Using a mouse model of inducible autoimmune arthritis that mimics some aspects of the human disease, Roord and colleagues identify a role for regulatory T cells (Tregs) both for modulation of disease and as a therapeutic component of autologous bone marrow transplantation (aBMT).
Since 1996, more than 1000 patients worldwide have undergone autologous hematopoietic stem cell transplantation (HSCT) as therapy for refractory autoimmune diseases, including systemic sclerosis, multiple sclerosis, systemic lupus, Crohn disease, and rheumatoid arthritis (RA). Although autologous HSCT yields 5-year disease-free survival in 30% to 50% of patients, current therapies are likely not curative.1 Importantly, post-HSCT disease responses in RA patients are very common, but unfortunately, relatively short-lived. As such, one critical question investigators must address is how HSCT can be modified to induce sustained disease-free survival and, ideally, cure most patients with autoimmune disease. Clearly, it is desirable to elucidate the mechanisms whereby autologous HSCT controls autoimmunity. In this respect, the contributory role of Treg reconstitution identified by Roord et al points to a promising future for the field.
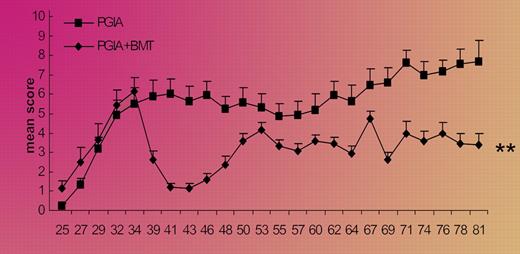
The proteoglycan-induced RA model used in this report appears to invoke both B-cell and T-cell immunity, and to produce a relapsing and remitting disease natural history that is perhaps clinically relevant. The investigators' discovery that in vivo depletion of CD25+ cells yielded a 100% incidence of the arthritic syndrome strongly supports the protective role of naturally occurring Tregs in this model. With respect to the authors' transplantation results, it was determined that Tregs were reconstituted after transplantation in an initial wave of functionally quiescent memory cells and a second wave of naive, competent, Foxp3+ Tregs. This latter Treg population represented an immunotherapeutic subset because in vivo antibody depletion resulted in disease relapse. Such cells appear to be analogous to thymus-derived Tregs, known to suppress graft-versus-host disease after murine allogeneic HSCT.2 Several points of discussion are of interest. First, similar to the clinical context, the arthritis syndrome in mice that underwent transplantation was ameliorated but not completely resolved (see figure). It will be of interest to determine whether methods to further improve Treg reconstitution, such as adoptive cell therapy3 or pharmacologic methods such as rapamycin,4 may consolidate the autoimmune arthritis remissions in this model. Second, further murine studies to compare and contrast the efficacy of autologous versus allogeneic transplantation would be informative in light of the potential role of allogeneic HSCT as an autoimmune therapy.5 Finally, in an attempt to evaluate a potentially more stringent model more analogous to the clinical context, it would be of interest to observe the therapeutic effect of transplantation in thymectomized murine hosts.
Conditioning followed by aBMT leads to a rapid decrease in the severity of arthritis; the clinical effect is reduced by depletion of CD25+ cells. See the complete figure in the article beginning on page 5233.
Conditioning followed by aBMT leads to a rapid decrease in the severity of arthritis; the clinical effect is reduced by depletion of CD25+ cells. See the complete figure in the article beginning on page 5233.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal