Abstract
Exocytosis of specialized endothelial cell secretory organelles, Weibel-Palade bodies (WPBs), is thought to play an important role in regulating hemostasis and intravascular inflammation. The major WPB core proteins are Von Willebrand factor (VWF) and its propolypeptide (Proregion), constituting more than 95% of the content. Although the composition of the WPBs can be fine-tuned to include cytokines and chemokines (eg, interleukin-8 [IL-8] and eotaxin-3), it is generally assumed that WPB exocytosis is inextricably associated with secretion of VWF. Here we show that WPBs can undergo a form of exocytosis during which VWF and Proregion are retained while smaller molecules, such as IL-8, are released. Imaging individual WPBs containing fluorescent cargo molecules revealed that during weak stimulation approximately 25% of fusion events result in a failure to release VWF or Proregion. The WPB membrane protein P-selectin was also retained; however, the membrane tetraspannin CD63 was released. Accumulation or exclusion of extracellular fluorescent dextran molecules ranging from 3 kDa to 2 mDa show that these events arise due to the formation of a fusion pore approximately 12 nm in diameter. The pore behaves as a molecular filter, allowing selective release of WPB core and membrane proteins. WPB exocytosis is not inextricably associated with secretion of VWF.
Introduction
Endothelial cells monitor and respond to changes in mechanical stresses and the chemical composition of their environment by synthesizing, expressing on the cell surface, or secreting a range of bioactive molecules. The acute secretion of preformed peptides and proteins in response to external signals is mediated primarily by exocytosis of Weibel-Palade bodies (WPBs), large rod-shaped secretory organelles unique to endothelial cells.1 The major WPB core proteins are the adhesive and procoagulant molecule Von Willebrand factor (VWF) and the VWF precursor propolypeptide (Proregion) that together constitute more than 95% of the WPBs' content. The best-characterized WPB membrane proteins are the leukocyte adhesion molecule P-selectin and the tetraspannin CD63.1 Under resting conditions other proteins may also be stored within WPBs,2 and recent studies have shown that the composition of WPBs can change rapidly to include small signaling molecules such as interleukin-8 (IL-8) following exposure to inflammatory signals.2
Although it is generally assumed that WPB exocytosis is inextricably associated with secretion of VWF, the realization that the WPB cargo can alter rapidly under different cellular conditions led us to ask the question, can WPBs selectively release different cargo molecules? If so, it could provide a mechanism by which processes such as inflammation might be regulated with a reduced risk of thrombosis. Such a mechanism would have important implications for our understanding of the role of WPBs in health and disease.
Considerable evidence, derived primarily from biophysical and optical studies in neuronal, neuroendocrine, and endocrine tissues, indicates that individual secretory organelles can undergo a type of fusion with the plasma membrane that allows the selective release or retention of granule contents. These fusion events involve the formation of a fluid-filled pore of variable dimension that has the potential to act as a molecular filter, allowing small molecules to exit while retaining larger polypeptides and proteins.3 In excitable cells the fusion pore may flicker open and close rapidly, a process called kiss-and-run fusion, before either closing with recovery of the vesicle membrane largely intact or fully dilating with collapse of the vesicle membrane into the plasma membrane.4,5 In other cell types, particularly nonexcitable cells such as pancreatic acinar cells and surfactant secreting alveolar type II cells, the pore may remain in a more stable open configuration for longer periods of time, a process called a lingering kiss, before closing to retrieve the vesicle membrane or fully dilating.6-8 Estimates of the size of fusion pores range from approximately 0.5 nm for spontaneous fusion events in pituitary lactotrophs, small enough to allow ions but not peptide content to exit9 ; 1 to 2 nm for beta cell large dense core vesicles, small enough to allow exit of adenosine triphosphate (ATP) for local paracrine signaling, but not insulin3 ; approximately 1 to 3 nm in neuronal and neuroendocrine cells, large enough to allow neurotransmitter release without loss of larger matrix proteins4 ; and up to 5 to 10 nm in pancreatic acinar cells that may allow small protein release and possible retention of larger components.10
Little is known about the biophysics of WPB fusion. High-resolution capacitance measurements in cultured human endothelial cells have revealed that during stimulation the distribution of amplitudes for discrete step increases in membrane capacitance, attributed to fusion of individual WPBs, was matched by a similar amplitude distribution for endocytic events, indicating that in some cases WPB fusion is followed by recovery of the membrane intact.11 Although it was not possible to establish directly if the insertion and retrieval of equivalent membrane areas reflected kiss-and-run or lingering kiss fusion events, those data were not inconsistent with such processes. Does such a process occur during WPB exocytosis, and if so, what is the size and molecular filter characteristics, if any, of the fusion pore that forms? Here we address these questions using multicolor fluorescence imaging of fluorescently tagged VWF, Proregion, P-selectin, CD63, and IL-8 transiently expressed in cultured human umbilical vein endothelial cells (HUVECs). We show that a population of WPBs undergoes membrane fusion during which a long-lived fusion pore of approximately 10 to 12 nm in diameter allows release of small core proteins, including the inflammatory cytokine IL-8, but not the major WPB core proteins VWF or Proregion. Evidence was also obtained for differential release of the membrane proteins P-selectin and CD63. These data show for the first time that WPB exocytosis can result in selective release of cargo molecules and that VWF secretion is not inextricably associated with WPB exocytosis.
Methods
Tissue culture and transfection
Primary HUVECs were purchased from TCS Cellworks (Botolph Clayton, United Kingdom) and grown as previously described.12 HUVECs were nucleofected using 2 to 4 μg of expression vector DNA using the Nucleofection device and buffers according to the manufacturer's instructions (Amaxa, Germany) and plated at confluent density in complete culture medium onto 35-mm diameter poly-D-lysine–coated glass-bottomed culture dishes (MatTeK, Ashland, MA) or 24-mm square glass coverslips for live cell imaging. Cells were maintained without further medium changes until their use 1 to 3 days after nucleofection.
Antibodies, reagents, and immunocytochemistry
Full-length VWF enhanced green fluorescent protein (EGFP) and Proregion-EGFP were made as previously described.12 EGFP-CD63 was a gift from Prof Paul Luzio, Cambridge University, United Kingdom. Lamp-1-YFP was a gift from Prof Norma Andrews, Yale University, New Haven, CT. cDNAs for mRFP1, mCherry, and tdTomato13 were gifts from Prof Roger Tsien, Howard Hughes Medical Institute, University of California San Diego. mRFP1 was polymerase chain reaction (PCR) amplified from the original cDNA using primers CAACCGGTCATGGCCTCCTCCGAGGACGTCATC (forward) and CTTGTACAGGGCGCCGGTGGAGTGGCGGCC (reverse), digested with AgeI/BsrGI and ligated into AgeI/BsrGI digested pEGFP-N1 (Clontech, Mountain View, CA), thereby swapping the fluorophores and producing mRFP-N1. mRFP-N2 was derived from mRFP-N1 by cutting with AgeI, filling in with T4 DNA polymerase and religating. Proregion-mRFP was made by transferring the entire Proregion cDNA from Proregion-EGFP into mRFP-N2 as an EcoRI/BamHI fragment. mCherry and tdTomato were amplified from their respective cDNAs using primers TCCACCGGTCGCCACCATGGTGAGCAAGGGCGAG (forward) and ACTTGTACAGCTCGTCCATGCCGC (reverse), digested with AgeI/BsrGI and ligated into AgeI/BsrGI–digested pEGFP-N1 (Clontech), producing mCherry-N1 and tdTomato-N1, respectively. VWF-mRFP and VWF-mCherry were made by transferring the VWF cDNA from VWF-EGFP into mRFP-N1 and mCherry-N1, respectively, as an EcoRI/AgeI fragment. mRFP-CD63 was made from EGFP-CD63 by replacing EGFP with mRFP as a AgeI/BsrGI fragment cut from mRFP-N1. Lamp-1-EGFP was made from Lamp-1-YFP by replacing YFP with EGFP as an AgeI/BsrGI fragment cut from pEGFP-N1. Lamp-1-tdTomato was made by transferring the Lamp-1 cDNA from Lamp-1-YFP into tdTomato-N1 as an EcoRI/BamHI fragment. IL-8-mCherry was made as follows: a sequence verified human IL-8 image clone (3882471 [AT17-e6]) was purchased from Geneservice (Cambridge, United Kingdom). IL-8 was amplified using primers CTCTCGAG ATGACTTCCAAGCTGGCC (forward) and CTTGGATCCCGTGAATTCTCAGCCCTCTTC (reverse), digested with XhoI/BamHI, and ligated into mCherry-N1. A cDNA for P-selectin flanked by BglII/SalI was generated by PCR from HUVEC cDNA using primers AAAGATCTGAGATGGCCAACTGCCAAATAGCC (forward) and GGTACCGTCGACAGGACTCGGGTCAAATGCAGCG (reverse). The PCR product was cloned into TOPO (Invitrogen, Paisley, United Kingdom) and the BglIl/SalI fragment encoding P-selectin cut out and ligated into BglII/XhoI digested pDsRed2-N1 (Clontech) to produce P-selectin-DsRed2. P-selectin-YFP was made by transferring the cDNA of P-selectin as a BglII/AgeI fragment from P-selectin-DsRed2 into BglII/AgeI digested YFP-N1. P-selectin-mRFP was derived from P-selectin-YFP by exchanging YFP with mRFP as an AgeI/BsrGI fragment cut from mRFP-N1. All PCR products were verified by sequencing. FM4-64, alexa-647, and the tetramethylrhodamine isothiocyanate (TRITC)–conjugated dextrans of 3, 10, 40, and 150 kDa and 2 mDa were purchased from Invitrogen. TRITC-conjugated 70 kDa dextran was purchased from Sigma-Aldrich (Poole, United Kingdom). TRITC-conjugated dextrans were purified from free fluorophore by gel-filtration through G-10 Sephadex (Sigma-Aldrich) prior to use.
Immunoblotting
Chinese hamster ovary cells were Nucleofected with mCherry-N1 or IL-8-mCherry, grown for 16 hours, and the cell monolayers washed and lysed in ice-cold phosphate buffered saline (PBS) containing 1% (v/v) TX-100, 5 mM ethylenediaminetetraacetic acid (EDTA), plus protease inhibitors. Triton insoluble material was removed from the lysates by centrifugation, and the soluble proteins in the supernatant were precipitated with methanol/chloroform.14 Precipitates were dissolved in sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis sample buffer (Bio-Rad, Hemel Hempstead, United Kingdom) containing 5% β-mercaptoethanol, heated at 95°C for 5 minutes, and samples resolved on 14% acrylamide gels. Recombinant human IL-8 (10 ng; Antigenix America, Huntington Station, NY) was made up in sample buffer and run alongside the lysates as a control. Electrophoresed proteins were transferred onto an Immobilon P membrane (Millipore, Watford, United Kingdom) using a Genie Blotter (Idea Scientific, Minneapolis, MN) and the membrane blocked in PBS containing 5% (w/v) milk powder and 0.3% (v/v) Tween-20. The blot was probed with a goat anti–IL-8 antibody (R&D Systems, Abingdon, United Kingdom) and a rabbit anti-red fluorescent protein (RFP) antibody (Millipore), followed by anti-goat and anti-rabbit secondary antibodies coupled to 2 different near infra-red fluorochromes (tebu-bio, Peterborough, United Kingdom), allowing the simultaneous detection of both antigens using the Li-cor Odyssey imaging system (Li-cor Biosciences, Cambridge, United Kingdom).
Confocal imaging of WPB exocytosis
Forty-eight hours after nucleofection, HUVECs were transfered into Ringer solution (140 mM NaCl, 5vmM KCl, 1.8 mM CaCl2, 2 mM MgCl2, 10 mM glucose, 20 mM HEPES-NaOH pH 7.40). Images were collected using a Leica SP2 Laser Scanning Spectral Confocal Microscope (TCS SP2) equipped with either an 63×/1.32 NA or 100×/1.40 NA objective (Leica) and enclosed within a microscope incubator (Solent Scientific, Segensworth, United Kingdom) allowing 37°C temperature control. Prior to stimulation the bathing solution was exchanged for one containing alexa-647 (7 μM) and a dextran-TRITC conjugate (1 mM). The molecular weights of the dextrans used were 3, 10, 40, 70, 150, or 2000 kDa. WPB exocytosis was initiated by addition of 50 μL alexa-647/dextran-TRITC conjugate solution supplemented with 100 μM histamine or 1 μM ionomycin. EGFP, dextran-TRITC, and alexa-647 were simultaneously excited with 488-nm, 561-nm, and 633-nm lasers, respectively. Emitted light was separated by an acousto-optical tunable filter (498-550 nm: EGFP; 590-625 nm: Dextran-TRITC; 665-850 nm: alexa-647) onto 3 photomultiplier channels. The contribution of EGFP fluorescence emission to signals detected in the dextran-TRITC channel were determined using Proregion-EGFP expressing WPBs in the presence of 20 mM NH4Cl to clamp the intra-WPBs pH to more than 7.4.15 A maximum of 8% of the EGFP signal was detected in the dextran-TRITC channel, and corrections for this cross-talk were made in each experiment. In some experiments simultaneous dual-color imaging of either alexa-647 (7 μM) or FM 4-64 (10 μM) and Proregion-EGFP was carried out. In these experiments EGFP and alexa-647 or FM4-64 were simultaneously excited by 488 nm and 561 nm laser light, and the emitted light separated by an acousto-optical tuneable filter (498-550 nm; EGFP, 600-850 nm; alexa-647) onto 2 photomultiplier channels. Confocal images were acquired at approximately 7.4 frames per second.
Epifluorescence imaging of WPB exocytosis in living cells
Nucleofected HUVECs were transferred to the stage of an Olympus IX71 inverted fluorescence microscope and maintained at approximately 37°C using a stage heater, objective heater, and in-line solution heater (Harvard Apparatus, Edenbridge, United Kingdom). The imaging system comprised a fast (∼1 ms) wavelength switching monochromator (Cairn Research, Faversham, United Kingdom), an Olympus U Apochromat 150×/1.45 NA objective and an Ixon EMCCD camera (Andor, Belfast, United Kingdom). Wavelength switching was synchronized with image capture using WinFluor software (Dr John Dempster, Strathclyde University, Glasgow, United Kingdom). For EGFP imaging cells were illuminated with 480 (± 10) nm light. The emission filter set comprised a 500DCXR dichrochic mirror (Chroma, Rockingham, VT) and 535 (± 50) nm emission filter. EMCCD camera was operated in frame transfer mode at full gain, cooled to −65°C, and images acquired at 30 frames per second. Dual EGFP and red fluorescent protein (RFP; mRFP or mCherry) imaging was carried out by sequential illumination with 480 (± 20) nm and 555 (± 20) nm light (30 frames per second). Excitation filter set comprised a GFP/DsRed dual band dichrochic mirror (Chroma part 51019) and a GFP/DsRed dual band emitter. Histamine or ionomycin were applied to the cell by pressure injection from a glass micropipette positioned close to the cell.15 For some experiments a Deltavision Imaging system (Applied Precision, Seattle, WA) housed within a temperature-controlled incubator was used for dual GFP and RFP imaging as previously described.12
Data analysis and measurements
Image analysis was carried out using custom-written plugins and macros in ImageJ software (http://rsb.info.nih.gov/ij/). Confocal images were filtered with 3-pixel radius mean ImageJ filter. The mean background subtracted fluorescence was measured in minimal regions of interest including the whole WPBs. Dataset plotting, fitting, and analysis were performed in Excel and Microcal Origin 7. Results are expressed as means plus or minus SD unless otherwise indicated. The proportion of WPBs that underwent exocytosis was determined in epifluorescence experiments as previously described.15 Those that underwent lingering kiss events were determined from the profile of the fluorescence increase following collapse (long-lived maintained fluorescence increases).
Results
WPB fusion without loss of major core proteins
Stimulation of cells using the Ca2+-elevating hormone histamine resulted in 2 distinct types of WPB-plasma membrane fusion; the most common form was complete exocytosis in which the major WPB core proteins were released. Figure 1Ai (and Video S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) shows a typical example of such an event for a WPBs co-expressing VWF-EGFP and Proregion-mRFP. Following the increase in VWF-EGFP fluorescence due to the pH change within the WPBs on fusion,12,15 VWF-EGFP remained associated with the cell surface and dispersed slowly (Figure 1Ai top panel and black trace in Aiii). The fluorescence of mRFP, like that of DsRed from which it is derived,16 is largely insensitive to changes in pH (Prescott et al17 and Figure S1A) and declines abruptly following fusion due to a rapid dispersal of Proregion-mRFP into the extracellular medium15 (Figure 1Ai bottom panel and gray trace in Aiii). Full fusion events accounted for approximately 88% of all exocytotic events at high hormone concentrations (100 μM; 495 of 560 exocytotic events, n = 15 cells) and approximately 75% of exocytotic events at low hormone concentrations (0.3-1 μM; 303 of 402 events, n = 22 cells). A second form of fusion comprised an abrupt collapse in WPB morphology with retention of the major WPB core proteins within a spherical structure (illustrated in Figure 1Aii, iv, and Video S2 for WPBs co-expressing VWF-EGFP and Proregion-mRFP). These events were characterized by a maintained increase in WPB-EGFP fluorescence and no loss of Proregion-mRFP fluorescence. To establish that collapsed WPBs are membrane bounded structures arising from the formation of a fusion pore, we looked for the accumulation of extracellular FM4-64 within these structures. Like the more commonly used FM1-43, FM4-64 is nonfluorescent in solution but becomes intensely fluorescent when intercalated into lipid membranes, and crucially, like FM1-43, it is unable to cross lipid bilayers.18 Thus, appearance of FM4-64 fluorescence in the WPB membrane will only result from direct contact of the WPB membrane with the extracellular fluid phase or plasma membrane–associated FM4-64 following formation of a fusion pore. Accumulation of FM4-64 within the membrane of collapsed WPBs (Figure 1B and Video S3) provides strong evidence that the morphological change in the organelle coincides with the formation of a fusion pore and that the collapsed structure formed is membrane bounded. The formation of collapsed structures retaining VWF or Proregion accounted for approximately 12% of all fusion events at high hormone concentrations (100 μM; 65 of 560 fusion events, n = 15 cells) and approximately 25% of fusion events at low hormone concentrations (0.3-1 μM; 99 of 402 fusion events, n = 22 cells). For most subsequent experiments, unless otherwise stated, Proregion-EGFP was used as the major core protein marker; its smaller size relative to VWF, and rapid release from WPBs in which the fusion pore expands fully,12 allowed clear discrimination between collapsed structures, in which no loss of Proregion-EGFP fluorescence is seen (Figure 1C) and events in which full expansion of the pore allowed complete (Figure 1C) or partial (eg, Video S4) release of Proregion-EGFP.
Retention of major WPB core proteins during lingering kiss fusion events. (A) The fate of VWF-EGFP and Proregion-mRFP, co-packaged within the same WPB, following a complete exocytotic event (Ai,iii) or following fusion-evoked collapse (Aii,iv). Ai and ii show montages of images of VWF-EGFP (top panels) and Proregion-mRFP (bottom panels) fluorescence from time-lapse sequences, and Aiii and iv show the time-course for the fluorescence changes for VWF-EGFP (black traces) and Proregion-mRFP (gray traces) during the 2 distinct fusion events. Scale bars are 2 μm, dual-color images were acquired at 0.83 frames per second. (B) A montage of images of a single Proregion-EGFP containing WPBs undergoing fusion in the presence of 7 μM extracellular FM 4-64. FM4-64 labels the WPB membrane only following formation of a fusion pore. The top panel shows Proregion-EGFP fluorescence, the lower panel shows FM 4-64 fluorescence. Images were acquired simultaneously on a Leica SP2 confocal microscope at 7.4 frames per second. The cell was stimulated with histamine (100 μM), the scale bar is 2 μm. (C) In the same format as Panel A, examples of complete exocytosis and collapsed WPB structures containing Proregion-EGFP alone. Images were acquired at 30 frames per second on an epi-fluorescence microscope. For display, images were resampled to a frame every 2 seconds. Frames indicated by the white asterisks correspond to the point on the time-course plot indicated by the black asterisk.
Retention of major WPB core proteins during lingering kiss fusion events. (A) The fate of VWF-EGFP and Proregion-mRFP, co-packaged within the same WPB, following a complete exocytotic event (Ai,iii) or following fusion-evoked collapse (Aii,iv). Ai and ii show montages of images of VWF-EGFP (top panels) and Proregion-mRFP (bottom panels) fluorescence from time-lapse sequences, and Aiii and iv show the time-course for the fluorescence changes for VWF-EGFP (black traces) and Proregion-mRFP (gray traces) during the 2 distinct fusion events. Scale bars are 2 μm, dual-color images were acquired at 0.83 frames per second. (B) A montage of images of a single Proregion-EGFP containing WPBs undergoing fusion in the presence of 7 μM extracellular FM 4-64. FM4-64 labels the WPB membrane only following formation of a fusion pore. The top panel shows Proregion-EGFP fluorescence, the lower panel shows FM 4-64 fluorescence. Images were acquired simultaneously on a Leica SP2 confocal microscope at 7.4 frames per second. The cell was stimulated with histamine (100 μM), the scale bar is 2 μm. (C) In the same format as Panel A, examples of complete exocytosis and collapsed WPB structures containing Proregion-EGFP alone. Images were acquired at 30 frames per second on an epi-fluorescence microscope. For display, images were resampled to a frame every 2 seconds. Frames indicated by the white asterisks correspond to the point on the time-course plot indicated by the black asterisk.
Size of the fusion pore formed during WPB collapse
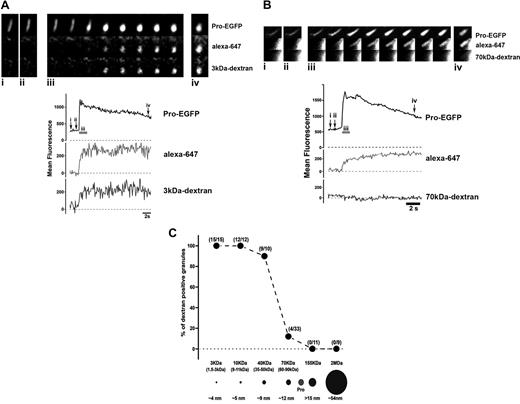
The size of the fusion pore formed in collapsed WPBs was estimated from the accumulation or exclusion, within these membrane-bound structures, of extracellular Dextran-TRITC conjugates ranging in size from 3 kDa to 2000 kDa. Fusion pore formation was monitored by changes in both WPB EGFP fluorescence and the intra-WPBs accumulation of a small extracellular fluid phase marker, alexa-647. Dextran-TRITC in the size range 3 kDa to 40 kDa were found to enter collapsed WPBs (Figure 2A and Video S5); however, dextran-TRITC of 70 kDa or greater were excluded (Figure 2B, and Video S6). These results are summarized in Figure 2C. The mean diameter of 70-kDa dextran is approximately 12 nm, suggesting that the size of the fusion pore that forms in these cases has an upper limit of this order.
Size of the fusion pore formed during a lingering kiss. In each case (panels A and B) the image montages show the fluorescence of a Proregion-EGFP (top panels) containing WPBs prior to (i and ii) and during (iii and iv) a lingering kiss evoked by histamine (100 μM), in the presence of extracellular alexa-647 (middle panels) and 3 kDa dextran-TRITC (A; bottom panel) or 70 kDa dextran-TRITC (B; bottom panel). The time-courses for the changes in WPB-EGFP (top traces), alexa-647 (middle traces), and dextran-TRITC (bottom traces) fluorescence are shown below each montage. The time points corresponding to the images in the montages are indicated (i-iv), and scale bars are 1 μm. Images were acquired simultaneously on a Leica SP2 confocal microscope at 7.4 frames per second. Panel C summaries the proportion of WPBs that accumulated dextran-TRITC, over the size range 3 kDa to 2000 kDa, in collapsed WPBs. The numbers of WPBs analyzed at each dextran size is indicated in parenthesis by each point. The size range for the dextrans used (where known) are shown in parenthesis below the ordinate for each point, and scale drawings of the mean diameters of the dextrans used are show as black circles. The diameter of Proregion (Pro) estimated by gel filtration28 is indicated as a gray circle.
Size of the fusion pore formed during a lingering kiss. In each case (panels A and B) the image montages show the fluorescence of a Proregion-EGFP (top panels) containing WPBs prior to (i and ii) and during (iii and iv) a lingering kiss evoked by histamine (100 μM), in the presence of extracellular alexa-647 (middle panels) and 3 kDa dextran-TRITC (A; bottom panel) or 70 kDa dextran-TRITC (B; bottom panel). The time-courses for the changes in WPB-EGFP (top traces), alexa-647 (middle traces), and dextran-TRITC (bottom traces) fluorescence are shown below each montage. The time points corresponding to the images in the montages are indicated (i-iv), and scale bars are 1 μm. Images were acquired simultaneously on a Leica SP2 confocal microscope at 7.4 frames per second. Panel C summaries the proportion of WPBs that accumulated dextran-TRITC, over the size range 3 kDa to 2000 kDa, in collapsed WPBs. The numbers of WPBs analyzed at each dextran size is indicated in parenthesis by each point. The size range for the dextrans used (where known) are shown in parenthesis below the ordinate for each point, and scale drawings of the mean diameters of the dextrans used are show as black circles. The diameter of Proregion (Pro) estimated by gel filtration28 is indicated as a gray circle.
Loss of IL-8-mCherry from collapsed WPBs
To determine if release of small core molecules might occur from collapsed WPBs, we constructed an mCherry fusion protein of IL-8 and coexpressed this with Proregion-EGFP. IL-8 was chosen not only because of its biological interest, being a potent chemo-attractant for neutrophils,19,20 but also because its small size allowed the generation of a fluorescent fusion protein with a molecular mass (including the ∼27-kDa predicted mass of mCherry) in the range likely to retain the ability to pass through the pore (≤ 40 kDa; Figure S2A). Expression of IL-8-mCherry labeled WPBs in living HUVECs (Figure S2B). The fluorescence change of Proregion-EGFP was used to determine the point of WPB fusion. The time-course for release of Proregion-EGFP and IL-8-mCherry following complete WPB exocytosis and during WPBs collapse evoked by histamine are shown in Figure 3. During complete exocytosis, Proregion-EGFP and IL-8-mCherry rapidly dispersed from the site of fusion with half-times of 3.7 (± 5.5) s and 1.45 (± 1.3) s (n = 30 WPBs), respectively (Figure 3A). In collapsed WPBs, where Proregion-EGFP is retained, IL-8-mCherry fluorescence was also lost, although more slowly, with a mean half-time of 9.36 (± 9.21) s (n = 5 WPBs; Figure 3B). In contrast, no loss of mCherry fluorescence was seen in collapsed WPBs co-expressing Proregion-EGFP and VWF-mCherry (Figure S3).
Selective loss of IL-8-mCherry but not Proregion-EGFP from WPBs during a lingering kiss. The image montages in panels A and B show individual WPBs containing both Proregion-EGFP (top panels) and IL-8-mCherry (bottom panels) during a complete exocytotic event (A) or a lingering kiss event (B). Scale bars are 2 μm. Cells were stimulated with 100 μM histamine. In each case the time-course for loss of fluorescence for each protein (as indicated) is shown below. Images were acquired at 30 frames per second. Images shown in the montages were taken at points indicated by the markers i, ii, and iii. Images shown in montages Aii and Bii were at 12 frame intervals during the period indicated by the gray bar. The transient drop in fluorescence of the Proregion-EGFP signal (*) in B was due to a focus change during recording.
Selective loss of IL-8-mCherry but not Proregion-EGFP from WPBs during a lingering kiss. The image montages in panels A and B show individual WPBs containing both Proregion-EGFP (top panels) and IL-8-mCherry (bottom panels) during a complete exocytotic event (A) or a lingering kiss event (B). Scale bars are 2 μm. Cells were stimulated with 100 μM histamine. In each case the time-course for loss of fluorescence for each protein (as indicated) is shown below. Images were acquired at 30 frames per second. Images shown in the montages were taken at points indicated by the markers i, ii, and iii. Images shown in montages Aii and Bii were at 12 frame intervals during the period indicated by the gray bar. The transient drop in fluorescence of the Proregion-EGFP signal (*) in B was due to a focus change during recording.
Selective loss of WPB membrane proteins during a lingering kiss
Our preliminary data indicate that complete exocytosis of WPBs results in rapid dispersal of both P-selectin-mRFP and mRFP-CD63 from the site of fusion.21 What happens to these proteins during a lingering kiss? To address this, we imaged exocytosis of individual WPBs in cells coexpressing Proregion-EGFP and P-selectin-mRFP or mRFP-CD63 (Figure S4). The increase in Proregion-EGFP fluorescence was used to mark the opening of the fusion pore, and the loss of membrane proteins was determined for WPBs that underwent complete exocytosis or collapsed and retained Proregion-EGFP. Consistent with preliminary observations, both P-selectin-mRFP and mRFP-CD63 rapidly dispersed following complete exocytosis (Figure 4A,C, respectively). Half-times for dispersal of Proregion-EGFP from WPBs co-expressing P-selectin-mRFP or mRFP-CD63 were not significantly different (3.3 ± 3.8 s, n = 25 WPBs and 2.7 ± 4.0 s, n = 25 WPBs, respectively) and the half-times for dispersal of P-selectin-mRFP and mRFP-CD63 were 2.9 (± 3.8 s; n = 25 WPBs) and 2.6 (± 4.2 s; n = 25 WPBs), respectively. In contrast, during a lingering kiss P-selectin was retained within the collapsed structure (n = 8 WPBs; Figure 4B); however, mRFP-CD63 was lost with a time-course similar to that for full exocytosis (2.6 ± 1.2 s; n = 9 WPBs; Figure 4D).
Selective release of WPB membrane proteins during a lingering kiss. In each case the image montages in panels A to D show the fluorescence of a Proregion-EGFP (Pro-EGFP; top panels) containing WPBs during complete exocytosis (panels A and C) or during a lingering kiss (panels B and D) evoked by histamine (100 μM). The fluorescence of P-selectin-mRFP (panels A and B) or mRFP-CD63 (panels C and D) coexpressed with Proregion-EGFP are shown in the lower panels. Scale bars are 2 μm. The time-course for dispersal of Proregion-EGFP (black traces) and P-selectin-mRFP or mRFP-CD63 (gray traces) from the WPBs shown are plotted below each montage. Data in panels A and B were acquired at 0.83 frames per second, and in panels C and D at 30 frames per second.
Selective release of WPB membrane proteins during a lingering kiss. In each case the image montages in panels A to D show the fluorescence of a Proregion-EGFP (Pro-EGFP; top panels) containing WPBs during complete exocytosis (panels A and C) or during a lingering kiss (panels B and D) evoked by histamine (100 μM). The fluorescence of P-selectin-mRFP (panels A and B) or mRFP-CD63 (panels C and D) coexpressed with Proregion-EGFP are shown in the lower panels. Scale bars are 2 μm. The time-course for dispersal of Proregion-EGFP (black traces) and P-selectin-mRFP or mRFP-CD63 (gray traces) from the WPBs shown are plotted below each montage. Data in panels A and B were acquired at 0.83 frames per second, and in panels C and D at 30 frames per second.
Discussion
Expression of VWF- and Proregion-EGFP in living HUVECs results in the specific labeling of WPBs, allowing direct visualization of their exocytosis.12,15 An abrupt increase in EGFP fluorescence arising from the elevation of WPB pH from resting levels (∼pH 5.5) to that of the external solution was used to mark the time of WPB fusion pore formation.15 In most cases WPBs undergo full fusion, resulting in the complete loss of the major core proteins, VWF and Proregion with distinctive time-courses.12 For Proregion-EGFP the time-course for dispersal is rapid with a half-time of a few seconds (Figure 1). However, a small but significant population of WPBs was found to undergo a distinct form of fusion in which the WPB morphology collapsed from a rod-like to spherical shape and in which the major WPB core proteins were retained within a membrane-bound structure (Figure 1). The increase in fluorescence of WPB EGFP was identical in amplitude to that seen during complete fusion, however, it was maintained, suggesting that no loss of fluorescent core proteins occur. In a small proportion of these cases (1%-2% during strong stimulation), the collapse of the WPBs was associated with abrupt transient increases of WPB EGFP fluorescence coupled with discrete stepwise accumulation of extracellular fluorescent tracer molecules, suggesting brief cyclic periods of fusion pore opening and closure (Figure S5). The crucial observation from those experiments is that pore closure results in a decline of WPB EGFP fluorescence (arrows in Figure S5), presumably due to reacidification of the organelle, similar to that reported following resealing of secretory organelles in other cells.22 Thus, the maintained fluorescence increase seen in collapsed WPBs suggests 2 things: first, that the pore remains open for long periods of time during which the fluorescence of intra-WPB EGFP is determined by the extracellular pH; and second, that VWF-EGFP and, crucially, the much smaller Proregion (see next paragraph) do not escape, indicating that the pore is of limited size. In neuronal cells an extended vesicle kiss phase has been termed a “long lingering kiss.”23 The lingering kiss events observed here are in many ways similar to those seen in other nonexcitable cells,6-8 suggesting that they may be a common feature of exocytosis in these cell types. The ultimate fate of WPBs that undergo a lingering kiss is not clear. WPBs could remain in a lingering kiss configuration for in excess of 80 seconds before resealing, indicated by a decline in fluorescence due to reacidification of the organelle (data not shown). Collapsed structures often remained close to their site of fusion for prolonged periods before being trafficked deeper into the cell. Dual-color imaging of collapsed WPBs containing VWF-mCherry and EGFP-CD63 showed that in some cases EGFP-CD63, initially lost during the lingering kiss (eg, Video S7), could be partially recovered, presumably through interactions with EGFP-CD63–positive endo-membrane structures (Figure S6). Dual-color imaging experiments using Proregion-EGFP and LAMP-1-tdTomato coexpressing cells showed no evidence for a direct transfer of Proregion-EGFP from collapsed WPBs to lysosomes over time periods of up to 1.5 hours after fusion (data not shown). Technical improvements allowing longer-term dual-color imaging are needed to follow the ultimate fate of these structures.
The accumulation or exclusion of dextran-TRITC conjugates ranging in size from 3 kDa to 2000 kDa suggest that the pore formed during a lingering kiss has an upper size limit of approximately 12 nm. How large are VWF or Proregion? A mature wild-type VWF monomer has a predicted molecular mass of approximately 275 kDa; however, VWF is glycosylated and within WPBs exists as covalently linked polymers ranging in size from 0.5 to 20 mDa.24 Within WPB VWF forms a highly ordered quasi-crystalline structure,25 however, in solution the VWF polymers assume large diameter tangled “ball of yarn” configurations.26 Solution structures determined for VWF multimers show that they resemble prolate ellipsoids with radii of gyration of approximately 75 to 100 nm,27 too large to pass through a 12-nm pore. Proregion is thought to exist as a homodimer28 ; the monomer has a predicted molecular mass of approximately 81 kDa but is glycosylated and thought to be closer to 90 to 100 kDa. The Stokes radius of Proregion, estimated by gel filtration, is approximately 6.7 nm,28 corresponding to a diameter of approximately 13.4 nm, too large to pass through a 12-nm pore and consistent with the inability Proregion-EGFP (∼120 kDa) to exit WPBs during a lingering kiss.
Many secreted proteins reported to be copackaged in WPBs are considerably smaller than VWF and Proregion. The predicted molecular masses (not including glycans) of angiopoietin-2, osteoprotegerin, tissue plasminogen activator, endothelin-1, IL-8, and eotaxin-3 are 56 kDa, 44 kDa, 59 kDa, 4.3 kDa, 8.9 kDa, and 8.3 kDa, respectively, although some (eg, osteoprotegerin and angiopoietin-2) are reported to exist as dimers or higher order multimers29,30 ; amino acid sequence data obtained from UniProt http://www.ebi.uniprot.org). A 12-nm fusion pore formed during lingering kiss events is potentially large enough to allow some of these proteins to exit the WPBs. Here we show that IL-8-mCherry fluorescence is lost from WPBs during both complete exocytosis and a lingering kiss fusion event. The slower time-course for the loss of IL-8-mCherry fluorescence during a lingering kiss is most likely due to both a restriction to free diffusion produced by the crowded milieu within the collapsed organelle and the small size of the fusion pore. The loss of IL-8-mCherry fluorescence during a lingering kiss is unlikely to be due to rapid destruction or quenching of mCherry following WPB fusion. mCherry is reported to be resistant to degradation,31 and its fluorescence is insensitive to pH changes (Pankiv et al31 and Figure S1B). Consistent with this was the observation that VWF-mCherry fluorescence was maintained within collapsed WPBs (Figure S3).
Selective release of WPB membrane proteins could also occur during a lingering kiss. What might be the basis for this? CD63, like other members of the tetraspannin family of integral membrane proteins, are buried within the lipid bilayer in which they reside, extending to only a few nano-meters beyond the membrane boundary.32 Being buried within the membrane CD63 is less likely to present a significant physical obstacle within the pore lumen. Our preliminary data have shown that this molecule is mobile within the limiting membrane of the WPBs,33 and its ability to exit these structures suggests that the pore is composed partially or wholly of lipids. These data also imply that plasma membrane proteins and lipids may enter the membrane of these structures. In contrast, the extracellular domain of P-selectin (that projects into the WPB lumen) is thought to form a rod-like structure approximately 48 nm in length,34 and biochemical studies suggest that the protein can exist as either a monomer or dimer within the WPBs.35 The conformation of the extracellular domain of P-selectin within the resting WPBs is not known. In the extended form it would project to a depth of approximately one third of the diameter of the average WPB11 and could constitute a significant physical obstacle to passage through a 12-nm diameter fusion pore (Figure 5). Even if tightly folded in the resting WPBs, a fusion-evoked hydration, pH change, and disordering of core proteins is likely to result in some unfolding to form a partially or fully extended molecule. In addition, potential interactions between P-selectin and VWF36 may help to retain this molecule within the collapsed structure. The physiological consequences for the retention of P-selectin within these structures are not clear. Both P-selectin and VWF are able to support neutrophil adhesion.37,38 Retention of these molecules may play a role in fine-tuning the adhesiveness of the endothelial cell membrane to such cells during mild activation (see next paragraph).
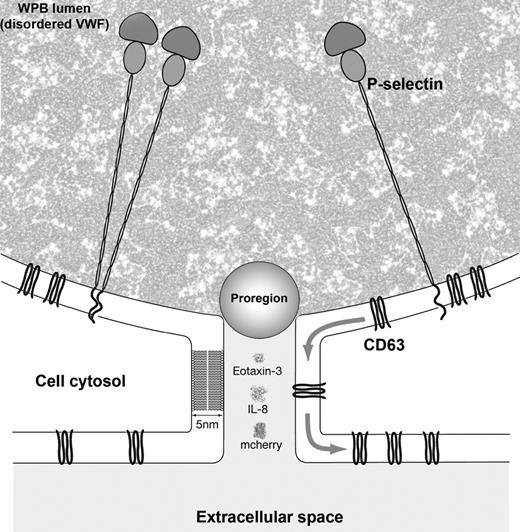
The fusion pore formed during a lingering kiss acts as a molecular filter mediating selective content release. Cartoon showing the solution structures of IL-8 (data from PDB structure 2IL8), mCherry (data from PDB structure 2H5Q), eotaxin-3 (data from PDB structure 1G2T), and the estimated size of Proregion28 relative to the dimensions of the fusion pore formed during a lingering kiss. Also shown are cartoons of the membrane proteins CD63 and P-selectin (full crystal structures are not available for either protein). P-selectin is shown as both a monomer and a dimer35 in the extended conformation observed for the detergent-purified membrane form of this molecule.34 Disordered polymers of multimeric VWF form a “ball of yarn” configuration under nonshear conditions26 and are shown here as a mottled background within the WPB lumen.
The fusion pore formed during a lingering kiss acts as a molecular filter mediating selective content release. Cartoon showing the solution structures of IL-8 (data from PDB structure 2IL8), mCherry (data from PDB structure 2H5Q), eotaxin-3 (data from PDB structure 1G2T), and the estimated size of Proregion28 relative to the dimensions of the fusion pore formed during a lingering kiss. Also shown are cartoons of the membrane proteins CD63 and P-selectin (full crystal structures are not available for either protein). P-selectin is shown as both a monomer and a dimer35 in the extended conformation observed for the detergent-purified membrane form of this molecule.34 Disordered polymers of multimeric VWF form a “ball of yarn” configuration under nonshear conditions26 and are shown here as a mottled background within the WPB lumen.
Together these data show that the fusion pore formed during a lingering kiss can act as a molecular filter retaining large core proteins while allowing the release of ions (eg, H+), small core molecules, some membrane components (summarized in Figure 5), and offering the potential to accumulate plasma membrane or soluble plasma components. The latter may allow the endothelial cell to sample the extracellular milieu, although whether this represents a physiologically significant mechanism remains to be established. It is interesting to note that the proportion of lingering kiss events is greater during weak stimulation. Under such conditions fewer WPB fuse15 and the local concentrations of molecules released will be much lower than those seen during strong stimulation. Under these conditions small changes in the local concentrations of secreted agonist molecules may substantially influence biological responses, particularly where a dose-response relationship is steep. Thus, under conditions of mild activation the substantial fraction of small molecules contributed by WPBs that undergo a lingering kiss (∼25%) might be expected to play a significant role in modulating the strength of the endothelial cell signal to local inflammatory cells. During strong stimulation, where many more WPB fuse, the contribution of small molecules provided by the small fraction of WPBs undergoing a lingering kiss may have a less significant impact, particularly if local concentrations of such molecules are supramaximally effective.
What is perhaps more important to consider are those molecules that are not released during lingering kiss events. In this case the question is whether the physiological effect mediated by the molecules would be attenuated significantly by loss (due to retention within the collapsed organelle) of a proportion of these molecules. In the case of strong stimulation the retention of VWF, Proregion, and P-selectin by approximately 10% of fused WPBs may indeed not significantly affect neutrophil or platelet capture and coagulation and therefore be of little physiological significance. However, under conditions of mild stimulation where the numbers of granules that fuse is already low, a further reduction of 25% in secreted VWF and Proregion may well have a disproportionate effect on the ability to recruit or activate target cells. This could be advantageous as the requirement for a coagulant response during a weak stimulus might be less important than efficient signaling of a potential inflammatory situation. Thus, under conditions of low-level activation, the endothelial cell may be able to fine-tune the secretory phenotype to control processes such as inflammation, while limiting the risk of thrombosis. This may have important implications for our understanding of the role of WPBs and their exocytosis in the development and progression of vascular disease. Large numbers of WPBs are found in endothelial cells near vascular branch points and bifurcations, areas prone to complex shear stresses, cellular activation, and the development of atherosclerotic lesions. Histological studies show a marked increase in WPB number in endothelial cells from early atherosclerotic lesions, and evidence of WPB exocytosis.39 A lack of VWF results in the failure to form WPBs and is associated with a resistance to atherosclerosis.40,41 A failure to form WPBs also results in a failure to store and express the leukocyte adhesion molecule P-selectin; a deficiency of this molecule is associated with delayed onset and reduced severity of atherosclerotic lesions.41-44 Indeed, failure to form WPBs will also prevent the long-term storage and limit the regulated secretion of the pro-inflammatory molecules, such as IL-8, that are also implicated in the development of vascular disease.45 Together, these studies suggest an important role for WPBs, their cargo proteins, and their modes of exocytosis in the complex processes that contribute to the development of vascular disease.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We would like to thank Dr David Ogden for useful discussions.
Authorship
Contribution: V.B. contributed vital new reagents, performed research, and analyzed data; A.M. and L.K. performed research; J.E.D. contributed vital analytical tools; P.S. contributed vital reagents; M.J.H. contributed vital reagents; and T.C. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: T. Carter, MRC National Institute for Medical Research, The Ridgeway, Mill Hill, London, NW7 1AA; e-mail: tcarter@nimr.mrc.ac.uk.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal