Abstract
Despite the earlier use of potent immunosuppressive or cytostatic drugs and the recent emergence of biologicals as treatment for human autoimmune diseases (AIDs), some patients still remain unresponsive to treatment. To those severely ill patients, autologous bone marrow transplantation (aBMT) is applied as a last resource, leading to disease remission in a majority of patients. The underlying mechanism of action of aBMT is still largely unknown. Here, we showed that regulatory T cells (Tregs) play a role in the natural disease course of proteoglycan-induced arthritis (PGIA) and in disease remission by aBMT. aBMT led to an initial phase of rapid disease improvement corresponding with a relative increase in CD4+CD25+ T cells. At this time, the CD4+CD25+ cells did not yet show an increase in Foxp3 expression and showed less potent suppression. After this initial improvement, disease relapsed but stabilized at a level below the severity before aBMT. This second phase was actively regulated by potently suppressive CD4+CD25+Foxp3+ Tregs. This work provided further insight into the role of Tregs in restoration of the immune balance by aBMT and can open the way to explore therapeutic interventions to further improve treatment of AID and disease relapses.
Introduction
Human autoimmune diseases (AIDs) are often difficult to treat and the cause of major disability. In recent years, treatment has become increasingly more aggressive, with the earlier use of potent immunosuppressive or cytostatic drugs.1-3 These methods are based on a generalized and nonspecific inhibition of immune response and inflammation, thus having considerable side effects, and they are often not curative. In autoimmune arthritis, such as rheumatoid arthritis (RA) and juvenile idiopathic arthritis (JIA), considerable progress has been made by the introduction of immunotherapy with biologic agents, which aims to interfere with the molecular processes that are involved in the immune-mediated pathogenesis of AID.4-12 Despite the progress in treatment methods, some patients are still unresponsive. For those severely ill children, autologous bone marrow transplantation (aBMT) has been the last resource. The results of aBMT in JIA and other AIDs are remarkably good, since it induces drug-free disease remission in a majority of JIA patients during a follow up of 12 to 60 months after transplantation.13,14 Obviously, it is questionable whether the achieved disease remission will be long lasting, as the assumed genetic predisposition does not change in an autologous BMT setting. Indeed, recently, this problem has arisen, since some of the patients who underwent aBMT have experienced severe disease relapse after many years of full disease remission (N.W., personal communication), suggesting that the arthritis-suppressive effect of aBMT is not indefinite.
The underlying mechanism of action of aBMT in AID is still largely unknown. It has been hypothesized that due to the stringent conditioning regimen, leading to severe aplasia, an environment is created where renewed acquisition of self-tolerance can be acquired after rescue by autologous BM.15,16 This self-tolerance may be guided by regulatory T cells (Tregs), and relapses may and probably will occur in time when this self-tolerance is broken again. Several types of Tregs have been identified to date, of which CD4+CD25+Foxp3+ Tregs have been studied most extensively in the BMT setting (eg, for their role in graft-versus-host disease).17
Indeed, in previous work in JIA patients undergoing aBMT, we showed that aBMT induces a restoration of the frequency of CD4+CD25brightFoxp3+ Tregs from severely reduced levels before aBMT to normal levels after. The study suggested that 2 mechanisms may be in place: on one hand (preferential) homeostatic proliferation of Tregs during the lymphopenic phase of the reconstitution of the immune system and on the other hand renewed thymopoiesis of naive Tregs. Furthermore, a deviation of autoreactive T cells from a proinflammatory phenotype before aBMT to a tolerant phenotype after aBMT was seen.18 To further explore the mechanism of aBMT and develop strategies that may help to improve the efficacy and prevent late relapses of the disease, it is necessary to turn to an experimental arthritis model.
Earlier pioneering work in animal models by van Bekkum provided the experimental basis for the treatment of AID by autologous BMT. He successfully treated adjuvant arthritis in buffalo rats by aBMT and defined conditioning regimens and levels of T-cell depletion.19 In addition, in collagen-induced arthritis, an antibody-mediated mouse model, a decrease of arthritis occurred after irradiation and BMT.20 This work has provided pivotal insight; however, to study the maintenance and the break of tolerance as seen in humans in the long term, we wanted to turn to a model that is mediated by both T and B cells and that is relapsing and remitting, as is the human disease.
Proteoglycan-induced arthritis (PGIA) fits both these criteria. It can be induced in retired BALB/c mice by 2 injections of human cartilage PG in the adjuvant DDA. This causes a progressive polyarthritis that is initiated by a cross recognition of mouse PG by CD4+ T cells, however, B cells are also crucial for the development of disease.21,22 PGIA is extensively studied; has clinical, immunologic, and histopathological resemblance to human arthritis; and has a chronic relapsing remitting course. We recently succeeded in setting up an aBMT model in PGIA (S.T.A.R. et al, manuscript in preparation). The role of CD4+CD25+ Tregs in the natural disease course as well as after aBMT has not been studied yet and was a focus of the work presented here.
In this study, we showed a role for Tregs in the natural disease course of PGIA, as well as a role for Tregs in the restoration of the immune homeostasis after aBMT. We found that Tregs were crucial for the development of PGIA, while after autologous BMT, a rapid decrease of arthritis was observed, which could be (partially) reversed by in vivo depletion of Tregs. Interestingly, the initial phase of clinical improvement corresponded with a relative increase in CD4+CD25+ T cells, but not yet with an increase in Foxp3 expression in those cells, or with in vitro suppressive capacity. However, at a later stage, when disease activity stabilized, the newly reinstated immune balance was regulated by potently suppressive CD4+CD25+Foxp3+ Tregs.
Methods
Antigens, immunization, and assessment of arthritis
Mice.
Female retired BALB/c mice were obtained from Charles River (Sulzfeld, Germany) and served as recipients of the autologous bone marrow. Male donor BALB/c mice of 7 weeks of age were also obtained from Charles River and served as donors for autologous bone marrow.
The mice were kept in the animal facility of the Utrecht University under regular conditions until aBMT, after which they were housed under sterile conditions in filter-top cages and received distilled drinking water containing the antibiotic ciprofloxacin (100 μg/mL).
The experiments were approved by the Animal Experiment Committee of the Faculty of Veterinary Medicine (Utrecht University).
Proteoglycan preparation. Proteoglycan
(PG) was purified from human articular cartilage and removed during knee joint replacement surgery by 4 M guanidinium chloride extraction, and the human GAG side chains were depleted by digestion with chondroitinase ABC as described previously.22,23 The PG was kindly provided by the Faculty of Veterinary Medicine in Utrecht, The Netherlands.
Induction and assessment of arthritis.
Arthritis was induced in BALB/c mice by 2 intraperitoneal injections of 0.4 mg human deglycosylated PG in 2 mg of the synthetic adjuvant dimethyl dioctadecyl ammonium bromide (DDA; Sigma Aldrich, Zwijndrecht, The Netherlands) on days 0 and 21. The onset and severity of arthritis were assessed 3 times a week in a blinded fashion by a visual scoring system as described previously.22,24 In brief, the degree of joint swelling, redness, and deformation of each paw (scored from 0-4) was determined to express a total arthritis score, with a maximum severity index of 16 per animal. In accordance with the Animal Experiment Committee, mice were killed when they achieved a total score of 12, or exhibited a cumulative weight loss of 20% or a weight loss of more than 15% within 3 days.
Treatment protocols
Bone marrow transplantation.
On day 35 after the first injection of PG in DDA, recipient mice that had become arthritic were conditioned by 7.5 Gy irradiation. Subsequently, bone marrow transplantation (BMT) was performed by intravenous injection of 2 × 106 BM cells from syngeneic donor animals that were at the same stage of disease. As the composition of pseudoautologous and autologous BM is identical, the term autologous BMT (aBMT) is used for both throughout this paper.
BM suspensions were prepared as follows: donor mice were killed and BM was harvested by flushing tibia and femur with Iscoves modified Dulbecco medium (IMDM) containing 2% fetal calf serum (FCS), 2 mM l-glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin (all from Gibco BRL, Grand Island, NY). Cells (2 × 106) were resuspended in 200 μL 0.2% BSA before injection intravenously. Sick untreated mice were used as controls.
Anti-CD25 administration.
To assess the role of CD4+CD25+ Tregs in the natural course of PGIA, anti-CD25 was administered to animals that had received 2 PG injections on days 0 and 21, but had not yet developed arthritis. Two doses of 200 or 250 μg anti-CD25 Ab PC61 (kindly provided by L. Boon) were administered on days 35 and 42 after the initial PG injection.
To assess the role of CD4+CD25+ Tregs in the disease course following aBMT, 250 or 200 μg anti-CD25 mAb PC61 was administered to BALB/c mice undergoing aBMT on the day of BMT (day 35) and on day 42, and the dose-dependent effect was assessed.
In vitro assays
Repopulation white blood cell count.
On a weekly basis following aBMT, blood was drawn from the tail vein and collected in heparin-containing tubes. Subsequently, the white blood cell (WBC) count was measured on a Coulter cell counter (Beckman Coulter, Hialeah, FL).
Repopulation of CD3+, CD4+, CD4+CD25+ cells in peripheral blood, spleen, and/or lymph nodes by FACS analysis.
To assess repopulation in peripheral blood, on a weekly basis following aBMT, blood was drawn from the tail and collected in heparin-containing tubes. Erythrocytes were lysed for 15 minutes in the dark in fluorescence-activated cell sorting (FACS) lysing solution (BD Pharmingen, San Diego, CA).
To assess repopulation in spleen and lymph nodes (LNs), mice were killed on day 42, day 55, and day 81, and cells were mashed through a 70-μm cell strainer (BD Biosciences, San Jose, CA). Repopulation of cells was assessed by FACS analysis and FACS staining was performed as follows: cells were washed twice in FACS buffer (PBS + 2% FCS). Nonspecific staining was limited by incubating the cells for 15 minutes in 2% normal rat serum (NRS). Subsequently, cells were stained with one or more of the following surface marker antibodies: antimouse CD3ϵ PercP (clone 145-2C11), CD4 PercP (RM4-5), CD25 FITC (PC61.5), CD44 FITC (Pgp-1, Ly-24, IM7), CD45RB PE (16A), respectively (all from BD Pharmingen) for 30 minutes at 4°C. Unbound antibodies were washed away by 2 washes with FACS buffer. For Foxp3 analysis, cells were incubated in Fix/perm concentrate diluted 1:4 in Fix/perm diluent (eBioscience, San Diego, CA) overnight. The next day, they were washed twice in Perm buffer (eBioscience) and blocked in 2% NRS. Subsequently, the cells were stained with Foxp3 APC (clone FJK-16s; eBioscience) for 30 minutes at 4°C. Then they were washed twice in Perm buffer and finally resuspended in FACS buffer to be acquired right away on a FACS Calibur machine.
Suppression assays.
On day 42 and day 55 after the first PG/DDA injection, mice were killed and spleens were harvested. Cells were mashed through a 70-μm cell strainer (BD Biosciences) and stained by antimouse CD4 PercP and antimouse CD25 APC (clone RM4-5 and PC61, respectively; both from BD Pharmingen). CD4+CD25− cells (10 000) were sorted by FACS Aria directly into a 96-well plate, and CD4+CD25+ sorted cells were added at a ratio of 1:5 and 1:1. A CD4-depleted fraction of spleen cells (using antimouse CD4 microbeads and a single run on an LS column [MACS; Miltenyi Biotec, Auburn, CA]) was irradiated at 35 Gy and served as a source for antigen-presenting cells (APCs). CD4+CD25+ and CD4+CD25− cells were cultured in IMDM supplemented with 10% FCS, 2 mM l-glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin, and 5 × 10−5 M 2-mercaptoethanol in the presence of 50 000 APCs and 1 μg/mL soluble anti-CD3 (clone 145-2C11; BD Pharmingen) for 120 hours. During the last 16 to 18 hours, 1 μCi (0.037 MBq) 3H (3H-TdR; GE Healthcare, Little Chalfont, United Kingdom) was added per well and 3H uptake was measured using a liquid scintillation beta counter. Proliferative responses were calculated as the mean 3H incorporation (cpm) of triplicate wells.
Statistical analysis
Differences in clinical scores were determined by a Student t test. A P value less than .05 was considered significant.
Results
CD25+ T cells actively suppress arthritis in animals that are resistant to the induction of PGIA despite a proper immunization protocol
The PGIA model has been described extensively by Glant et al21 and Glant and Mikecz22 as a relapsing remitting chronic disease. By 2 injections of human PG in the adjuvant DDA, arthritis is induced in BALB/c mice within a few days after the second injection. However, a small percentage of animals do not develop arthritis within the first few days, although eventually nearly 100% of animals develop the disease. The reason for this delay in disease induction in some animals is not yet known. Either the injection of antigen in those animals may be insufficient to induce disease efficiently or regulatory mechanisms may actively play a role in suppressing disease. To assess whether CD25+ Tregs played a role, we tested the effect of depletion of those cells by administration of an anti-CD25 mAb in vivo to animals that were tolerant to the induction of PGIA despite 2 injections of PG in DDA.
Arthritis was induced on day 0 and day 21 by an injection of PG in DDA adjuvant. From day 21 onward, the arthritis score was assessed 3 times a week by a visual scoring system of redness, swelling, and deformities of the paws. On day 35, animals that were resistant to arthritis induction received an intraperitoneal injection of the anti-CD25 mAb PC61, followed by a second injection on day 42.
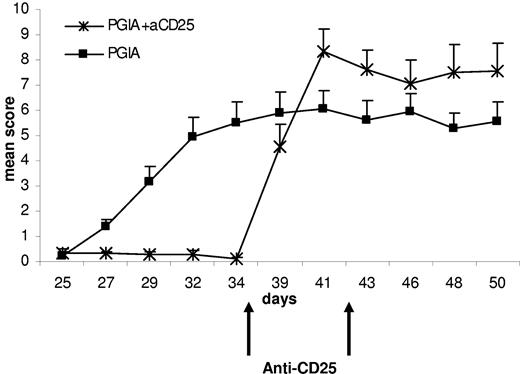
Remarkably, in the animals that did not develop arthritis despite a proper immunization protocol, CD25 depletion was able to rapidly induce an even more severe arthritis, apparent after just one dose of the CD25-depleting mAb (Figure 1). This indicates that active suppression of arthritis by CD25+ Tregs is vital for the tolerance to disease induction.
Depletion of CD25+ cells in animals resistant to arthritis induction leads to a rapid induction of disease. The effect of anti-CD25 administration on the course of PGIA in animals resistant to arthritis induction. Arthritis was induced by 2 injections of PG in DDA on day 0 and day 21. On day 35, animals that had not developed arthritis by then received 2 injections of the anti-CD25 mAb PC61 with a 1-week interval, and mean arthritis scores are shown (+ SEM; N = 9).
Depletion of CD25+ cells in animals resistant to arthritis induction leads to a rapid induction of disease. The effect of anti-CD25 administration on the course of PGIA in animals resistant to arthritis induction. Arthritis was induced by 2 injections of PG in DDA on day 0 and day 21. On day 35, animals that had not developed arthritis by then received 2 injections of the anti-CD25 mAb PC61 with a 1-week interval, and mean arthritis scores are shown (+ SEM; N = 9).
Depletion of CD4+CD25+ T cells interferes with long-term disease remission after aBMT treatment
These data suggested that presence or absence of CD4+CD25+ T cells could influence the disease severity in PGIA, thus providing a rationale for further studying the role of these cells in PGIA after aBMT. Next, we wanted to assess the effect of aBMT in PGIA and the role of CD4+CD25+ Tregs in the clinical effect seen after aBMT. We recently showed that PGIA is inhibited by aBMT (S.T.A.R. et al, manuscript in preparation).
Arthritis was induced according to the protocol. On day 35, the mean arthritis score of all recipient mice was 6.2, and animals resistant to arthritis induction were excluded. On this day, conditioning was performed by a lethal 7.5-Gy irradiation dose followed by bone marrow transplant from sick syngeneic animals that were at the same stage of disease. Sick untreated mice were used as controls.
As shown in Figure 2A, conditioning followed by aBMT on day 35 led to a rapid improvement in disease scores, which reached its maximum effect by day 42. Fairly rapidly after aBMT, relapses occurred in the animals. Interestingly, however, shortly after the initial relapse, by day 55, the arthritis stabilized at a degree that was significantly lower than the arthritis of untreated animals (P = .006). As a consequence, after aBMT, despite the relapse, the severity of arthritis never returned to the level of before aBMT.
Conditioning followed by autologous bone marrow transplantation leads to a rapid decrease in the severity of arthritis: the clinical effect is reduced by depletion of CD25+ cells. (A) The effect of aBMT in PGIA. Arthritis was induced by 2 injections of PG in DDA on day 0 and day 21. On day 35, mice received a lethal irradiation dose of 7.5 Gy followed by autologous BMT from syngeneic donor animals at the same stage of the disease. Mean arthritis scores are shown (+ SEM; N = 17). (B) The effect of anti-CD25 treatment after aBMT. Following aBMT on day 35, the anti-CD25 mAb PC61 was administered on day 35 and day 42 at 2 different doses: 250 μg (N = 2) or 200 μg (N = 6). Mean arthritis scores are shown (+ SEM).
Conditioning followed by autologous bone marrow transplantation leads to a rapid decrease in the severity of arthritis: the clinical effect is reduced by depletion of CD25+ cells. (A) The effect of aBMT in PGIA. Arthritis was induced by 2 injections of PG in DDA on day 0 and day 21. On day 35, mice received a lethal irradiation dose of 7.5 Gy followed by autologous BMT from syngeneic donor animals at the same stage of the disease. Mean arthritis scores are shown (+ SEM; N = 17). (B) The effect of anti-CD25 treatment after aBMT. Following aBMT on day 35, the anti-CD25 mAb PC61 was administered on day 35 and day 42 at 2 different doses: 250 μg (N = 2) or 200 μg (N = 6). Mean arthritis scores are shown (+ SEM).
To assess whether CD25+ Tregs play a role in the long-term disease remission after aBMT, those cells were depleted in vivo by administration of an anti-CD25 mAb at 2 different doses (250 μg and 200 μg) on days 35 and 42.
Administration of anti-CD25 on the day of aBMT did not influence the initial arthritis-suppressive effect, independent of the dose of anti-CD25 given (Figure 2B). However, administration of anti-CD25 1 week after aBMT during the initial reconstitution phase had dramatic effects. After anti-CD25 treatment, the clinical improvement of aBMT was rapidly lost in a dose-dependent fashion. After a dose of 250 μg, the arthritis worsened to a level far beyond the arthritis severity of sick untreated animals. The effect of the lower anti-CD25 dose (200 μg) was similar, though less powerful. Altogether, these results imply a role for CD4+CD25+ Tregs in disease remission after aBMT, albeit not in causing the initial clinical improvement, but during the subsequent phase of clinical stabilization of the disease at a lower level than before aBMT.
Steep increase in CD4+CD25+ T cells in peripheral blood shortly after aBMT
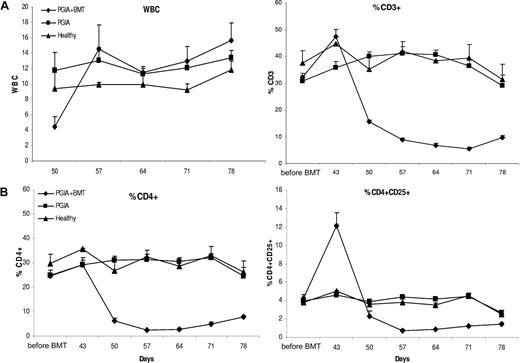
To gain insight into the repopulation of CD4+CD25+ T cells after aBMT, blood was drawn on a weekly basis. From day 50 onward, the white blood cell (WBC) count was assessed, and from day 43 onward, repopulation of CD3+, CD4+, and CD4+CD25+ T cells was measured in peripheral blood lymphocytes (PBLs) by FACS analysis. As to be expected, irradiation and aBMT led to an initial decrease in the WBC count, which recovered by day 57, at which time the WBC count had returned to levels before aBMT (Figure 3A). CD3+ T cells increased initially on day 42, followed by a clear decrease that lasted until the end of the experiment. As shown in Figure 3B, on day 42 the percentage of CD4+ cells of the lymphocyte population increased slightly, while within this population, there was a clear steep relative increase in CD4+CD25+ cells. Thus, although the WBC count after BMT treatment had recovered by day 57 in this model, CD3+, CD4+, and CD4+CD25+ lymphocytes did not fully recover yet by the end of the observation period (43 days after aBMT). Intriguingly, shortly after aBMT there was a relative increase in CD4+CD25+ cells coinciding with the time of maximum clinical improvement. We next questioned whether those CD4+CD25+ T cells were true Tregs that may have been involved in the early recovery following aBMT.
Peripheral blood repopulation of total white blood cells, and CD3+, CD4+, and CD4+CD25+ T cells after BMT. (A) Repopulation of WBCs and T lymphocytes in PBLs. After aBMT, blood was drawn from the tail vein of the mice on a weekly basis and the WBC count was determined on a Coulter cell counter as well as the repopulation of the percentage of CD3+ T cells by FACS, shown (+ SEM). (B) Repopulation of CD4+ and CD4+CD25+ T cells in PBLs. FACS analysis on PBLs was performed on repopulation of the percentage of CD4+ and CD4+CD25+ T cells, shown (+ SEM).
Peripheral blood repopulation of total white blood cells, and CD3+, CD4+, and CD4+CD25+ T cells after BMT. (A) Repopulation of WBCs and T lymphocytes in PBLs. After aBMT, blood was drawn from the tail vein of the mice on a weekly basis and the WBC count was determined on a Coulter cell counter as well as the repopulation of the percentage of CD3+ T cells by FACS, shown (+ SEM). (B) Repopulation of CD4+ and CD4+CD25+ T cells in PBLs. FACS analysis on PBLs was performed on repopulation of the percentage of CD4+ and CD4+CD25+ T cells, shown (+ SEM).
CD4+CD25+Foxp3+ Tregs in spleen and local lymph nodes reach a maximum relative increase at the time of clinical stabilization of disease after aBMT
Obviously, the presence of CD4 and CD25 on T cells is not a satisfactory characterization of Tregs, while also the peripheral blood may not be the best tissue to monitor reacquired immune tolerance following aBMT. We therefore in subsequent experiments assessed the presence of CD4+CD25+ Tregs locally in draining lymph nodes (LNs) and spleen, by applying Foxp3 as an additional marker.
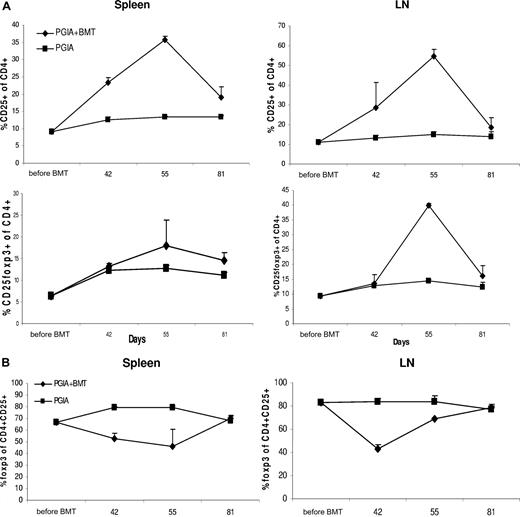
For this purpose, 3 mice per group were killed before aBMT and on day 42, day 55, and day 81. By FACS analysis, the percentage of CD25+ and CD25+Foxp3+ T cells within the CD4+ population was measured as well as the percentage of Foxp3+ cells within the CD4+CD25+ T-cell population. As can be seen in Figure 4A, after aBMT there was a clear increase by day 42 of CD25+ cells within the CD4+ lymphocyte population in both LNs and spleen. This paralleled the increase in CD4+CD25+ T cells in peripheral blood documented before. The percentage of Foxp3+ cells within the CD4+ T cells was, however, not increased. Within the CD4+CD25+ T-cell population, even a relative decrease of Foxp3+ cells was found (Figure 4B). This indicates that at this early time point after BMT not all CD4+CD25+ T cells display a regulatory phenotype. By day 55, a peak in CD25 expression was reached, now also corresponding with an increase and peak in Foxp3 expression within the CD4+ T cells, especially in the LNs (Figure 4A). Accordingly, the relative expression of Foxp3 within the CD4+CD25+ T-cell population also showed an increase (Figure 4B). These results indicate that by day 55 most of the CD4+CD25+ T cells (re)gained a regulatory phenotype.
After aBMT, the early relative increase in CD4+CD25+ T cells in spleen and lymph nodes is initially not accompanied by an increase in Foxp3-expressing cells. Repopulation of CD4+CD25+Foxp3+ cells in spleen and lymph nodes (LNs). (A) Before BMT and on days 35, 42, 55, and 81, N = 3 animals were killed per group, and FACS analysis was performed on the relative amount of CD25+ and CD25+ Foxp3+ cells within the CD4+ T-cell population, shown (+ SEM). (B) The relative amount of Foxp3+ cells within the CD4+CD25+ T-cell compartment was determined by FACS, shown (+ SEM).
After aBMT, the early relative increase in CD4+CD25+ T cells in spleen and lymph nodes is initially not accompanied by an increase in Foxp3-expressing cells. Repopulation of CD4+CD25+Foxp3+ cells in spleen and lymph nodes (LNs). (A) Before BMT and on days 35, 42, 55, and 81, N = 3 animals were killed per group, and FACS analysis was performed on the relative amount of CD25+ and CD25+ Foxp3+ cells within the CD4+ T-cell population, shown (+ SEM). (B) The relative amount of Foxp3+ cells within the CD4+CD25+ T-cell compartment was determined by FACS, shown (+ SEM).
Thus, at the time of maximum clinical improvement, there is a marked relative increase in CD4+CD25+ T cells not only in PBLs, but also in LNs and spleen. A large proportion of these cells, however, do not express Foxp3, while 2 weeks later, at the time of clinical stabilization most CD25+ cells do express Foxp3. Combined with the time-dependent effects of blocking CD25, shown in Figure 2B, this suggests that at day 55 indeed CD4+CD25+ T cells have (re)gained a regulatory phenotype and may be involved in the clinical stabilization of the disease. To further investigate whether these CD25+ T cells are indeed Tregs, their functionality was tested in vitro in a classical suppression assay.
The suppressive capacity of the CD4+CD25+ cells is reduced at the time of initial clinical improvement
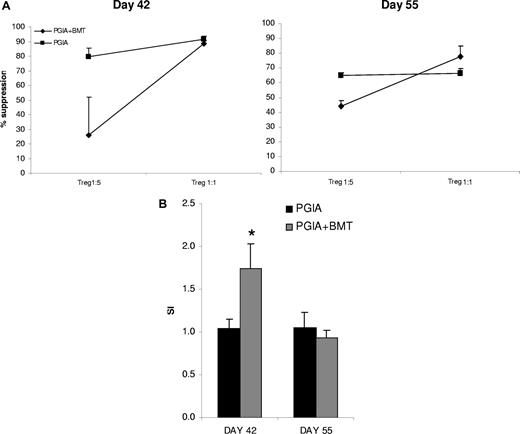
Next, we assessed the functionality of the CD4+CD25+ T cells in vitro during the repopulation phase after aBMT. CD4+CD25+ Tregs are in general anergic in vitro and should be capable of suppressing proliferation of CD4+CD25− T effector (Teff) cells in a contact-dependent manner.25 For this purpose, on day 42 and day 55 spleens were harvested from 3 mice per group; CD4+CD25+ T cells and CD4+CD25− T cells were FACS sorted and the suppressive capacity of CD4+CD25+ T cells on the proliferation of CD4+CD25− T cells was tested in a classical suppression assay. Figure 5A shows that on day 42, the CD4+CD25+ cells were less potent suppressors at a 1:5 ratio (mean 25.9% suppression vs 79.5% suppression by control animals). The suppression at a 1:5 ratio was fully restored by day 55. When added at a 5 times higher ratio (T suppressor/Teff: 1:1), CD4+CD25+ T cells were able to suppress effector T cells at both time points similar to normal controls.
Suppressive capacity of CD4+CD25+ T cells is reduced at day 42, but is recovered by day 55. (A) Suppressive capacity of CD4+CD25+ T cells. On days 42 and 55 after the first injection of PG/DDA spleens were harvested. CD4+CD25+ and CD4+CD25− T cells were FACS sorted, and the suppressive capacity of CD4+CD25+ cells on the proliferation of CD4+CD25− T effector cells was tested in the presence of APCs and anti-CD3. Shown is percentage suppression of 3 mice per group (+ SEM). (B) Anergy of CD4+CD25+ T cells. Sorted CD4+CD25+ T cells were stimulated with 1 μg/mL soluble anti-CD3 for 96 hours and proliferation was measured by 3H incorporation. Three mice per group were used. Shown are stimulation indexes (SIs) of stimulation by anti-CD3 divided by culture in medium (+ SEM).
Suppressive capacity of CD4+CD25+ T cells is reduced at day 42, but is recovered by day 55. (A) Suppressive capacity of CD4+CD25+ T cells. On days 42 and 55 after the first injection of PG/DDA spleens were harvested. CD4+CD25+ and CD4+CD25− T cells were FACS sorted, and the suppressive capacity of CD4+CD25+ cells on the proliferation of CD4+CD25− T effector cells was tested in the presence of APCs and anti-CD3. Shown is percentage suppression of 3 mice per group (+ SEM). (B) Anergy of CD4+CD25+ T cells. Sorted CD4+CD25+ T cells were stimulated with 1 μg/mL soluble anti-CD3 for 96 hours and proliferation was measured by 3H incorporation. Three mice per group were used. Shown are stimulation indexes (SIs) of stimulation by anti-CD3 divided by culture in medium (+ SEM).
In addition, at day 42, CD4+CD25+ T cells were significantly less anergic to stimulation by anti-CD3 (P = .01; Figure 4B), while at day 55 CD4+CD25+ cells were again fully anergic. Thus, altogether the lack of anergy and less potent suppressive capacity at day 42 underscore that at this time the CD4+CD25+ cells cannot be considered full T regulatory cells. These observations match the lack of Foxp3 expression in CD4+CD25+ cells during the first phase of immune reconstitution (day 42). This lack of Foxp3 expression had restored by day 55, the time of clinical stabilization of disease, as had the functionality of the Tregs.
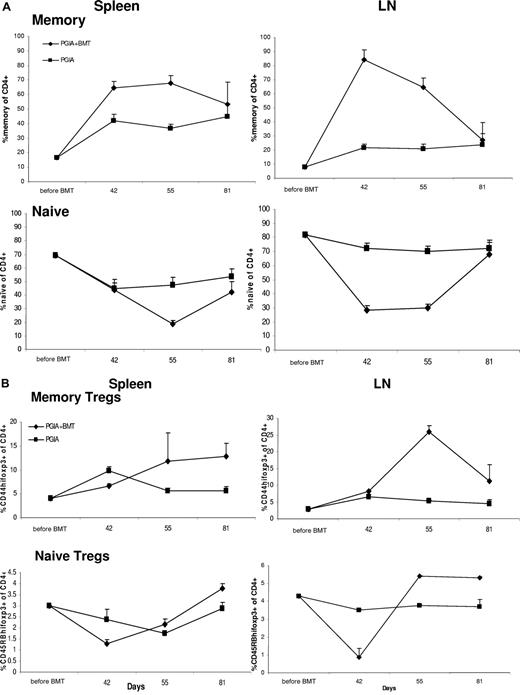
The initial repopulation phase is dominated by memory Foxp3+ T cells, but this is reversed during the second phase, when naive Foxp3+ T cells arise
Lastly, we wanted to assess whether the repopulating CD4+Foxp3+ cells were of a naive or memory phenotype. Therefore, FACS analysis was performed on spleens and LN cells prior to aBMT and at days 42, 55, and 81. The percentage of CD44high (memory) and CD45RBhigh (naive) cells was determined within the CD4+ T-cell and CD4+Foxp3+ T-cell population.
Prior to aBMT, both in spleen and LNs, the ratio between memory and naive cells was dominated by naive cells (Figure 6A). Shortly after aBMT this ratio changed, due to a steep relative increase in memory cells corresponding with a relative decrease in naive cells. This predominance in memory cells persisted until day 55, after which the contribution of the naive cell population emerged, and was back to levels it had before aBMT by day 81 (Figure 6A). Memory and naive CD4+Foxp3+ cells showed a similar trend, with a relative increase in memory Tregs and a relative decrease of naive Tregs shortly after aBMT. Naive Foxp3 cells recuperated faster (by day 55) compared with the overall naive cell population (day 81).
The dominance of memory CD4+ and CD4+Foxp3+ T cells over naive T cells shortly after BMT starts to reverse by day 55. Before BMT and on days 42, 55, and 81 spleens and LNs were harvested from killed animals (N = 3 per group), and by FACS analysis the percentage of CD44high (memory) and CD45RBhigh (naive) cells (A) as well as CD44highFoxp3+ (memory Tregs) and CD45RBhighFoxp3+ (naive Tregs) cells were determined within the CD4+ T-cell population (B). Error bars represent SEM.
The dominance of memory CD4+ and CD4+Foxp3+ T cells over naive T cells shortly after BMT starts to reverse by day 55. Before BMT and on days 42, 55, and 81 spleens and LNs were harvested from killed animals (N = 3 per group), and by FACS analysis the percentage of CD44high (memory) and CD45RBhigh (naive) cells (A) as well as CD44highFoxp3+ (memory Tregs) and CD45RBhighFoxp3+ (naive Tregs) cells were determined within the CD4+ T-cell population (B). Error bars represent SEM.
All together, T cells during the initial repopulation phase were of a memory phenotype, but during the second phase, naive cells came up, first in the Foxp3+ Treg population. Thus the emergence of naive Tregs from the thymus coincided with the time of clinical stabilization.
Discussion
aBMT is an effective last treatment option for severely ill children suffering from therapy-resistant JIA. It leads to drug-free disease remission in a majority of patients during a follow-up of 12 to 60 months after transplantation.13,14 Previous in vitro work in patients undergoing aBMT showed a restoration of the frequency of CD4+CD25brightFoxp3+ T cells from severely reduced levels before aBMT to normal levels after, suggesting a role for Tregs in disease remission after aBMT.18 A role for Tregs in BMT was also found in an antibody-mediated experimental arthritis model, in which the addition of CD4+CD25+ Tregs to the BM graft was found to have additional clinical effect.20 In this study, we aimed to explore whether CD4+CD25+Foxp3+ Tregs are crucial for the mechanism of action of aBMT. For this purpose, we turned to the relapsing remitting experimental arthritis model PGIA.
PGIA can serve as a valid model for aBMT in arthritis, as irradiation followed by aBMT with BM from syngeneic animals at the same stage of disease leads to a lasting clinical improvement (S.T.A.R. et al, manuscript in preparation). We now show in this paper that CD4+CD25+Foxp3+ Tregs are crucial for the establishment of the renewed immune tolerance after aBMT. First, in vivo depletion of CD25+ cells by the anti-CD25 mAb PC61 shortly after aBMT led to a dose-dependent enhancement of disease. Second, the clinical stabilization of arthritis corresponded with a rise in CD4+CD25+Foxp3+ T cells that have increased in vitro suppressive capacity. At a closer look, the correlation between disease improvement and Tregs was less straightforward than initially presumed. We found that the clinical improvement after aBMT could be split up into 2 phases: an initial rapid disease remission, followed by a second phase, consisting of a relapse but stabilizing long-term at a degree of arthritis that was significantly less severe than before aBMT.
The initial clinical improvement correlated with a relative increase in CD4+CD25+ T cells in PBLs, LNs, and spleen, again suggesting a critical role for Tregs. However, at this early time point, a large proportion of CD4+CD25+ T cells did not have a classical Treg phenotype, since they did not express the transcription factor Foxp3, nor were they potent suppressors in vitro. In fact, they were less anergic than their potently suppressive counterparts, which may explain their decrease in functionality.
Thus, in the early phase after aBMT, at the time of maximum clinical improvement, CD4+CD25+ cells consisted mainly of highly proliferative T cells, probably in a state of homeostatic proliferation during lymphopenia after irradiation. The absence of disease at that time may be explained by the relatively empty immune system after conditioning, where the lack of inflammation and inflammatory Teff cells may help to explain the clinical picture.
This initial stage was followed by a phase of relapse and subsequent long-term stabilization of disease. In contrast to the early phase, in this later stage, in vitro data pointed to a crucial role for Tregs. At this stage, the CD4+ cells reached a peak in CD25 as well as Foxp3 expression, and those cells now were anergic to nonspecific stimulation and had regained potent suppressive capacity in vitro. The consequent clinical stabilization was long lasting, since it persisted until the end of the experiment, and therefore suggested the establishment of a renewed immune balance.
The exact nature and origin of the regulatory T cells that emerge after BMT have yet to be determined. To date, several types of Tregs have been identified.25-27 They may be of donor or host origin and may be naturally occurring Tregs, educated in the thymus, or peripherally induced Tregs, educated by the encounter of disease-triggering antigens in a lymphopenic environment. Our data indicate that during the initial phase, the repopulating cells, including Foxp3+ cells, were mainly of a memory phenotype. However, this phenotype did not yet determine their origin. They may be “host” cells that have preferentially survived irradiation, as Tregs have been described to preferentially survive irradiation in another experimental model.28 On the other hand, memory cells have been shown to preferentially home to the BM, and therefore could be of “donor” origin as well.29,30 Either way, at the time of clinical stabilization, the contribution of naive Tregs came into play, indicating generation of novel naturally occurring Tregs, which were clearly important in restoring the immune balance. Further experiments are needed to determine whether cell therapy with Tregs may be of additional therapeutic value, for example by addition of Tregs to the BM graft.
Lastly, we established a role for CD4+CD25+ Tregs in the natural disease course of PGIA that had not yet been established previously.21 In animals that initially were resistant to PGIA induction, despite a proper immunization protocol, in vivo depletion of Tregs by the administration of anti-CD25 led to a rapid and dramatic induction of arthritis. These data are in line with similar observations in experimental autoimmune encephalomyelitis (EAE) as well as antigen-induced arthritis, where anti-CD25 administration led to enhancement of disease.31-34 Furthermore, CD25 depletion was found to inhibit the natural recovery from EAE, as was CD25 depletion after recovery from EAE able to remove the resistance to reinduction of EAE observed in this model.35 In our PGIA model, we hereby showed that not insufficient immunization but active suppression of arthritis by CD25+ Tregs was responsible for the initial tolerance to disease induction observed in a proportion of animals.
The establishment of a prominent role for Tregs in restoration of the immune balance by aBMT provided here is a conceptual step forward. It may have important therapeutic translational implications for improving clinical outcome by, for example, adding Tregs to the BM graft.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Suzanne Berlo, Corlinda ten Brink, and Femke Broere of the department of Veterinary Medicine Utrecht for their help and advice on the PGIA model and Henk Rozemuller from the Department of Hematology Utrecht for his help with the bone marrow transplantations. Furthermore, the authors thank Ellen Wehrens for her help with the experiments.
S.T.A.R. is financially supported by a grant from the Dutch Organization for Scientific Research (NWO). B.P. is supported by the Dutch Rheumatoid Arthritis Foundation (Nationaal Reumafonds) and an NWO Innovation Impulse grant (VIDI) from the NWO.
Authorship
Contribution: S.T.A.R. and F.v.W. designed and performed research, analyzed data, and wrote the paper; W.d.J. performed research; L.B. contributed vital new reagents; N.W. and A.M. designed research; B.P. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Berent Prakken, Department of Pediatric Immunology (KC010690), University Medical Center Utrecht, Lundlaan 6, 3584 EA Utrecht, The Netherlands; e-mail: b.prakken@umcutrecht.nl.
References
Author notes
*B.P. and F.v.W. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal