In this issue of Blood, Kim and colleagues propose a model describing the function of FANCM in chromatin recruitment of the Fanconi anemia core complex.
Fanconi anemia (FA) is a rare genetic disorder characterized by congenital abnormalities, bone marrow failure, and increased incidence of cancer, particularly acute myelogenous leukemia. Individuals with defects in FA genes are classified in 13 complementation groups. The interaction of 8 FA proteins forms a nuclear complex known as the FA core complex which, in response to DNA damage or during the S phase of the cell cycle, promotes the monoubiquitination of FANCD2 and FANCI. Once monoubiquitinated, these proteins facilitate the participation of other FA and DNA repair proteins in orchestrating cellular responses that mediate the repair of DNA. Defining the key steps that regulate the FA pathway is essential not only to understand the molecular biology of FA, but also to develop new therapies for the treatment of FA patients and patients with tumors defective in the FA pathway.
The study by Kim and colleagues focuses on defining the function of one of these FA proteins, FANCM. This protein was identified by Meetei et al in 2005 as a human ortholog of the archeal DNA repair protein Hef,1 suggesting an evolutionary link between FA-associated proteins and DNA repair. In that study, the authors proposed for the first time that FANCM may translocate the FA core complex along DNA.1 In another elegant study, Ciccia and colleagues identified an additional component of the FA core complex: FA-associated protein FAAP24.2 The authors showed that FAAP24 targets FANCM to branched DNA structures, and proposed that the FANCM/FAAP24 complex may play a key role in the recruitment of the FA core complex to damaged DNA.
The article by Kim and colleagues goes one step further in defining the role of FANCM/FAAP24 in the recruitment of the FA core complex to chromatin. Their study demonstrates that FANCM/FAAP24 interaction is essential for chromatin loading of the FA core complex, but not for its assembly. Interestingly, these authors show that FANCM is constitutively bound to chromatin in a process that depends on the presence of FAAP24. This contrasts with the association of the FA core complex to chromatin, which is particularly strong after DNA damage or during the S phase of the cell cycle, and is regulated by FANCM.
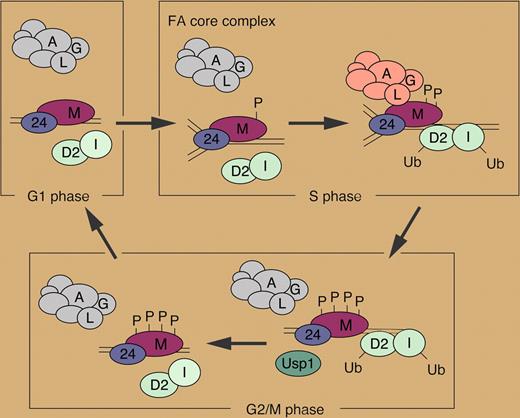
In the model proposed by the authors (see figure), when cells are in G1 phase, the FA core complex can be assembled but does not associate with FANCM/FAAP24 in chromatin. During the S phase, phosphorylated FANCM recruits the FA core complex to chromatin, facilitating the monoubiquitination of FANCD2 and FANCI. The hyperphosphorylation of FANCM during G2/M phases releases the FA core complex from chromatin. USP1 may then deubiquitinate FANCD2 and FANCI, shutting off the FA pathway.
Model for the role of FANCM in regulating the FA pathway. See the complete figure in the article beginning on page 5215.
Model for the role of FANCM in regulating the FA pathway. See the complete figure in the article beginning on page 5215.
It is still unclear whether FANCM directs the FA core complex specifically at sites of stalled replication forks; how FA core complex loading leads to DNA repair also remains to be determined. Nevertheless, the study by Kim and colleagues unequivocally shows that FANCM plays a regulatory role in the FA pathway, and provides evidence that the dysregulated loading of the FA core complex to chromatin accounts, at least partially, for the characteristic phenotype of FA cells.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal