AE1 was eluted from the protein A beads by boiling in IP buffer (with no NaCl added) containing 5% SDS and resuspended in 50 vol of IP buffer with no SDS added. The biotinylated AE1 was isolated from the pooled eluate using streptavidin beads (Pierce). A proportion of the AE1 immunoprecipitate was saved to give an indication of the input for the streptavidin isolation. The samples were washed and eluted from streptavidin beads and then run on an 8% SDS PAGE gel, electroblotted, and then probed with the C-terminal AE1 monoclonal BRIC155.
Preparation of mutant constructs and expression in MDCK1 cells
The mutant human kidney band 3 cDNA (S667F-kB3) was TOPO-TA cloned into the pcDNA3 vector (Invitrogen, Paisley, United Kingdom) as previously described.16,17 MDCK cells type 1 (MDCK1) were grown in Dulbecco modified Eagle media containing 25 mM HEPES supplemented with 10% (vol/vol) fetal bovine serum (Invitrogen) and maintained at 37°C in 5% CO2. MDCK1-kB3 cells stably expressing kidney band 3 were already available.16,17 Stably transfected MDCK1-S667F-kB3 cells were obtained as described previously.17 Cells were seeded on to poly-lysine–coated coverslips (Sigma, Poole, United Kingdom) at either low density (∼5 × 104 cells/well) to obtain nonpolarized cells, or approximately 106 cells to obtain polarized cells. Polarized cells grown on filters were seeded at 5 × 105 as previously described.16 The nonpolarized cells were processed for immunofluorescence within 24 hours. The polarized cells were cultured for 2 to 3 days, then incubated with 5 mM sodium butyrate for 12 to 16 hours prior to fixation to increase protein expression.16
Immunofluorescence microscopy
Cells were processed for confocal microscopy as described previously.16 Briefly, the cells were fixed in methanol/acetone (6:4 vol/vol), blocked with 4% bovine serum albumin (BSA) in phosphate-buffered saline (PBS), incubated with anti–band 3 antibodies (mouse monoclonal BRIC170,8 rabbit anti-Ct peptide band 3 [raised against a synthetic peptide to band 3 residues 881-911: αrbB3Ct], or sheep polyclonal anti-band 3 [αshB3]18 ) and detected with suitable secondary antibodies. For antibody double-labeling, the cells were incubated with BRIC170 culture supernatant (approximately 39 μg/mL), washed 3 times with PBS, then incubated with rabbit anticalnexin (Stressgen, San Diego, CA), anti-TGN38,16 or anti–β-catenin (Abcam). After the final washes, the cells were mounted in Vectashield (Vector Labs, Burlingame, CA). Secondary antibodies used were goat anti–mouse Alexa fluor 488 and goat anti–rabbit Alexa fluor 594 (Invitrogen) and donkey anti–sheep TRITC (Stratec Scientific, Cambridgeshire, United Kingdom).
Fluorescence imaging was conducted using a Leica TCS-NT confocal laser-scanning microscope (Leica Microsystems, Milton Keynes, United Kingdom) using a 63×/1.32 oil-immersion objective equipped with a Kr/Ar laser. The dual-labeling Alexa fluor 488 or TRITC/Alexa fluor 594 images were taken sequentially with excitation/emission filters set at short pass 510 nm/band pass 530 nm for Alexa fluor 488 and band pass 568 nm/long pass 590 nm for TRITC/Alexa fluor 594. Images were assembled using Adobe Photoshop 8 software and Adobe Illustrator CS 11 software (Adobe, San Jose, CA).
Labeling of cell-surface band 3 with an extracellular anti–band 3 antibody
FITC-conjugated BRIC6 (FITC-BRIC6; anti–band 3 mouse monoclonal antibody labeled as described16 ) in 4% BSA was applied directly to PBS-washed intact unpolarized cells on coverslips for 1 hour at 37°C. Cells were incubated for a longer time period (1 hour compared with 30 minutes used in Toye et al16 ) to increase detectable levels of surface-bound or internalized FITC-BRIC6. We have found using this technique that even after an hour of incubation, the majority of FITC-BRIC6 attached to MDCK1-kAE1 cells remains at the plasma membrane (A.M.T., unpublished observations, July 2, 2007). No FITC-BRIC6 was found associated with control MDCK1 cells under these conditions (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The cells were washed twice and both fixed and permeabilized with methanol/acetone (6/4 vol/vol) at −20°C for 5 minutes. The cells were then labeled with αrbB3Ct, detected using a goat anti–rabbit Alexa fluor 594 secondary antibody, and imaged and processed as described.
Endoglycosidase digestion of immunoprecipitates
A total of 2 separate immunoprecipitations from confluent MDCK1-kB3 or MDCK1-S667F-kB3 cells grown on 10-cm2 dishes were conducted as described previously but using αrbB3Ct.16 The immunoprecipitated proteins were eluted from the protein A beads by boiling in SDS-PAGE sample buffer. A total of 2 vol of 2.5% NP40 (wt/vol) in 125 mM sodium phosphate buffer (pH 7.5) were added. Then, equal aliquots of the sample were either not digested or digested with endoglycosidase H (Endo H) or N-glycanase F (PNGase F; New England Biolabs, Beverly, MA). The samples were run on 8% SDS-PAGE gels, electroblotted, and then probed with BRIC170.
Results
Phenotype and genotype
Blood smears from the proband showed many spherocytes plus other abnormal cells (Figure 1A). Osmotic gradient ektacytometry displayed a dramatically disturbed curve in keeping with severe spherocytosis (Figure 1B). The heterozygous parents had almost 100% spherocytes and both showed typical HS ektacytometry curves (Figure 1). DNA from the patient was analyzed for SSCPs using primers to exons 2 to 20 of SLC4A1. A band shift was noted in exon 16. DNA sequencing showed that the patient was homozygous, and the parents heterozygous, for a single base mutation, c.2000C>T, which led to the substitution Ser667Phe (Figure S2).
Erythrocyte membrane protein analysis
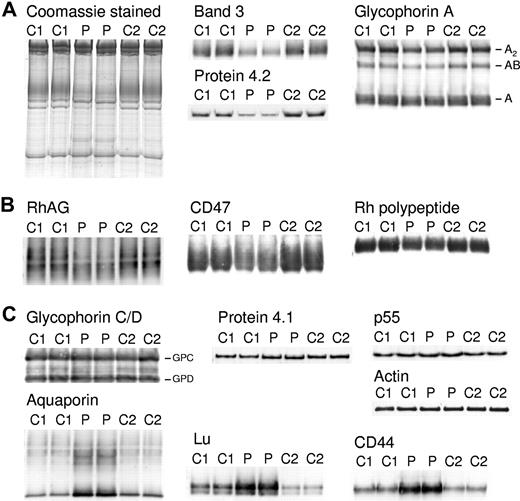
Erythrocyte membrane ghost preparations were separated by SDS-PAGE and immunoblotted using a range of erythrocyte membrane protein antibodies (Figure 2). The patient's erythrocytes serotyped as Rh phenotype R1R1, and 2 R1R1 controls were used for the SDS-PAGE so that direct comparison of the amount of Rh proteins was possible. The amount of band 3 present in the patient's erythrocytes was about 35% of the 2 wild-type controls; protein 4.2 was similarly reduced (Figure 2A; Coomassie-stained gel and immunoblots). GPA was reduced to about 60% normal (Figure 2A). This reduction is most obvious in the dimer (A2) and heterodimer (AB) forms of GPA, whose formation in SDS is dependent on the amount of monomeric GPA present. Proteins of the Rh complex (RhAG, Rh polypeptides, CD47) were reduced to about 60% of normal (Figure 2B). Proteins of the GPC complex (GPC, protein 4.1, p55) and ankyrin (data not shown) were present in normal amounts; however, 4.1b was increased, and 4.1a was decreased, due to reticulocytosis (Figure 2C). Spectrin was present in normal amounts, in contrast to the reduced amount present in band 3 Coimbra (data not shown). Aquaporin 1 (AQP1) was increased again at variance to band 3 Coimbra, as were CD44 and Lu, possibly a result of selective enrichment during erythrocyte membrane loss in the circulation (Figure 2C).8
Coomassie and immunostaining of erythrocyte membrane proteins. Erythrocyte membranes were separated on 10% Laemmli gels and immunoblotted using antibodies as shown. Loading C1, C2, controls 1 and 2, respectively. P indicates proband. (A) Proteins of the band 3 complex: immunoblotting used the monoclonal antibody BRIC170 (N-terminal band 3) and antipeptide antibodies against C-terminal of protein 4.2 and GPA. (B) Proteins of the Rh complex: immunoblotting used antipeptide antibodies against C-terminal of RhAG, Rh polypeptides, and CD47. (C) Proteins of the glycophorin C (GPC) complex: immunoblotting used antipeptide antibodies against C-terminal of GPC and GPD, protein 4.1, and p55. Other proteins: immunoblotting used monoclonal antibodies anti–β-actin (Abcam), BRIC235 (CD44), and BRIC221 (Lu), and an antipeptide antibody against C-terminal aquaporin (AQP1). All antibodies used as described in “Erythrocyte membrane protein analysis.”
Coomassie and immunostaining of erythrocyte membrane proteins. Erythrocyte membranes were separated on 10% Laemmli gels and immunoblotted using antibodies as shown. Loading C1, C2, controls 1 and 2, respectively. P indicates proband. (A) Proteins of the band 3 complex: immunoblotting used the monoclonal antibody BRIC170 (N-terminal band 3) and antipeptide antibodies against C-terminal of protein 4.2 and GPA. (B) Proteins of the Rh complex: immunoblotting used antipeptide antibodies against C-terminal of RhAG, Rh polypeptides, and CD47. (C) Proteins of the glycophorin C (GPC) complex: immunoblotting used antipeptide antibodies against C-terminal of GPC and GPD, protein 4.1, and p55. Other proteins: immunoblotting used monoclonal antibodies anti–β-actin (Abcam), BRIC235 (CD44), and BRIC221 (Lu), and an antipeptide antibody against C-terminal aquaporin (AQP1). All antibodies used as described in “Erythrocyte membrane protein analysis.”
Anion transport studies
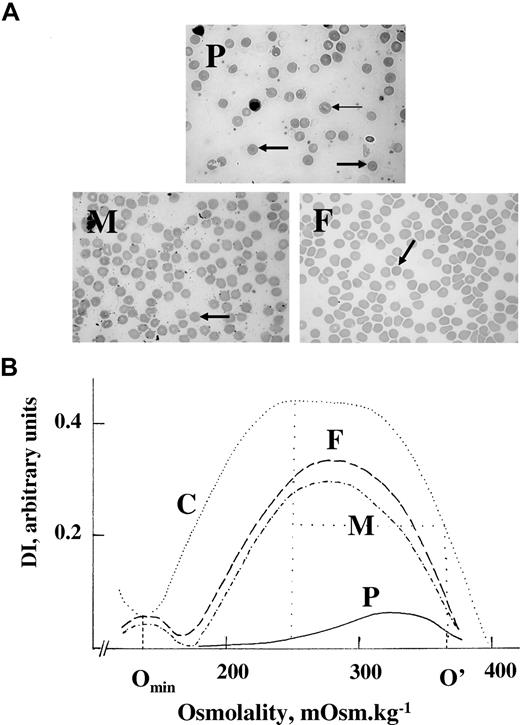
A DIDS titration of sulfate transport was done comparing the influx of sulfate in a 10% hematocrit of erythrocytes from the patient, mother, and controls over 5 minutes in the presence of increasing concentration of the anion transport inhibitor DIDS (Figure 3). DIDS binds to band 3 on a 1:1 basis with extremely high affinity, and hence the titration plots as a straight line, with the point at which the line crosses the y-axis giving the relative amount of sulfate influx and the point at which the line crosses the x-axis giving the number of DIDS-binding sites, which is equivalent to the number of band 3 molecules in the sample. The relative amount of sulfate influx and the number of DIDS-binding sites in the patient's erythrocytes were 39% and 44%, respectively, compared with the control. The relative amount of sulfate influx and the number of DIDS-binding sites in the mother's erythrocytes were both 95% compared with the control. As the amount of band 3 present in the patients erythrocytes was about 35% that of the controls (see “Erythrocyte membrane protein analysis”), this result suggests that the anion transport activity of the mutant protein is normal.
DIDS titration of sulfate transport. The influx of [35S]sulfate into the cells was measured at 10% hematocrit in isotonic citrate buffer (84 mM sodium citrate, 1 mM EGTA, 4 mM sodium sulfate [pH 6.5]). Influx was determined after 5 minutes at 30°C in the presence of different concentrations of DIDS. The lines show the results of linear regression analysis of the data. ● indicates control; ○, mother; and ▵, patient.
DIDS titration of sulfate transport. The influx of [35S]sulfate into the cells was measured at 10% hematocrit in isotonic citrate buffer (84 mM sodium citrate, 1 mM EGTA, 4 mM sodium sulfate [pH 6.5]). Influx was determined after 5 minutes at 30°C in the presence of different concentrations of DIDS. The lines show the results of linear regression analysis of the data. ● indicates control; ○, mother; and ▵, patient.
Expression in Xenopus oocytes
Coexpression of GPA partially rescues the chloride influx of band 3 Courcouronnes.
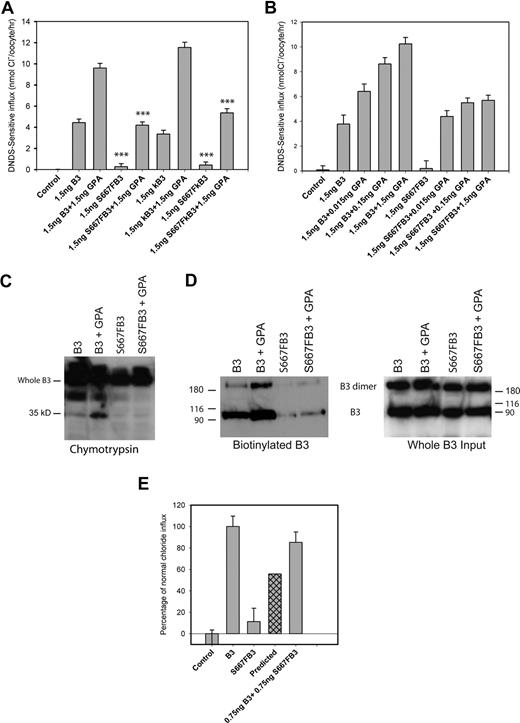
The mutant band 3 constructs prepared in both full-length erythroid band 3 (S667F-B3) and truncated kidney band 3 (S667F-kB3) were expressed in Xenopus oocytes, and the relative amount of chloride transport was compared with uninjected oocytes and oocytes expressing wild-type band 3 (B3) or kidney band 3 (kB3), with and without GPA (Figure 4A). As expected, oocytes expressing wild-type B3 or kB3 had substantial DNDS (4,4′-dinitro-2,2′-stilbene-disulfonic acid) sensitive anion transport, which was increased by coexpression of GPA.14 Both S667F-B3 and S667F-kB3 consistently gave very low levels of chloride influx that was not significantly above that of the uninjected control oocytes. Chloride influx was increased for both S667F-B3 and S667F-kB3 when GPA was coexpressed, but this was substantially lower (55% ± 4%; n = 4 for S667F-B3) than the chloride influx observed for oocytes expressing wild-type B3 or kB3 coexpressed with GPA.
Chloride influx and surface assays in Xenopus oocytes. Band 3 cRNA (B3), kidney band 3 cRNA (kB3), S667F band 3 cRNA (S667F-B3), or S667F kidney band 3 cRNA (S667F-kB3) was injected into Xenopus oocytes either alone or coinjected with GPA or band 3 at concentrations indicated. DNDS-sensitive chloride influx (1 hour) was measured 24 hours after injection with the cRNA using groups of 12 to 15 oocytes. Results are shown as means plus or minus SEM. Significance level is for comparisons of influx between B3 and S667F-B3, or B3 plus GPA and S667F-B3 plus GPA, or kB3 and S667F-kB3, or kB3 plus GPA and S667F-kB3 plus GPA (sample by Student t test; ***P < .001). For the chymotrypsin assay and the biotinylation assay, oocytes were injected with 5 ng of B3 or S667F-B3 and 1.5 ng of GPA and allowed to express protein for 24 hours. The oocytes were then subjected to a chymotrypsin assay or biotinylated as outlined in “Cell-surface protease assay and biotinylation of oocytes.” (A) S667F-B3 has very little chloride influx when expressed alone in oocytes, and this is incompletely rescued by coexpression of GPA in both S667F-B3 and S667F-kB3 to approximately 50% of wild-type B3 + GPA or kB3 + GPA. (B) Comparison of the effects of coexpression of 0.015 to 1.5 ng GPA cRNA on normal B3 and S667F-B3 chloride influx. Although GPA dose-dependently increased wild-type chloride influx, the enhancement effect of GPA was observed to be maximal at 0.15 ng with S667F-B3 (representative of 3 independent experiments) and was saturated. (C,D) Representative chymotrypsin and biotinylation blots after 24 hours' expression using anti–band 3 C-terminal antibody BRIC155 to detect B3. The chymotrypsin gel was loaded with 10 oocytes per lane and is representative of 2 separate experiments conducted in duplicate. The biotinylation assay used material from 3 immunoprecipitations (10 oocytes per IP), and the biotinylated fraction from this pooled material was isolated using strepavidin beads (representative of 2 separate experiments). One-twentieth of the input from the 3 IPs is also shown. Both methods confirm that GPA increases the level of wild-type B3 at the cell surface as previously reported, but only a small amount of S667F-B3 is detected at the cell surface; this does not appear to increase upon coexpression with GPA under the conditions used. (E) Effects of coexpression of 0.75 ng normal B3 with 0.75 ng S667F-B3 on chloride influx. Results are expressed as percentages of the chloride influx obtained with normal B3 and are shown as means plus or minus SEM. The predicted amount of activity is also shown, which represents the expected contribution of 50% B3 and 50% S667F-B3 chloride influx assuming that each population is independent. This result shows that coexpression of wild-type B3 with S667F-B3 rescues the chloride influx of S667F-B3 beyond the predicted level (representative of 4 independent experiments).
Chloride influx and surface assays in Xenopus oocytes. Band 3 cRNA (B3), kidney band 3 cRNA (kB3), S667F band 3 cRNA (S667F-B3), or S667F kidney band 3 cRNA (S667F-kB3) was injected into Xenopus oocytes either alone or coinjected with GPA or band 3 at concentrations indicated. DNDS-sensitive chloride influx (1 hour) was measured 24 hours after injection with the cRNA using groups of 12 to 15 oocytes. Results are shown as means plus or minus SEM. Significance level is for comparisons of influx between B3 and S667F-B3, or B3 plus GPA and S667F-B3 plus GPA, or kB3 and S667F-kB3, or kB3 plus GPA and S667F-kB3 plus GPA (sample by Student t test; ***P < .001). For the chymotrypsin assay and the biotinylation assay, oocytes were injected with 5 ng of B3 or S667F-B3 and 1.5 ng of GPA and allowed to express protein for 24 hours. The oocytes were then subjected to a chymotrypsin assay or biotinylated as outlined in “Cell-surface protease assay and biotinylation of oocytes.” (A) S667F-B3 has very little chloride influx when expressed alone in oocytes, and this is incompletely rescued by coexpression of GPA in both S667F-B3 and S667F-kB3 to approximately 50% of wild-type B3 + GPA or kB3 + GPA. (B) Comparison of the effects of coexpression of 0.015 to 1.5 ng GPA cRNA on normal B3 and S667F-B3 chloride influx. Although GPA dose-dependently increased wild-type chloride influx, the enhancement effect of GPA was observed to be maximal at 0.15 ng with S667F-B3 (representative of 3 independent experiments) and was saturated. (C,D) Representative chymotrypsin and biotinylation blots after 24 hours' expression using anti–band 3 C-terminal antibody BRIC155 to detect B3. The chymotrypsin gel was loaded with 10 oocytes per lane and is representative of 2 separate experiments conducted in duplicate. The biotinylation assay used material from 3 immunoprecipitations (10 oocytes per IP), and the biotinylated fraction from this pooled material was isolated using strepavidin beads (representative of 2 separate experiments). One-twentieth of the input from the 3 IPs is also shown. Both methods confirm that GPA increases the level of wild-type B3 at the cell surface as previously reported, but only a small amount of S667F-B3 is detected at the cell surface; this does not appear to increase upon coexpression with GPA under the conditions used. (E) Effects of coexpression of 0.75 ng normal B3 with 0.75 ng S667F-B3 on chloride influx. Results are expressed as percentages of the chloride influx obtained with normal B3 and are shown as means plus or minus SEM. The predicted amount of activity is also shown, which represents the expected contribution of 50% B3 and 50% S667F-B3 chloride influx assuming that each population is independent. This result shows that coexpression of wild-type B3 with S667F-B3 rescues the chloride influx of S667F-B3 beyond the predicted level (representative of 4 independent experiments).
To investigate how little GPA cRNA was required to significantly increase the level of S667F-B3 DNDS-sensitive anion transport, oocytes were injected with a constant amount of band 3 cRNA (1.5 ng/oocyte) together with GPA cRNA concentrations ranging from 0.015 ng to 1.5 ng (Figure 4B). Coexpression of increasing amounts of GPA cRNA linearly increased the chloride influx of wild-type band 3 (Figure 4B). However, the rescue effect of GPA on the mutant S667F-B3 chloride influx was observed at the lowest levels of GPA cRNA coinjected (0.015 ng), and coexpression of increasing amounts of GPA cRNA (greater than 0.015 ng) did not significantly improve on this influx (Figure 4B).
To investigate whether coexpression of GPA increased the expression of the S667F-B3 at the oocyte membrane, we assessed the surface expression of S667F-B3 by chymotrypsin cleavage and biotinylation. Band 3 possesses a single extracellular chymotrypsin cleavage site between transmembrane spans 5 and 6, which can be used to assay the proportion of band 3 at the oocyte membrane.15 After protease treatment, intracellular band 3 remains intact, whereas surface-expressed band 3 can be detected as a 35-kDa C-terminal fragment using Western blotting.15 Coexpression of band 3 with GPA increased the amount of wild-type band 3 protein at the cell surface as expected14 (Figure 4C). There was very little S667F-B3 expressed at the surface in the absence of GPA, and coexpression with GPA did not enhance this (Figure 4C). Biotinylation studies gave a similar result (Figure 4D). Thus, since there was no obvious enhancement of S667F-B3 protein expression to the surface under these conditions, this suggests that the majority of the observed rescue of chloride influx seen on coexpression with GPA results from enhanced activity of the mutant protein already at the surface. Interestingly, S667F-B3 protein levels were further reduced in oocytes incubated for 48 hours, suggesting that the mutant protein is degraded (results not shown). Pulse-chase experiments in MDCK1 cells also show rapid degradation of the mutant protein (Figure S3).
Coexpression of wild-type band 3 significantly rescues the chloride influx of band 3 Courcouronnes.
We next investigated whether coexpression of wild-type B3 could influence the expression/anion transport of S667F-B3. If the DNDS-sensitive anion transport achieved was proportional to the separate activities of the 2 proteins, then we anticipated that we would observe combined anion transport equivalent to 50:50 of wild-type and mutant band 3 activity. To mimic the normal, homozygous, and heterozygous situations, oocytes were injected with B3 (1.5 ng) alone, S667F-B3 (1.5 ng) alone, or B3 (0.75 ng) and S667F-B3 (0.75 ng) combined, and chloride influx was measured (Figure 4E). DNDS-sensitive chloride influx in oocytes coexpressing B3 and S667F-B3 consistently ranged between 70% to 90% of normal (mean, 79.7% ± 3.8%; n = 7) compared with the predicted 55% activity (Figure 4E). This effect was unexpected, as generally coexpression of wild-type band 3 with other band 3 mutants results in anion transport equivalent to approx 50:50 that of the mutant and wild-type activities13 (Figure S4).
We tried to use biotinylation to measure the surface expression of S667F-B3 in the presence of wild-type pro tein using a combination of S667F-B3 and WT-kB3 in order to get a detectable size differential between the 2 proteins. It was not possible to discern any obvious difference between the levels of S667F-B3 protein when expressed alone and when the mutant protein was coexpressed with normal protein (Figure S5). However, where WT-kB3 and S667F-B3 were coexpressed, both proteins were expressed to the surface at similar levels (Figure S5), consistent with the idea that both mutant and wild-type protein equally contribute to the increase in chloride influx observed when the 2 proteins are coexpressed.
Expression in MDCK1 cells
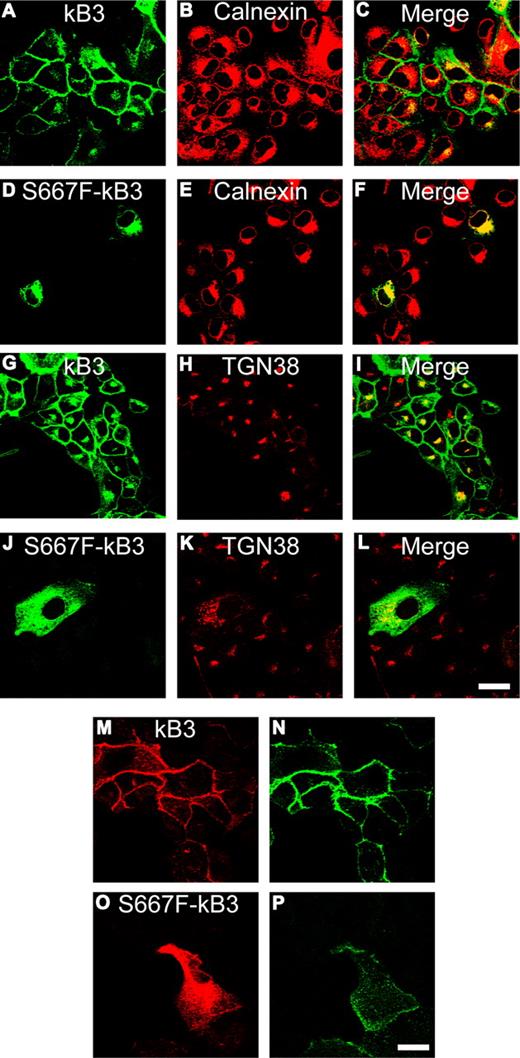
To examine the effects of the Ser667Phe mutation on the trafficking of kidney band 3 in nonpolarized and polarized cells, we stably expressed S667F-kB3 in MDCK1 cells. MDCK1 cells target kidney band 3 to the plasma membrane in nonpolarized cells and correctly target the protein to the basolateral membrane when these cells are seeded at high density and allowed to polarize.16,17 As previously observed, wild-type kidney band 3 is predominantly at the plasma membrane in MDCK1-kB3 cells (Figure 5A,G), with variable amounts of intracellular material that overlaps with the TGN marker TGN38 (Figure 5I; Figure S6 for higher-resolution images).
Expression of S667F-kB3 in nonpolarized MDCK1 cells. (A-P) MDCK1 cells stably expressing normal kB3 or mutant S667F-kB3 that were grown on coverslips and fixed (A-L), or washed, incubated with the extracellular anti–band 3 antibody BRIC6 for 1 hour, and then fixed (M-P) as outlined in “Methods.” (A-L) Comparison of kB3 and S667F-kB3 localization with intracellular markers for the ER (calnexin) or TGN (TGN38). kB3 had only a partial overlap with the ER marker (merge; panel C) and some overlap with TGN38 (merge; panel I); most of the protein is at plasma membrane as previously reported.16 The majority of mutant S667F-kB3 immunoreactive protein overlapped with the calnexin (merge; panel F), but there was some overlap with the TGN38 staining (merge; panel L), suggesting that a small proportion of the protein reaches the late stages of the secretory pathway. (M-P) rbB3Ct staining (M,O) and BRIC6 staining (N,P). All cells expressing wild-type kB3 (confirmed by double staining with the rbB3Ct: panel M) are labeled with substantial amount of FITC-BRIC6 (N). Cells expressing S667F-kB3, which were detected with the rbB3Ct antibody (O), also bound a small amount of FITC-BRIC6 (P), suggesting that a small amount of S667F-kB3 can reach the plasma membrane. Scale bar equals 30 μm.
Expression of S667F-kB3 in nonpolarized MDCK1 cells. (A-P) MDCK1 cells stably expressing normal kB3 or mutant S667F-kB3 that were grown on coverslips and fixed (A-L), or washed, incubated with the extracellular anti–band 3 antibody BRIC6 for 1 hour, and then fixed (M-P) as outlined in “Methods.” (A-L) Comparison of kB3 and S667F-kB3 localization with intracellular markers for the ER (calnexin) or TGN (TGN38). kB3 had only a partial overlap with the ER marker (merge; panel C) and some overlap with TGN38 (merge; panel I); most of the protein is at plasma membrane as previously reported.16 The majority of mutant S667F-kB3 immunoreactive protein overlapped with the calnexin (merge; panel F), but there was some overlap with the TGN38 staining (merge; panel L), suggesting that a small proportion of the protein reaches the late stages of the secretory pathway. (M-P) rbB3Ct staining (M,O) and BRIC6 staining (N,P). All cells expressing wild-type kB3 (confirmed by double staining with the rbB3Ct: panel M) are labeled with substantial amount of FITC-BRIC6 (N). Cells expressing S667F-kB3, which were detected with the rbB3Ct antibody (O), also bound a small amount of FITC-BRIC6 (P), suggesting that a small amount of S667F-kB3 can reach the plasma membrane. Scale bar equals 30 μm.
We successfully isolated stable clones expressing S667F-kB3, but only a small proportion of these cells contained detectable amounts of S667F-kB3 as determined by confocal microscopy using a variety of band 3 antibodies. Where detected, the majority of S667F-kB3 was localized to an intracellular compartment reminiscent of the endoplasmic reticulum (ER; Figure 5D,J). This localization was also seen in MDCK1 cells transiently transfected with S667F-kB3 (results not shown). Double antibody staining using the monoclonal antibody BRIC170 in combination with an antibody to the ER marker calnexin confirmed that the majority of the S667F-kB3 immunolocalized protein overlaps substantially with the ER (Figures 5F, S6). However, unlike other band 3 mutants that are exclusively trapped in the ER, such as Arg589His and Ser613Phe,16 S667F-kB3 immunostaining colocalized with the TGN marker TGN38 (Figure 5L), suggesting that some mutant protein is able to escape the ER and reach the later stages of the secretory pathway. Therefore, we used an extracellular band 3 antibody FITC-BRIC6 (which recognizes the third extracellular loop of band 3) to detect any kidney band 3 protein at the surface. All cells expressing wild-type kidney band 3 (Figure 5M) were labeled with substantial amount of FITC-BRIC6 (Figure 5N). All cells expressing mutant kidney band 3, detected with the αrbB3Ct antibody (Figure 5O), also bound a very small amount of FITC-BRIC6 (Figure 5P), suggesting that a small proportion of S667F-kB3 is able escape the ER and to reach the cell surface. Double-staining MDCK1-S667F-kB3 cells with both BRIC170 and αrbB3Ct showed that the cells expressing low levels of S667F-kB3 are not detected by αrbB3Ct (Figure S1). Therefore, it is the cells expressing higher levels of S667F-kB3 (visible using αrbB3Ct antibody) that show small amounts of mutant protein at the cell surface.
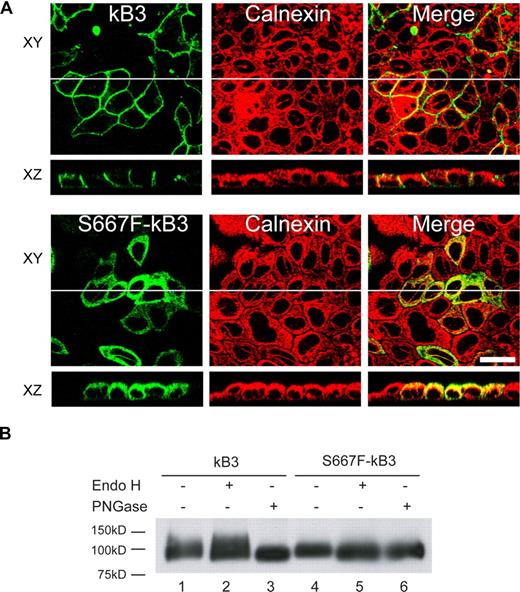
We also examined the localization of S667F-kB3 in polarized MDCK1 cells. Figure 6A shows polarized MDCK1 cells expressing wild-type kB3 or S667F-kB3 double-labeled with the BRIC170 antibody and a rabbit antibody to calnexin. The top portion of the panels (XY) display a view parallel to the epithelium showing the BRIC170 image, calnexin, and merged image, and panels underneath (XZ) show a perpendicular view of BRIC170, calnexin, and the merged image. Wild-type kB3 did not overlap with calnexin distribution, and is localized to the basolateral membrane as previously reported.16 In contrast, the majority of the S667F-kB3 immunoreactive protein overlaps with the localization of calnexin in polarized cells, confirming the intracellular localization of the majority of S667F-kB3 observed in nonpolarized MDCK1 cells. Polarized MDCK1 cells expressing kB3 or S667F-kB3 were also double-labeled with BRIC170 and the basolateral marker β-catenin. Wild-type kB3 clearly colocalizes with the basolateral marker, but no such colocalization was observed with S667F-kB3 (Figure S7). To investigate whether S667F-kB3 reaches the surface in polarized cells, kB3-and S667F-kB3–expressing cells were incubated with FITC-BRIC6. Although kB3 was detectable at the basolateral membrane, no S667F-kB3 was detectable for the MDCK1-S667F-kB3–expressing cells, probably due to the lower protein expression seen when MDCK1 cells are polarized17 (results not shown).
Polarized expression of S667F-kB3 in MDCK1 cells and endoglycosidases treatment. (A) MDCK1 cells stably expressing kB3 or S667F-kB3 that were allowed to polarize for 3 days; their protein expression was induced with sodium butyrate and fixed as described in “Methods.” The cells were then double-labeled with anti–band 3 mouse monoclonal BRIC170 and a rabbit antibody to calnexin, and the bound antibodies were detected with suitable goat anti-mouse or anti-rabbit secondary antibodies and imaged using confocal microscopy. The top panels (XY) show a view parallel to the epithelium, with the BRIC170 image, calnexin, and merged images. The images below (XZ) show a perpendicular view of BRIC170, calnexin, and the merged image of the same epithelium, as represented by the white line in the XY image. kB3 did not overlap with calnexin distribution, and is localized to the basolateral membrane. In contrast, the majority of the S667F-kB3 immunoreactive protein overlaps with the localization of calnexin in polarized cells. Scale bar equals 30 μM. (B) Western blot of kB3 or S667F-kB3 proteins immunoprecipitated with rbB3Ct from one confluent 10-cm2 plate of cells induced with sodium butyrate and treated with either nothing, Endo H (removes high mannose glycosylation), and PNGase (removes complex glycosylation). The immunoprecipitated proteins were eluted and detected by Western blotting using anti–band 3 BRIC170. Normal kB3 protein is complex glycosylated, as evidenced by a diffuse band present in lane 1, which is insensitive to Endo H treatment (lane 2) but runs as a lower-molecular-weight after treatment with PNGase (lane 3). In contrast, S667F-kB3 does not have a diffuse band (lane 4) and is sensitive to Endo H (compare lane 4 with 5), suggestive of high mannose glycosylation only, and consistent with this protein being retained in the ER.
Polarized expression of S667F-kB3 in MDCK1 cells and endoglycosidases treatment. (A) MDCK1 cells stably expressing kB3 or S667F-kB3 that were allowed to polarize for 3 days; their protein expression was induced with sodium butyrate and fixed as described in “Methods.” The cells were then double-labeled with anti–band 3 mouse monoclonal BRIC170 and a rabbit antibody to calnexin, and the bound antibodies were detected with suitable goat anti-mouse or anti-rabbit secondary antibodies and imaged using confocal microscopy. The top panels (XY) show a view parallel to the epithelium, with the BRIC170 image, calnexin, and merged images. The images below (XZ) show a perpendicular view of BRIC170, calnexin, and the merged image of the same epithelium, as represented by the white line in the XY image. kB3 did not overlap with calnexin distribution, and is localized to the basolateral membrane. In contrast, the majority of the S667F-kB3 immunoreactive protein overlaps with the localization of calnexin in polarized cells. Scale bar equals 30 μM. (B) Western blot of kB3 or S667F-kB3 proteins immunoprecipitated with rbB3Ct from one confluent 10-cm2 plate of cells induced with sodium butyrate and treated with either nothing, Endo H (removes high mannose glycosylation), and PNGase (removes complex glycosylation). The immunoprecipitated proteins were eluted and detected by Western blotting using anti–band 3 BRIC170. Normal kB3 protein is complex glycosylated, as evidenced by a diffuse band present in lane 1, which is insensitive to Endo H treatment (lane 2) but runs as a lower-molecular-weight after treatment with PNGase (lane 3). In contrast, S667F-kB3 does not have a diffuse band (lane 4) and is sensitive to Endo H (compare lane 4 with 5), suggestive of high mannose glycosylation only, and consistent with this protein being retained in the ER.
Kidney band 3 is known to be complex N-glycosylated when expressed in MDCK1 cells, consistent with the ability of this protein to exit the ER and reach the plasma membrane.16 In order to ascertain the glycosylation state of S667F-kB3, the kidney band 3 protein from MDCK1-kB3 and MDCK1-S667F-kB3 cells was immunoprecipitated using αrbB3Ct, and the susceptibility of the immunoprecipitate to endoglycosidases was examined by treatment with either Endo H or PNGase and Western blotting using anti–band 3 antibody BRIC170. Figure 6B demonstrates that at steady state, kidney band 3 runs as a diffuse band, the upper part manifests as Endo H–insensitive (compare lane 1 with 2) and PNGase F–sensitive bands (lane 3), indicative of complex glycosylation. In comparison, S667F-kB3 is Endo H sensitive (compare lane 4 with lane 5), consistent with the observation here that the majority of the S667F-kB3 is retained in the ER.
Discussion
A previous study on band 3 Coimbra red cells suggested the existence of a band 3/Rh macrocomplex that may function to facilitate more efficient gas exchange in the erythrocyte.8 Evidence for the macrocomplex was based on the observation that loss of band 3 expression caused the equivalent loss of a number of other proteins, including proteins of the Rh complex.8 In band 3 Courcouronnes erythrocytes, band 3 is reduced to about 35% of normal. Protein 4.2 is likewise reduced, but proteins of the Rh complex (RhAG, Rh polypeptides, CD47) are present at about 60% of normal. The band 3/Rh macrocomplex is thought to form around the tetrameric form of band 3, and the stoichiometry (in normal cells) suggests that all the Rh proteins associate with the macrocomplex. If the oligomeric ratio of band 3 in Courcouronnes red cells is maintained at 60:40, dimer to tetramer, then the Rh complex proteins should be present at about 14% of normal. The fact that the Rh complex proteins are present at about 60% of normal suggests that when the amount of band 3 is limited, tetrameric band 3 may form preferentially, perhaps in order to fill the normal amount of ankyrin-binding sites found in band 3 Courcouronnes erythrocytes.
Association with GPA is altered or S667F-B3 is degraded before association with GPA can occur
S667F-B3 was only partially rescued by the presence of GPA in erythrocytes (Figure 2A) or oocytes (Figure 4A), suggesting that the interaction of S667F-B3 with GPA is altered. Various studies locate the GPA interaction with either TM8 or TM9 of band 3, or with the C-terminal domain.19-21 Ser667 lies in putative TM8 of band 3 and is a Ser, Ala, or Cys in all band 3 homologs. The Ser667Phe mutation introduces a bulky, hydrophobic side chain that may sterically affect or destabilize the association of GPA with band 3, reducing the ability of GPA to interact with, and therefore rescue, the majority of S667F-B3 before it is turned over by cellular protein quality control mechanisms. Furthermore, previous work has shown that the N- and C-terminal regions of GPA contribute to the enhancement effects of GPA seen on B3 activity and translocation, respectively.15 Since GPA enhanced the activity of S667F but not its expression level at the surface in oocytes, this may be an indication of an altered interaction of S667F-B3 and the C-terminal tail region of GPA.
However, if the Ser667Phe mutation simply affected the affinity of the mutant band 3 for GPA, then a titration of GPA with S667F-B3 would be expected to show a linear relationship. Instead, we found that the ability of GPA to rescue S667F-B3 becomes saturated (Figure 4B). Our studies have shown that the majority of S667F-kB3 is located in the ER and is core glycosylated, indicating that the bulk of the mutant protein has not reached the Golgi. In contrast, the dRTA G701D-kB3 mutant, which like S667F-B3 does not reach the plasma membrane of oocytes, is localized to the TGN at steady state and is complex glycosylated22 (A.M.T., unpublished observations, July 2, 2007). G701D-B3 is almost completely rescued by GPA in both oocytes and erythrocytes,23,24 whereas S667F-B3 is only partially rescued by GPA. Together, these results suggest that the interaction between band 3 and GPA occurs in the late secretory pathway (TGN) and that S667F-B3 may be turned over by the protein quality control mechanisms before it can interact with GPA.
Coexpression of wild-type band 3 rescues band 3 Courcouronnes
Band 3 Courcouronnes is expressed, and functional, in the heterozygous parents, suggesting that the trafficking defect in these cells is compensated in some way. In Xenopus oocytes, the chloride influx of S667F-B3 is substantially rescued by coexpression of wild-type B3, suggesting that the mutant protein forms fully functional heterodimers with wild-type protein. Heterodimerisation of S667F-B3 with wild-type B3 probably occurs in the ER on formation of the nascent protein and may induce the correct folding of the mutant protein, or the association may provide the required trafficking signals or interaction sites for accessory proteins necessary for ER exit, which may be occluded in the S667F-B3 mutant homodimer.25 It is interesting to speculate that wild-type rescue could be a common compensatory mechanism in band 3–induced HS. It would explain the 20% to 30% reduction in band 3, and mild phenotype, in individuals with HS caused by heterozygous band 3 mutations.
Homozygous S667F-kB3 causes incomplete dRTA
Recent in vitro studies in polarized MDCK cells suggest that dRTA mutations cause kB3 to be retained intracellularly or mistargeted to the apical membrane.16,22,26 This has been corroborated in renal biopsy tissue from one patient with the S613F mutation who had cytosolic staining.27 Retention of the majority of S667F-kB3 in the ER would explain the observed dRTA in the homozygous patient. The fact that small amounts of S667F-kB3 can reach the plasma membrane in MDCK1 cells that overexpress S667F-kB3 may explain why this patient has a mild form or incomplete dRTA (especially if we consider that acidosis is known to up-regulate band 3 RNA and protein levels in the rabbit kidney28 ). However, we cannot exclude the possibility that there may be some unknown compensating mechanism.
In summary, this study describes a novel homozygous SLC4A1 mutation (Ser667Phe) that results in HS and dRTA. This is only the second case of combined HS and dRTA to be reported. The study provides further evidence to support the hypothesis that a site of interaction between band 3 and GPA involves TM8. It also provides evidence that the formation of heterodimers between B3 mutant proteins and wild-type protein can correct mistrafficking, explaining the mild clinical presentation in some individuals heterozygous with HS. The Ser667Phe mutant will be a useful tool for further investigating the trafficking of band 3 and its interactions with GPA.
The online version of this article contains a data supplement.
Presented in part at the 48th Annual meeting of the American Society of Hematology, Orlando, FL, December 9-12, 2006.29
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ms S. Paul for technical assistance, Mr P. Martin for DNA sequencing, and Dr K. Ridgwell for preparation of AQP1 antibody.
This work was supported by the UK National Health Service R&D Directorate (L.J.B.), a National Health Service (NHS) Blood and Transplant Wellcome Trust Career Development Fellowship (A.M.T., R.W.), the “Center de Référence des Maladies Constitutionnelles de l'Erythropoïèse et du Globule Rouge, APHP,” and the “Unité 779” of the Inserm (J.D.).
Wellcome Trust
Authorship
Contribution: A.M.T. and R.W. performed expression studies in Xenopus oocytes and MDCK1 cells and prepared the manuscript; M.K. assumed the day-to-day therapeutical decisions since the neonatal period and managed the family genetic analysis; B.B.-M. and G.T. assumed specialized decisions, including partial splenectomy; T.C. performed ektacytometry; M.T. performed SDS-PAGE; M.D. performed the ammonium chloride challenge; J.D. asserted the initial diagnosis of HS associated with a nearly certain homozygous defect in the SLC4A1 gene and prepared the manuscript; and L.J.B. analyzed erythrocyte protein and anion transport, performed DNA analysis, and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lesley Bruce, Bristol Institute for Transfusion Sciences, National Blood Service, Southmead, Bristol, BS10 5ND, United Kingdom; e-mail: lesley.bruce@nbs.nhs.uk; or Ashley Toye, Department of Biochemistry, University of Bristol, BS8 1TD, United Kingdom; e-mail: ash.m.toye@bristol.ac.uk.

![Figure 3. DIDS titration of sulfate transport. The influx of [35S]sulfate into the cells was measured at 10% hematocrit in isotonic citrate buffer (84 mM sodium citrate, 1 mM EGTA, 4 mM sodium sulfate [pH 6.5]). Influx was determined after 5 minutes at 30°C in the presence of different concentrations of DIDS. The lines show the results of linear regression analysis of the data. ● indicates control; ○, mother; and ▵, patient.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/11/10.1182_blood-2007-07-099473/6/m_zh80050816220003.jpeg?Expires=1769226151&Signature=xCl-LbdWZqtvNsoivRzi60QnnZNj1HAiseMKpvIUdn6I7Ey4aJE0qffNo2~yBNTL1KilsHOSwZYkilhQ14L8tb-onw3aNqxBqcamBB329BIuN~Jq7n2Ux5rS0slTvRv2u6J43yNHjhcTYaUN~6SI6CsOZPlUAWsckSz9TF7d2LeeGHDAycBWtDq52uDNTF22LM3Hatb1ygvARgoPfsSoxU83Pt7LZ~QcDMBGdFQjp0u5~183MBePX6lXcqzZ3JqXfd7njfy1zaYmXvUY7RElw6wmUznqfTUmWPJbI7X7fflQZ7IWm1DmKqT1C~1vWskpdPzF90i9dKMwVh0DJ~1W-w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)