Abstract
The JAK2V617F mutation was found in most patients with myeloproliferative disorders (MPDs), including polycythemia vera, essential thrombocythemia, and primary myelofibrosis. We have generated transgenic mice expressing the mutated enzyme in the hematopoietic system driven by a vav gene promoter. The mice are viable and fertile. One line of the transgenic mice, which expressed a lower level of JAK2V617F, showed moderate elevations of blood cell counts, whereas another line with a higher level of JAK2V617F expression displayed marked increases in blood counts and developed phenotypes that closely resembled human essential thrombocythemia and polycythemia vera. The latter line of mice also developed primary myelofibrosis-like symptoms as they aged. The transgenic mice showed erythroid, megakaryocytic, and granulocytic hyperplasia in the bone marrow and spleen, displayed splenomegaly, and had reduced levels of plasma erythropoietin and thrombopoietin. They possessed an increased number of hematopoietic progenitor cells in peripheral blood, spleen, and bone marrow, and these cells formed autonomous colonies in the absence of growth factors and cytokines. The data show that JAK2V617F can cause MPDs in mice. Our study thus provides a mouse model to study the pathologic role of JAK2V617F and to develop treatment for MPDs.
Introduction
Myeloproliferative disorders are a group of conditions characterized by chronic increases in some or all of the blood cells (platelets, white blood cells, and red blood cells).1-3 This group of blood disorders includes polycythemia vera (PV), essential (or primary) thrombocythemia (ET), primary myelofibrosis (PMF), and chronic myeloid leukemia (CML). PV is characterized by increased production of all 3 types of cells, whereas ET is manifest in the elevation of platelets. PMF is a disease in which fibrous (scar-like) tissues form in the marrow as a result of abnormal production of red cells, white cells, and platelets. CML is characterized by the increased and unregulated growth of predominantly myeloid cells in the bone marrow and the accumulation of these cells in the peripheral blood. It is generally thought that MPDs arise from a transformation in a hematopoietic stem cell. Indeed, CML is now defined by its causative molecular lesion, the BCR-ABL fusion gene, which most commonly results from the Philadelphia translocation (Ph). Because of this defined molecular defect, a highly effective drug, namely, imatinib mesylate (Gleevec; Novartis, Basel, Switzerland), has been developed to treat CML.4 So far, there is no effective cure for the 3 Ph-negative MPDs. Recently, 5 groups have identified a gain-of-function mutation of tyrosine kinase JAK2, which likely represents a major molecular defect in approximately 90% patients with PV and in approximately 50% of patients with ET or PMF.5-10 The JAK2 mutant displays deregulated kinase activity and generates a PV-like phenotype in mouse bone marrow transplant models.11-14 Studies also demonstrated infrequent occurrence of this mutation in chronic myelomonocytic leukemia, atypical myeloproliferative disorders, myelodysplastic syndrome, systemic mastocytosis, chronic neutrophilic leukemia, and acute myeloid leukemia.15-19 Interestingly, our recent studies also demonstrated that nearly 1% of blood samples collected from a hospital population bear the JAK2V617F mutation.20 Most of these JAK2V617F-positive patients do not meet the criteria for diagnosis of MPDs but developed vascular diseases, including thrombosis, coronary heart disease, arteriosclerosis, cerebral ischemia, and cerebral infarction. The data suggest that MPDs and pre-MDPs conditions may represent a more profound public health problem than we had anticipated.20 This further emphasizes the pathologic importance of the JAK2V617F mutation
The pathologic implication of JAK2V617F in MPDs has generally been accepted. However, it is not clear why a single point mutation is associated with such a wide spectrum of phenotypes. Although retrovirus-mediated expression of JAK2V617F in mouse bone marrow transplant models produces PV phenotype,11-14 there are strong indications that mutation of JAK2 may not be the primary cause of the disease because JAK2V617F mutation does not provide a proliferative/survival advantage to the PV clone during in vitro expansion.21,22 To further define the pathologic role of JAK2V617F, we have generated transgenic mice expressing the mutated enzyme in the hematopoietic system driven by a tissue-specific promoter. Our data indicate that transgenic expression of JAK2V617F causes phenotypes resembling human ET, PV, and PMF in mice.
Methods
Construction of the transgene and generation of transgenic mice
The entire coding region of human JAK2V617F plus the 3′ noncoding region were cloned into the HS321/45-vav vector provided by Dr Jerry M. Adams (Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia).23 The plasmid DNA was digested with SacII to remove the pBSIISK backbone and was used for injection into pronuclei of eggs from C57BL/6XDBA/2 F2 mice using the core facility at the Oklahoma Medical Research Foundation. The transgenic mice were identified by PCR amplification of tail genomic DNAs with primers TACAACCTCAGTGGGACAAAGAAGAAC and CCATGCCAACTGTTTAGCAACTTCA. The primers cover a 594 bp region in the coding sequence of JAK2V617F. Transgenic founder mice were crossed with wild-type C57BL/6 mice. JAK2V617F negative siblings of transgenic mice were used as controls throughout the study.
Analyses of mice
Peripheral blood samples (∼50 μL) were collected by tail bleeding into tubes containing potassium salt of ethylenediamine tetraacetic acid. Complete blood counts were obtained using a HESKA Vet ABC-Diff Hematology analyzer. Each analysis was done in duplicate. Blood samples with a platelet level of more than 2000 × 109/L were diluted 3-fold in a RPMI medium containing 10% fetal bovine serum before analyses. Blood smears were stained with the May-Grünwald-Giemsa method. For histopathology analyses, femurs were fixed in formaldehyde, decalcified, and paraffin embedded. Spleens were treated in a similar way except that decalcification was omitted. Sections (4.5 μm) were cut and stained with hematoxylin and eosin. Reticulin staining was performed using the Accustain Reticulin Stain kit from Sigma-Aldrich (St Louis, MO). Levels of erythropoietin (Epo) and thrombopoitin (Tpo) in the plasma of mouse blood were determined using the mouse Epo and Tpo Quantikine enzyme-linked immunosorbent assay (ELISA) kits from R&D Systems (Minneapolis, MN) according to the manufacturer's protocol.
Flow cytometric analysis and progenitor cell culture
Total white blood cells were obtained after lysis of peripheral blood with a red cell lysis buffer. Single-cell suspensions were prepared from bone marrow (from femurs) and spleen, and red cells were removed with the red cell lysis buffer. For flow cytometric analysis, the cells were stained with phycoerythrin-conjugated anti-B220 and fluorescein isothiocyanate-conjugated antibodies against Gr-1, Ter-119, CD71, and CD41 (BD Biosciences, San Jose, CA). The analyses were performed with a FACSCalibur Flow Cytometer at the Flow and Image Cytometry Laboratory of University of Oklahoma Health Sciences Center. All the cell cultures were carried out using media, growth factors, and cytokines from StemCell Technologies (Vancouver, BC). These included methylcellulose-based MethoCult and collagen-based MegaCult-C media. Cell cultures were done in triplicates, and colonies were scored after 2, 7, and 10 days for erythrocyte colony-forming unit, colony-forming units-megakaryocyte, and all other colonies, respectively, according to the manufacturer's protocols. Bone marrow stromal cells were cultured and expanded in the MesenCult Medium (Mouse) with Mesenchymal Stem Cell Stimulatory Supplements. The second passage cells were harvested for isolation of total RNAs.
Real-time polymerase chain reaction analysis
Total RNAs were isolated from peripheral blood, bone marrow, spleen, stromal cells, and various other tissues of mice using the Trizol reagent (Invitrogen, Carlsbad, CA) and were then treated with RQ1 RNase-free DNase (Promega, Madison, WI) to remove contaminated genomic DNAs. First-strand cDNA was synthesized using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Real-time polymerase chain reaction (PCR) was performed in an IQ5 Multicolor Real-Time PCR Detection System using iQ SYBR Green Supermix (Bio-Rad). PCR amplifications were performed in triplicate with multiple dilutions for human JAK2V617F and mouse Jak2 along with parallel measurements of mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA (an internal control). To confirm specific amplification of desired PCR product, melting curves were analyzed and PCR products were separated on a 3.0% agarose gel. As an additional control, reverse transcription (RT–PCR) was also performed with equivalent amounts of RNA samples without reverse transcription. The following primers were used for PCR amplification: Mouse GAPDH, ACTCCCACTCTTCCACCTTCG (forward) and CACCCTGTTGCTGTAGCCGTA (reverse); human JAK2V617F, AGATGTGAAAATATCTGCTCAAAACT (forward) and AAACTTCTTACAAAATCCTTGCTAAG (reverse); mouse Jak2, AGACTTCCAGAACCAGAACAAAG (forward), and TCACAGTTTCTTCTGCCTAGCTA (reverse). The primer sets for human JAK2 and mouse Jak2 were derived from the last exons corresponding to the 3′noncoding regions of each cDNA where the 2 genes share essentially no sequence identity. The primers work for cDNAs as well as genomic DNAs. The PCR conditions were 95°C for 20 seconds, 59°C for 20 seconds, 72°C for 20 seconds, for 45 cycles. To determine the copy number of transgenic JAK2V617F, PCR products of human JAK2 and mouse Jak2 amplified with the above primer sets were cloned into the pBluescript KS vector, and the purified plasmid DNAs, with concentration being accurately measured, were used as standards in real-time PCR analyses. The absolute amounts of human JAK2V617F and mouse Jak2 in a given DNA samples were thereby determined, and the ratios of these gene DNAs represent relative gene copy numbers.
Statistical analysis
Statistical analyses were performed using the Excel program. Differences between 2 groups of samples were accessed using t tests. P values less than .05 (2-tailed) are considered significantly different.
Image acquisition
Slides were viewed with an Olympus BX-51 upright microscope equipped with U Plan Fluorite objectives at 40×/0.75 and 100× oil/1.30 (Olympus, Tokyo, Japan) and Cytoseal mounting medium (Richard-Allan Scientific, Kalamazoo, MI). Images were acquired using a DP71 digital camera with DP-BSW-V3.1 camera control software (Olympus) and were processed with Adobe Photoshop version 7.0 software (Adobe Systems, San Jose, CA).
Results
Generation of JAK2V617F transgenic mice
By transplanting bone marrow cells expressing JAK2V617F mediated by retroviral vectors, several studies have shown that the JAK2 mutant produces PV-like phenotype in mice.11-14 These studies have verified the pathogenicity of JAK2V617F. However, these mouse models are not permanent mouse lines. To create a stable mouse MPD model, we have chosen a transgenic approach. Considering the general effects of JAK2 on the development of cells other than hematopoietic cells, we chose a HS321/45-vav vector, which uses the vav gene promoter to control gene expression. Based on the previous studies, the vav promoter drove expression of human CD4 efficiently and stably in virtually all nucleated cells of adult hematopoietic tissues, including B and T lymphocytes, granulocytes, monocytes, megakaryocytes, eosinophils, and nucleated erythroid, but not in nonhematopoietic cell types.23 By following a similar procedure, we generated 3 JAK2V617F transgenic founder mice, designated AF0, BF0, and CF0, which give rise to A, B, and C lines of progeny, respectively. The AF0 and BF0 mice are viable and fertile. AF0 became very inactive and essentially moribund at 69 weeks and was thus killed for analyses. BF0 died at 93 weeks. Both A and B founder mice displayed enlarged spleens. CF0 did not look healthy from the beginning. It died suddenly at the age of 4 weeks when it was picked up from the back. The cause of death remains unknown. It also had an enlarged spleen. The founder line transgenic mice have the C57BL/6XDBA hybrid background, and they were crossed with wild-type C57BL/6 mice to reach purer genetic background. This study was carried out with F0 to F6 generation mice.
Analyses of transgenic JAK2V617F gene expression
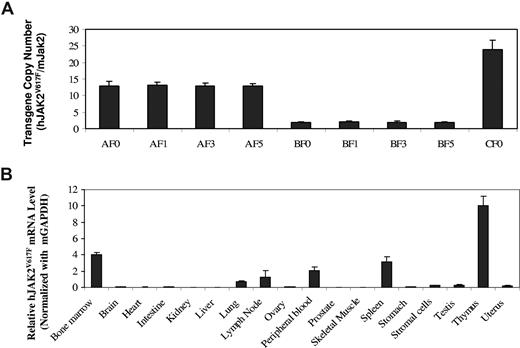
We used real-time PCR analyses to determine the gene copy numbers of JAK2V617F in mice. Our real-time PCR analyses yielded products showing single bands of expected size on agarose gels and single peaks on melting curve analyses (data not shown). Using purified plasmid DNAs as standards, we first generated linear standard curves as shown in Figure S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Based on the standard curves, we were able to determine the absolute amounts of human JAK2 and mouse Jak2 DNAs in a given sample and thereby calculate copy numbers of the JAK2V617F transgene (Figure 1A). The gene copy numbers are 13 plus or minus 1.4, 1.9 plus or minus 0.3, and 24 plus or minus 2.7 for the A, B, and C founder mice, respectively. Over 6 generations, the A and B line mice kept the same transgene gene copy numbers as their founder mice. This implies that the JAK2V617F transgene was inserted into a single chromosome in each mouse line, likely through one integration site. This was further supported by the fact that all JAK2V617F mice were born with the expected Mendelian ratio. To verify the tissue specificity of the vav promoter we used, we analyzed the mRNA level of JAK2V617F in various tissues of line A mice. As shown in Figure 1B, exogenously introduced human JAK2V617F is expressed in hematopoietic tissues, including thymus, bone marrow, spleen, peripheral blood, and lymph nodes, but hardly at all in various nonhematopoietic tissues except for lung. Interestingly, endogenous mouse Jak2 is highly expressed in all the hematologic tissues but also at a significant level in other tissues, such as lung, ovary, intestine, uterus, and stomach (Figure S2). We further analyzed the expression of JAK2V617F in bone marrow stromal cells obtained from in vitro culture. The stromal cells also expressed the JAK2V617F transgene gene, but at a level approximately one-tenth of that seen in the whole bone marrow cells (Figure 1B). A contribution of this expression to the observed phenotypes described in “Phenotypes of JAK2V617F mice” may be less likely but cannot be totally ruled out. We also compared the relative expression levels of JAK2V617F mRNA in A and B line mice. On average, JAK2V617F expression in line A mice was 4 times higher than that in line B, which is correlated with the gene copy numbers (data not shown). The fact that mRNA levels were not directly proportional to the gene copy numbers may be the result of the so-called repeat-induced gene silencing effect seen in transgenic animals with multiple transgene copies in concatameric arrays.24 However, within each line of mice, the expression level is consistent among different members over generations, despite some variations in phenotypes. With the standard curves shown in Figure S1, we were also able to calculate the ratio of reversely transcribed cDNAs of JAK2V617F and mouse Jak2 in a given sample. With bone marrow, spleen, and peripheral blood samples, the cDNA level of JAK2V617F was no more than 1/16th that of mouse Jak2, even for the line A transgenic mice. Nonetheless, this result should not be overinterpreted. Because the reverse transcription efficiencies for mJak2 and hJAK2V617F may be different, the data do not necessarily mean that the mRNA level of human JAK2V617F is less than 1/16th of that of mouse JAK2. In addition, even if the hJAK2 mRNA is that much lower, it does not necessarily produce less JAK2V617F protein because the translation efficiencies of these 2 mRNAs may also be different.
Detection of JAK2V617F expression in transgenic mice. (A) JAK2V617F copy numbers were determined by real-time PCR assays of genomic DNAs from founder (F0), F1, F3, and F5 generations of A, B, and C line transgenic mice. Data represent ratios of human JAK2V617F and mouse Jak2 (mean ± SD, n ≥ 6). (B) Real-time PCR assays of JAK2V617F mRNA expression in the indicated tissues of line A transgenic mice. Data represent relative mRNA levels (mean ± SD, n ≥ 3) normalized to mouse GAPDH in arbitrary units.
Detection of JAK2V617F expression in transgenic mice. (A) JAK2V617F copy numbers were determined by real-time PCR assays of genomic DNAs from founder (F0), F1, F3, and F5 generations of A, B, and C line transgenic mice. Data represent ratios of human JAK2V617F and mouse Jak2 (mean ± SD, n ≥ 6). (B) Real-time PCR assays of JAK2V617F mRNA expression in the indicated tissues of line A transgenic mice. Data represent relative mRNA levels (mean ± SD, n ≥ 3) normalized to mouse GAPDH in arbitrary units.
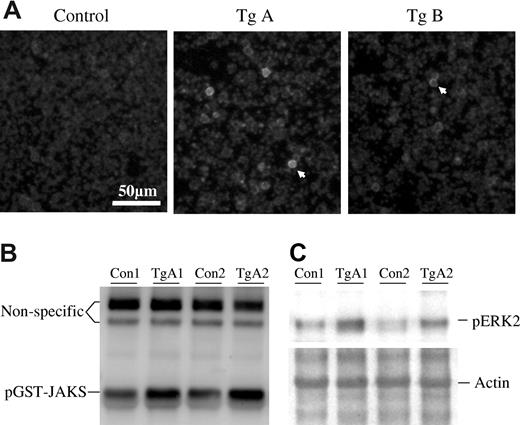
We attempted to use Western blotting analyses to detect the expression of JAK2V617F in white blood cells from peripheral blood, bone marrow, and spleen. The antibody worked well, but samples from transgenic mice did not show a significantly higher level of JAK2 expression over the basal level of mouse JAK2 in control mice (data not shown). So far, we don't have a specific antibody that recognizes human JAK2 but not mouse JAK2. The data suggest that the overall expression level of exogenously introduced JAK2V617F was not very high, further suggesting that the vav promoter is a relatively mild promoter as previously reported.23 We then performed cell staining to detect expression of JAK2V617F in individual cells. Figure 2A shows that a small fraction (<1%) of peripheral blood white cells expressed a high level of JAK2. In consistence with the mRNA expression levels, cells from line A mice appeared to be stained brighter than those of the line B mice. We observed a similar expression pattern of JAK2V617F in cells from bone marrow and spleen (not shown). This explains why Western blotting analyses of whole cell extracts failed to show a significant increase in the JAK2 protein level in transgenic mice. The nature of these positively stained cells is to be determined. We further detected JAK2 kinase activity in the extracts of mouse tissues using a sensitive assay system recently developed.25,26 As shown in Figure 2B, a markedly increased phosphorylation of GST-JAKS was seen in samples from transgenic mice compared with the control. This increased activity is presumably attributable to the expression of JAK2V617F. As a deregulated enzyme, JAK2V617F was known to cause constitutive cell signaling as revealed by many studies.5-14 This was found to be the case as illustrated by a significantly increased level of ERK2 phosphorylation in bone marrow cells of line A mice in comparison with the control mice (Figure 2C). We also analyzed phospho-ERK2 in line B mice and phospho-Akt and phospho-STAT5 in all the transgenic mice but detected only subtle increases over the control mice (data not shown).
Detection of JAK2V617F protein levels and activities in transgenic mice. (A) Peripheral blood white cells from line A and B transgenic mice and their nontransgenic siblings were attached to polylysine-coated coverslips, fixed with formaldehyde, and then stained with an anti-JAK2 antibody (Cell Signaling Technology, Danvers, MA) and further probed with a Cy3-conjugated antirabbit secondary antibody. Photos were taken with a 40× objective. The arrowheads point to positively stained cells. (B) Cell extracts from bone marrow (after red cell lysis) of 2 nontransgenic control and 2 line A transgenic mice were analyzed for JAK2 tyrosine kinase activity with GST-JAKS as a substrate as described earlier.25 Detections were made using an antiphosphotyrosine antibody. Equal protein loadings are indicated by 2 irrelevant (nonspecific protein) bands recognized by the antibody. (C) Analyses of ERK activation in bone marrow cells from 2 nontransgenic control and 2 line A transgenic mice with anti-pERK antibody (Cell Signaling Technology). Coomassie blue staining of the PVDF membrane revealed equal amounts of actin, indicating equal protein loadings. Note that actin and ERK2 have similar molecular size.
Detection of JAK2V617F protein levels and activities in transgenic mice. (A) Peripheral blood white cells from line A and B transgenic mice and their nontransgenic siblings were attached to polylysine-coated coverslips, fixed with formaldehyde, and then stained with an anti-JAK2 antibody (Cell Signaling Technology, Danvers, MA) and further probed with a Cy3-conjugated antirabbit secondary antibody. Photos were taken with a 40× objective. The arrowheads point to positively stained cells. (B) Cell extracts from bone marrow (after red cell lysis) of 2 nontransgenic control and 2 line A transgenic mice were analyzed for JAK2 tyrosine kinase activity with GST-JAKS as a substrate as described earlier.25 Detections were made using an antiphosphotyrosine antibody. Equal protein loadings are indicated by 2 irrelevant (nonspecific protein) bands recognized by the antibody. (C) Analyses of ERK activation in bone marrow cells from 2 nontransgenic control and 2 line A transgenic mice with anti-pERK antibody (Cell Signaling Technology). Coomassie blue staining of the PVDF membrane revealed equal amounts of actin, indicating equal protein loadings. Note that actin and ERK2 have similar molecular size.
Phenotypes of JAK2V617F mice
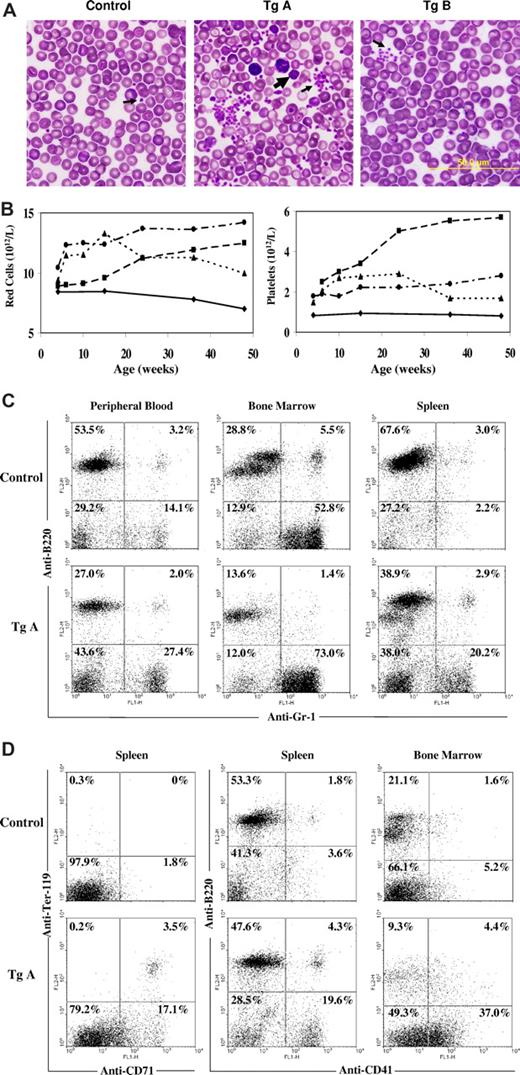
We have performed complete blood counts for more than 200 transgenic mice and their nontransgenic siblings and observed significant increases in blood counts, particularly for the line A mice. Table 1 summarizes data obtained with mice 10 to 12 weeks of age. Compared with the nontransgenic control mice, line A transgenic mice demonstrated markedly increased counts of white cells, red cells, hemoglobin, hematocrit, and platelets. To a lesser degree, the line B mice which had a lower level of JAK2V617F expression also produced increased blood counts. Red blood cell counts, hemoglobin, hematocrit, and platelet counts in the line B mice are considerably higher than the control mice, although their white cell counts were not significantly different. Interestingly, statistical analyses revealed that mean corpuscular volume and mean corpuscular hemoglobin of red cells from transgenic mice, especially line A, were significantly below those of control mice. Staining of blood smears revealed that the shape of red cells is apparently normal, but line A mice have many obviously smaller red cells (Figure 3A). Line A transgenic mice also displayed a markedly increased number of platelets in the blood smear with the occasional presence of giant platelets. Within the line A mice, approximately 75% showed strong elevations in the platelets (usually 3000 × 109/L) but with normal or slightly increased counts in white cells and red cells. The phenotype of these mice resembles that of human ET patients. The remaining approximately 25% of line A mice displayed abnormally high levels of red blood cells and white blood cells as well as platelet. This puts the mice into the PV category. Overall, essentially all the line A mice had elevated red cells, white cell, and/or platelets. Many line B transgenic mice exhibited moderately elevated blood counts, which did not reach levels that can be defined as ET or PV. The data provided in Table 1 were obtained from F2 to F6 generation mice at 10 to 12 weeks of age. Normal variation may contribute to the differences in phenotypes observed within each line of mice, although genetic background may be a factor as well. Age also affected blood counts of mice. Figure 3B illustrates several typical cases. Some mice started with high blood counts at the age of 4 weeks, whereas others started with normal or near-normal counts but increased to abnormal levels over time. Some mice showed a continual increase in blood counts over a long period, whereas others were stabilized or even showed some decline. So far, none of these mice have had their blood level decline to a point of anemia or thrombocytopenia. Finally, male and female mice appeared to respond similarly to transgenic expression of JAK2V617F.
Summary of blood analyses of control and JAK2V617F transgenic mice*
| . | WBC, ×109/L . | RBC, ×1012/L . | HGB, g/L . | HCT, % . | PLT, ×109/L . | MCV, fm3 . | MCH, pg . | MCHC, g/L . |
|---|---|---|---|---|---|---|---|---|
| Nontransgenic control, n = 30 | ||||||||
| Mean | 8.29 | 9.61 | 15.2 | 45.4 | 968 | 46.9 | 15.6 | 34.8 |
| SD | 2.54 | 0.55 | 0.76 | 1.78 | 89 | 1.4 | 0.60 | 1.45 |
| Range | 5.0-10.9 | 8.2-10.9 | 14-17.2 | 40.2-49.8 | 765-1254 | 45-50 | 14.9-17.1 | 33.6-39.3 |
| Transgenic line A, n = 38 | ||||||||
| Mean | 11.8 | 12.9 | 18.1 | 50.9 | 2708 | 43.1 | 14.4 | 34.1 |
| SD | 2.85 | 1.05 | 1.40 | 4.42 | 712 | 2.09 | 0.59 | 1.2 |
| Range | 6.2-17.7 | 9.50-16.7 | 15-21.9 | 42.7-65.4 | 1226-5621 | 38-48 | 13.5-15.9 | 32.2-37.5 |
| P value | < .05 | < .05 | < .05 | < .05 | < .05 | < .05 | < .05 | > .05 |
| Transgenic line B, n = 34 | ||||||||
| Mean | 9.14 | 11.7 | 16.5 | 48.0 | 1278 | 45.1 | 15.1 | 34.3 |
| SD | 2.63 | 0.64 | 0.59 | 2.87 | 215 | 1.58 | 0.59 | 1.2 |
| Range | 4.9-19.0 | 9.60-12.5 | 14.7-17.8 | 42.0-54.1 | 904-2021 | 42-48 | 13.5-16.2 | 32-37 |
| P value | > .05 | < .05 | < .05 | < .05 | < .05 | < .05 | < .05 | > .05 |
| . | WBC, ×109/L . | RBC, ×1012/L . | HGB, g/L . | HCT, % . | PLT, ×109/L . | MCV, fm3 . | MCH, pg . | MCHC, g/L . |
|---|---|---|---|---|---|---|---|---|
| Nontransgenic control, n = 30 | ||||||||
| Mean | 8.29 | 9.61 | 15.2 | 45.4 | 968 | 46.9 | 15.6 | 34.8 |
| SD | 2.54 | 0.55 | 0.76 | 1.78 | 89 | 1.4 | 0.60 | 1.45 |
| Range | 5.0-10.9 | 8.2-10.9 | 14-17.2 | 40.2-49.8 | 765-1254 | 45-50 | 14.9-17.1 | 33.6-39.3 |
| Transgenic line A, n = 38 | ||||||||
| Mean | 11.8 | 12.9 | 18.1 | 50.9 | 2708 | 43.1 | 14.4 | 34.1 |
| SD | 2.85 | 1.05 | 1.40 | 4.42 | 712 | 2.09 | 0.59 | 1.2 |
| Range | 6.2-17.7 | 9.50-16.7 | 15-21.9 | 42.7-65.4 | 1226-5621 | 38-48 | 13.5-15.9 | 32.2-37.5 |
| P value | < .05 | < .05 | < .05 | < .05 | < .05 | < .05 | < .05 | > .05 |
| Transgenic line B, n = 34 | ||||||||
| Mean | 9.14 | 11.7 | 16.5 | 48.0 | 1278 | 45.1 | 15.1 | 34.3 |
| SD | 2.63 | 0.64 | 0.59 | 2.87 | 215 | 1.58 | 0.59 | 1.2 |
| Range | 4.9-19.0 | 9.60-12.5 | 14.7-17.8 | 42.0-54.1 | 904-2021 | 42-48 | 13.5-16.2 | 32-37 |
| P value | > .05 | < .05 | < .05 | < .05 | < .05 | < .05 | < .05 | > .05 |
WBC indicates white blood cells; RBC, red blood cells; HGB, hemoglobin; HCT, hematocrit; PLT, platelet; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; and MCHC, mean corpuscular hemoglobin concentration.
Complete blood count analyses were performed with 10- to 12-week-old transgenic mice (F2 to F6 generations, about equal numbers of male and female) and their nontransgenic siblings. P values were calculated in reference to the nontransgenic control.
Analyses of hematopoietic cells of JAK2V617F transgenic mice. (A) Blood cell smears stained with the Wright and Giemsa reagent. Arrows point to platelets. The big arrow indicates a giant platelet. (B) Blood cell counts of a representative nontransgenic control (—) and 3 line A transgenic ( and …) mice at different ages. (C,D) Flow cytometric analyses of peripheral blood, bone marrow, and spleen cells from nontransgenic and line A transgenic mice. Cells were stained with phycoerythrin-conjugated anti-B220 and fluorescein isothiocyanate-conjugated anti-Gr-1, anti-Ter119, anti-CD71, or anti-CD41. Percentages of cells in each quadrant are indicated.
and …) mice at different ages. (C,D) Flow cytometric analyses of peripheral blood, bone marrow, and spleen cells from nontransgenic and line A transgenic mice. Cells were stained with phycoerythrin-conjugated anti-B220 and fluorescein isothiocyanate-conjugated anti-Gr-1, anti-Ter119, anti-CD71, or anti-CD41. Percentages of cells in each quadrant are indicated.
Analyses of hematopoietic cells of JAK2V617F transgenic mice. (A) Blood cell smears stained with the Wright and Giemsa reagent. Arrows point to platelets. The big arrow indicates a giant platelet. (B) Blood cell counts of a representative nontransgenic control (—) and 3 line A transgenic ( and …) mice at different ages. (C,D) Flow cytometric analyses of peripheral blood, bone marrow, and spleen cells from nontransgenic and line A transgenic mice. Cells were stained with phycoerythrin-conjugated anti-B220 and fluorescein isothiocyanate-conjugated anti-Gr-1, anti-Ter119, anti-CD71, or anti-CD41. Percentages of cells in each quadrant are indicated.
and …) mice at different ages. (C,D) Flow cytometric analyses of peripheral blood, bone marrow, and spleen cells from nontransgenic and line A transgenic mice. Cells were stained with phycoerythrin-conjugated anti-B220 and fluorescein isothiocyanate-conjugated anti-Gr-1, anti-Ter119, anti-CD71, or anti-CD41. Percentages of cells in each quadrant are indicated.
Flow cytometric analyses were conducted with single cell suspension of peripheral blood, bone marrow, and spleen cells from nontransgenic and line A transgenic mice. Forward and side scattering data revealed a profound increase in the proportion of nonlymphocytic cells in these 3 tissues (not shown). Staining of cells with anti-B220, a B-cell marker, and anti–Gr-1, a myeloid cell marker, verified the elevation in granulocytes in all 3 hematopoietic tissues (Figure 3C). Further staining of spleen cells with anti-Ter-119 and anti-CD71 for erythroid cells and anti-CD41 for megakaryocytic cells demonstrated erythroid and megakaryocytic hyperplasia in the spleen and bone marrow (Figure 3D).
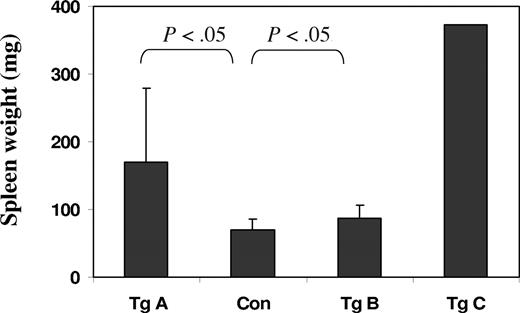
Splenomegaly is also a clear phenotype of the JAK2V617F transgenic mice (Figure 4). Overall, the line A mice have strikingly enlarged spleens, weighing 2 to 8 times more than those of control mice. Line B mice also exhibited enlarged spleens but to a much lesser degree, which is consistent with their relatively moderate elevations in blood cell counts. The line C founder mouse, which died at the age of 4 weeks, had a spleen 10 times the size of that of control mice of comparable ages.
JAK2V617F transgenic mice have enlarged spleens. Graphic representation of spleen weights of control and transgenic mice (11-12 weeks old except for Tg C, which was 4 weeks old). Error bars denote SD (n = 7, except for the Tg C bar, which represents only one sample). P values obtained from t tests are indicated. The weights of spleens range from 53 to 96 mg for control mice, 90 to 410 for transgenic line A (Tg A), and 68 to 117 for transgenic line B.
JAK2V617F transgenic mice have enlarged spleens. Graphic representation of spleen weights of control and transgenic mice (11-12 weeks old except for Tg C, which was 4 weeks old). Error bars denote SD (n = 7, except for the Tg C bar, which represents only one sample). P values obtained from t tests are indicated. The weights of spleens range from 53 to 96 mg for control mice, 90 to 410 for transgenic line A (Tg A), and 68 to 117 for transgenic line B.
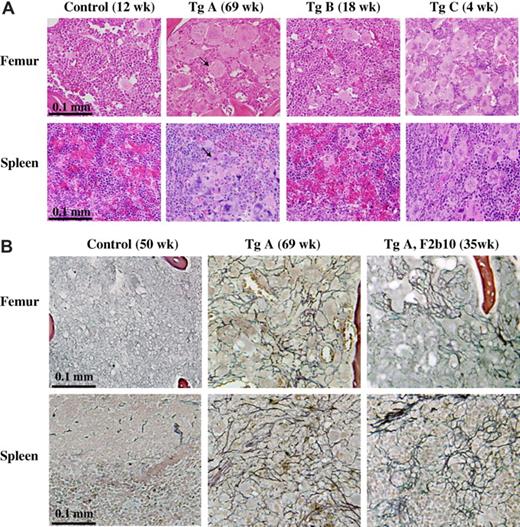
Histologic staining of bone marrow and spleen is shown in Figure 5A. All the transgenic mice displayed marrow megakaryocytic hyperplasia, most remarkably for line A and C mice in which the marrow displayed a remarkably increased number of megakaryocytes. The spleens of the transgenic mice were also infiltrated with megakaryocytes, which were not found with the control mice. We also performed reticulin staining to detect development of myelofibrosis in the transgenic mice (Figure 5B). Among 26 line A mice analyzed, 10 developed various degrees of fibrosis in the bone marrow and spleen. These 10 mice were all over the age of 30 weeks and showed more severe PV-like or ET-like phenotypes than those without fibrosis. Interestingly, fibrosis was not found in 10 of the line A mice, which were younger than 30 weeks, suggesting a strong correlation between fibrosis and aging. Figure 5B illustrates results obtained with the 69-week-old line A founder mouse, which had elevated platelet counts only, and a 35-week-old F2 generation line A mouse, which displayed elevations in red cell, white cell, and platelet counts. The data demonstrated clear fibrosis in both bone marrow and spleen, which was not present in the control mice. So far, we have not discovered the occurrence of fibrosis in more than a dozen of line B mice except for the founder mouse, which died at the age of 93 weeks with an enlarged spleen (data not shown).
JAK2V617F transgenic mice display megakaryocytic hyperplasia and develop fibrosis. Hematoxylin and eosin (A) and reticulin (B) staining of paraffin-embedded sections of spleen and femur (for bone marrow) from control and transgenic mice. Photos in panel A are representative results obtained with all 3 lines of transgenic mice, whereas those in panel B represent selected cases of line A mice that developed fibrosis. Arrows point to typical megakaryocytes, which are not present in the spleen of control mice. Pictures were taken with a 40× objective.
JAK2V617F transgenic mice display megakaryocytic hyperplasia and develop fibrosis. Hematoxylin and eosin (A) and reticulin (B) staining of paraffin-embedded sections of spleen and femur (for bone marrow) from control and transgenic mice. Photos in panel A are representative results obtained with all 3 lines of transgenic mice, whereas those in panel B represent selected cases of line A mice that developed fibrosis. Arrows point to typical megakaryocytes, which are not present in the spleen of control mice. Pictures were taken with a 40× objective.
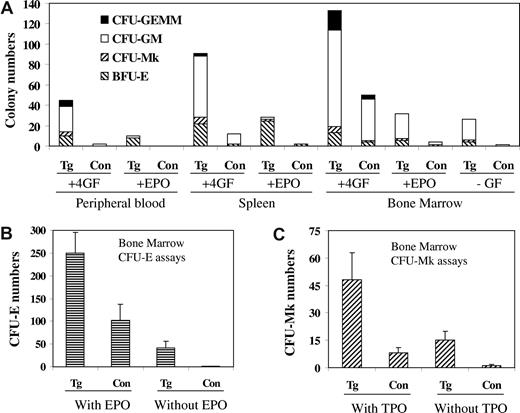
We further performed hematopoietic colony assays to compare the number and the property of progenitor cells in the peripheral blood, spleen, and bone marrow from control and line A transgenic mice. The data are shown in Figure 6. When cultured with optimal concentrations of growth factor and cytokines, peripheral blood from line A transgenic mice produced a significant number of colonies, which were essentially not found with the control mice. This indicates an occurrence of stem-progenitor mobilization in the transgenic mice, a feature also found in MPD patients. The bone marrow and spleen from the transgenic mice also gave rise to much increased colonies, indicating the presence of a larger percentage of progenitor cells in these tissues. For the bone marrow, however, the total number of progenitor cells in the transgenic mice may be reduced because the total nucleated cells obtained from their femurs were usually less than 50% of those from control wild-type mice. We have also cultured bone marrow cells in the absence of any added growth factors and cytokines, and the data shown in Figure 6A demonstrate that cells from line A transgenic mice produced a significant number of granulocyte-macrophage colony-forming cell colonies, whereas control cells hardly formed any colony. A hallmark of MPDs is autonomous growth of hematopoietic progenitor cells in vitro in the absence of growth factors. This was found to be true for the JAK2V617F transgenic mice. Figure 6B demonstrates autonomous formation of erythrocyte colony-forming unit colonies with cells from bone marrow of line A transgenic mice. Analyses with the MegaCult-C collagen-based media also demonstrated a much increased number of megakaryocytic progenitor cells in the bone marrow of line A transgenic mice and the ability of the cells to form colonies in the absence of Tpo (Figure 6C). Figure 6 shows data obtained with line A mice only. With line B mice, the trend is the same, although the changes were less remarkable (data not shown).
JAK2V617F transgenic mice have markedly increased progenitor cells numbers and form autonomous hematopoietic colonies. Progenitor cell assays were carried out in MethoCult methylcellulose media in the presence or absence of Epo alone or a 4-growth factor/cytokine cocktail (mSCF, mIL-3, hIL-6, hEpo; A,B) or in MegaCult-C collagen-based media supplemented 10 ng/mL rm IL-3 and 20 ng/mL rh IL-6 with or without 50 ng/mL rh Tpo (C). Nucleated cells from peripheral blood (105), spleen (105), and bone marrow (2 × 104 for A, 105 for B and C) of control and transgenic line A mice were seeded, and colonies were scored after culture for 10 (A), 2 (B), and 7 (C) days. Data represent at least 3 independent experiments.
JAK2V617F transgenic mice have markedly increased progenitor cells numbers and form autonomous hematopoietic colonies. Progenitor cell assays were carried out in MethoCult methylcellulose media in the presence or absence of Epo alone or a 4-growth factor/cytokine cocktail (mSCF, mIL-3, hIL-6, hEpo; A,B) or in MegaCult-C collagen-based media supplemented 10 ng/mL rm IL-3 and 20 ng/mL rh IL-6 with or without 50 ng/mL rh Tpo (C). Nucleated cells from peripheral blood (105), spleen (105), and bone marrow (2 × 104 for A, 105 for B and C) of control and transgenic line A mice were seeded, and colonies were scored after culture for 10 (A), 2 (B), and 7 (C) days. Data represent at least 3 independent experiments.
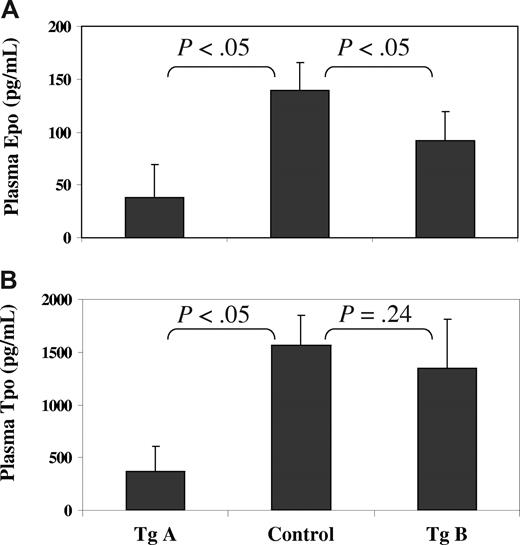
Finally, a major feature of PV is a reduced level of Epo in the blood serum.1-3 This was also found in our JAK2V617F transgenic mice, especially with the line A mice (Figure 7A). Indeed, the average level of Epo in line A mice was less than one fourth that found in control mice. Epo levels found in individual line A mice ranged from 9 to 80 pg/mL compared with 115 to 180 pg/mL found with control mice. To a lesser extent, line B mice also showed a significant reduction in their plasma Epo. We further analyzed the plasma level of Tpo (Figure 7B). Whereas the line A mice displayed a profound decrease in the Tpo level, the line B mice did not show a significant change. In all, the reduced levels of Epo and Tpo are inversely correlated with the increased red blood cell and platelet counts in transgenic mice. This presumably reflects a feedback inhibition on the syntheses of Epo and Tpo by the increased red cells and platelets in the peripheral blood of transgenic mice.
JAK2V617F transgenic mice have much reduced levels of Epo and Tpo in peripheral blood. Plasma samples were obtained from 18- to 20-week-old control and transgenic mice. The levels of Epo (A) and Tpo (B) in the plasma (with EDTA) were determined using the mouse Epo and Tpo Quantikine ELISA kits from R&D Systems. Error bars denote standard derivation with n = 5 (P < .05, significant difference).
JAK2V617F transgenic mice have much reduced levels of Epo and Tpo in peripheral blood. Plasma samples were obtained from 18- to 20-week-old control and transgenic mice. The levels of Epo (A) and Tpo (B) in the plasma (with EDTA) were determined using the mouse Epo and Tpo Quantikine ELISA kits from R&D Systems. Error bars denote standard derivation with n = 5 (P < .05, significant difference).
Discussion
By expressing JAK2V617F in the hematopoietic system of mice using a tissue specific promoter, we have generated a stable transgenic mouse model of ET, PV, and PMF. Our data provide unequivocal evidence that JAK2V617F is able to cause MPDs. We have generated 3 lines of transgenic mice. All show characteristics of MPDs despite different degrees of severity. The line B transgenic mice, which express a lower level of JAK2V617F, caused significantly elevated blood counts of red cells and platelets but not to a degree to be defined as PV- or ET-like phenotype. The line A transgenic mice, which had a higher level of JAK2V617F expression, displayed a much higher level of blood counts and developed a clear ET or PV-like phenotype. This occurred to males and females of all ages, although in many cases higher blood counts were seen as the animals aged. Interestingly, many of the line A mice also developed reticulin fibrosis in the bone marrow and spleen when they got older (beyond 30 weeks), but we have not seen such cases with line B mice except for the very old founder mouse. With real-time PCR analyses, we were able to determine gene copy numbers and the expression levels of the JAK2V617F transgene in the transgenic mice. The data demonstrate that line A mice had a much higher number of gene copies and expressed a higher level of JAK2V617F mRNA than line B mice, providing a good correlation between gene expression and disease phenotype. The line C mouse, which had the highest JAK2V617F gene copy numbers, also displayed the worst splenomegaly. We think that a more severe MPD phenotype might have contributed to the early death of this mouse at the age of 4 weeks. Previous studies with bone marrow transplant models expressed JAK2V617F by retrovirus. This yielded a severe PV-like phenotype accompanied by myelofibrosis.11-14 A relatively high expression of JAK2V617F may be the cause, although many other factors may also be involved. Recent studies have suggested a correlation between the JAK2V617F mutational load and disease phenotypes. For example, homozygous JAK2V617F mutation occurs frequently in PV patients but rarely in ET,27,28 and an increasing JAK2V617F allele burden was observed from ET, over PV to PMF.29 One may postulate that a high level of JAK2V617F expression causes PV, but a low level is sufficient for ET. Comparison of our line A and B transgenic mice indeed suggests that the expression levels of JAK2V617F determine phenotypes. A sufficiently high level of JAK2V617F expression is required to cause ET-, PV-, and PMF-like phenotypes in mice. However, with our current mice, we are not able to address exactly how much JAK2V617F is enough to induce a particular MPD phenotype. Interestingly, within the line A mice, some had clear ET-like symptoms, whereas others displayed PV-like phenotypes. Position-effect variegation caused by repeat-induced gene silencing24 and normal variations among mice resulting from epigenetic factors may contribute to such a phenomenon. In addition, our transgenic mice started with a C57BL/6XDBA hybrid background and were further crossed with wild-type C57BL/6. Our data were collected from mice of mixed genetic backgrounds, which may partly explain the variations in blood counts and phenotype observed within each line of transgenic mice. Indeed, earlier studies with bone marrow transplant models have revealed strain-specific differences in phenotype, with Balb/c mice showing a more severely diseased phenotype, including myelofibrosis, than with C57Bl/6 mice.12 We are in the process of breeding our transgenic mice to obtain different mouse strain backgrounds.
The vav promoter used in our study has been shown to drive expression of transgenes in virtually all nucleated cells of hematopoietic tissues but not in nonhematopoietic cell types.23 Our current study demonstrates expression of JAK2V617F in bone marrow, spleen, peripheral blood, and thymus, but not in many nonhematopoietic tissues. This further proves the specificity of the promoter. The phenotype observed with our transgenic mice involves the myelocytic system. Whether or not JAK2V617F affects development and activation of lymphocytes of transgenic mice is to be investigated. Based on our Western blotting analyses, the vav promoter is rather moderate. It failed to produce robust expressions of JAK2V617F but was sufficient to cause phenotypes and thus represented a good choice for the expression of transgenes in the hematopoietic system.
Earlier studies have been focused on the bone marrow transplant approach.11-14 For this purpose, mice were lethally irradiated and reconstituted with bone marrow cells infected with JAK2V617F-expressing retroviruses. These mice exhibited severe PV-like hematologic disorders accompanied by myelofibrosis. These studies have provided strong evidence that JAK2V617F indeed possesses an oncogenic potential to transform hematopoietic precursor cells. We have now generated JAK2V617F transgenic mice with MPD phenotypes, thereby further supporting such a notion. The transgenic model we developed provides an excellent tool for further studies. First, the transgenic mice transmit the transgene stably to the progeny; thus, they can be crossed with other transgenic and knockout mice for investigating interaction and crosstalk among different proteins. Second, it provides unlimited resources for primary hematopoietic stem and progenitor cells to study the functions of JAK2V617F in vitro and in vivo after transplant into recipient mice. Finally, our transgenic mice develop ET, PV, and myelofibrosis. This model will be valuable not only for investigating the pathologic mechanism underlying MPDs, but also for finding a therapeutic drug and procedure to treat the diseases.
While this manuscript was in revision, 2 other groups reported their JAK2V617F transgenic mice with phenotypes similar to what we found.30,31
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the the National Institutes of Health (grants HL076309 and HL079441) and the Oklahoma Center for the Advancement of Science & Technology (Z.J.Z.).
National Institutes of Health
Authorship
Contribution: S.X., W.T.H., J.M., S.W., and W.Z. performed the research; X.X., Q.L., X.F., and M.X. provided assistance in designing the study and analyzing data; Z.J.Z. designed the study, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zhizhuang Joe Zhao, Department of Pathology, University of Oklahoma Health Sciences Center, 940 Stanton L. Young Blvd, BMSB 451, Oklahoma City, OK 73104; e-mail: joe-zhao@ouhsc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal