Abstract
Decoy receptor 3 (DcR3) is a member of the TNF receptor superfamily and is up-regulated in tumors originating from a diversity of lineages. DcR3 is capable of promoting angiogenesis, inducing dendritic cell apoptosis, and modulating macrophage differentiation. Since tumor-associated macrophages (TAMs) are the major infiltrating leukocytes in most malignant tumors, we used microarray technology to investigate whether DcR3 contributes to the development of TAMs. Among the DcR3-modulated genes expressed by TAMs, those that encode proteins involved in MHC class II (MHC-II)–dependent antigen presentation were down-regulated substantially, together with the master regulator of MHC-II expression (the class II transactivator, CIITA). The ERK- and JNK-induced deacetylation of histones associated with the CIITA promoters was responsible for DcR3-mediated down-regulation of MHC-II expression. Furthermore, the expression level of DcR3 in cancer cells correlated inversely with HLA-DR levels on TAMs and with the overall survival time of pancreatic cancer patients. The role of DcR3 in the development of TAMs was further confirmed using transgenic mice overexpressing DcR3. This elucidates the molecular mechanism of impaired MHC-II–mediated antigen presentation by TAMs, and raises the possibility that subversion of TAM-induced immunosuppression via inhibition of DcR3 expression might represent a target for the design of new therapeutics.
Introduction
Cancer cells escape from the attack of host immune system by a progressively suppressed process called immune editing, which provides a selective pressure in the tumor microenvironment that ultimately leads to tumor progression and metastasis.1 A variety of tumor-derived factors contribute to the immunosuppressive networks, including vascular endothelial growth factor, interleukin-10, transforming growth factor-β, prostaglandin E2, and several soluble members of tumor necrosis factor (TNF) and TNF receptor superfamily.2 Established solid tumors not only consist of malignant cells, but also contain stroma cells, immune cells, and extracellular matrix. Macrophages within the tumors, referred to as tumor-associated macrophages (TAMs), are the pivotal member of infiltrating leukocytes.3,4
By analogy to the Th1/Th2 dichotomy described for T-cell responses, macrophages activated by exposure to IFN-γ and IL-4 have been referred to as type I and type II macrophages (M1 and M2), respectively.5 The M1 phenotype represents potent effector cells that are capable of killing microorganisms and tumor cells and of producing proinflammatory cytokines. In contrast, M2 macrophages suppress inflammatory responses, promote angiogenesis, and enhance tumor invasion.6 The available information suggests that macrophages that infiltrate tumors become polarized to the M2 phenotype.7 Thus, by acquiring the properties of M2 cells, TAMs participate in processes that regulate tumor growth and progression, in addition to modulating adaptive immunity and angiogenesis.8,9
TAMs are derived from circulating monocyte precursors and preferentially localize at the stroma-tumor interface.8,10 The protumoral role of TAMs is further supported by clinical and experimental studies that link a high frequency of infiltrating macrophages with poor patient prognosis.11,12 Moreover, TAMs are characteristically of poor antigen-presenting capacity and are capable of inducing T-cell tolerance to help tumors evade attack from immune system.7,13 One of the major features of TAMs is the down-regulation of MHC class II (MHC-II) molecules, but the underlying mechanism has not been elucidated yet.13,14
MHC-II molecules play a pivotal role in the induction and regulation of adaptive immune responses. Cell type–specific and IFN-γ–induced MHC-II expression is predominantly regulated at the transcriptional level. The promoters of MHC-II genes characteristically contain the conserved regulatory SXY motif, which is a docking site for the MHC-II enhanceosome.15,16 However, the enhanceosome components are expressed ubiquitously and fail to dictate the complex expression pattern displayed by MHC-II genes. Therefore, the precise control of MHC-II gene expression is achieved by a very peculiarly and tightly regulated coactivator called class II transactivator (CIITA).17,18 CIITA expression is regulated mainly at the level of transcription, which is driven by the alternative usage of 3 distinct promoters (pI, pIII, and pIV) that are activated differentially in various cell types and in response to specific stimuli.19,20
Decoy receptor 3 (DcR3), a member of the TNF receptor superfamily, is up-regulated in tumors that originate from a diversity of lineages,21-24 and a high-level expression of DcR3 is associated with poor prognoses.25 We have reported previously that DcR3 is capable of promoting angiogenesis,26 inducing dendritic cell apoptosis,27 and modulating macrophages differentiation.28 In addition, DcR3 up-regulated mannose receptor (M2 marker) and down-regulated interleukin-1β, TNF-α, and iNOS (M1 marker) in macrophages exposed to LPS and IFN-γ.28 Moreover, T-cell activation is biased to Th2 phenotype,29 and Th1-mediated insulitis is suppressed in transgenic mice overexpressing DcR3.30 We therefore asked whether DcR3 is a critical factor in the development of TAMs. To address this question, we first used Affymetrix oligonucleotide chips (Santa Clara, CA) to investigate whether the gene expression profile of DcR3-treated macrophages correlates with that observed for TAMs. In this study, we show that DcR3 not only up-regulates genes characteristic of TAMs, but also represses the expression of MHC-II on macrophages via histone deacetylation of the CIITA promoters. Moreover, HLA-DR expression is down-regulated in TAMs in biopsies from tumors with high levels of DcR3. The role of DcR3 in down-regulating MHC-II is further confirmed by examining MHC-II expression level of TAMs in DcR3-transgenic mice. Our studies indicate that DcR3 is able to modulate genes characteristic of TAMs and M2, and inhibition of DcR3 expression might be able to restore the ability of the host immunity to inhibit tumor growth.
Methods
Cell lines, reagents, and recombinant fusion protein
SW480 colorectal adenocarcinoma cells (ATCC, Manassas, VA) were cultured in Leibovitz L-15 medium supplemented with 10% FCS. Human IgG1, anti-Flag M2 affinity gel, trichostatin A, and 5-azacytidine were purchased from Sigma-Aldrich (St Louis, MO). Human macrophage colony-stimulating factor (M-CSF) and DcR3 enzyme-linked immunosorbent assay (ELISA) detection kit were from R&D Systems (Minneapolis, MN). Kinase inhibitors (SP600125, PD98059, and SB203580) were purchased from Calbiochem (San Diego, CA). Anti–phospho-ERK (T202/Y204), anti–phospho-JNK (T183/Y185), and anti–phospho-p38 (T180/Y182) Abs were purchased from Cell Signaling Technology (Beverly, MA). Antibodies recognizing ERK2 (C-14), JNK1 (C-17), and p38α (C-20) were from Santa Cruz (Santa Cruz, CA). Antibodies used for immunohistochemical staining include antibodies recognizing DcR3 (3H5; BioLegend, San Diego, CA), CD68 (Dako, Glostrup, Denmark), and HLA-DR (Biogenex, San Ramon, CA). DcR3 recombinant fusion proteins were produced using the FreeStyle 293 expression system (Invitrogen, Carlsbad, CA) and purified as previously described.31
Flow cytometry and macrophage preparation
Flow cytometric analysis was performed using a fluorescence-activated cell sorting (FACS) Calibur and data were analyzed using CellQuest (BD Biosciences, Mountain View, CA). The sources of antibodies are as follows: allophycocyanin- or PE-conjugated mAb against HLA-DR (L243) was purchased from BioLegend; FITC-conjugated mAb against HLA-ABC (W6/32) and PE-conjugated mAb against HLA-DR, -DP, and -DQ (WR18) was purchased from Serotec (Oxford, United Kingdom). Human CD14+ monocytes were purified from peripheral blood mononuclear cells (PBMCs) and monocyte-derived macrophages were cultured as described previously.28
Transfection and PKH67 labeling
The DcR3 siRNA duplex (sense: 5′-UCGACUUUGUGGCUUUCCATT-3′, antisense: 5′-UGGAAAGCCACAAAGUCGATT-3′) and a control siRNA duplex were purchased from Proligo (Sigma-Aldrich). SW480 cells (2.5 × 105) were seeded into 6-well plates and transfected with DcR3 siRNA duplex using Lipofectamine 2000 (Invitrogen). Monocytes (107 in 1 mL) were incubated with 2 μM PKH67 for 4 minutes at room temperature, followed by addition of FCS to stop the reaction.
RNA isolation, array processing, and data mining
Monocytes were treated with hIgG1 or DcR3 for 48 hours in the presence of M-CSF. Total RNA was extracted using Trizol (Invitrogen Life Technologies, Midland, TX) according to the vendor's instructions, followed by purification using RNeasy Mini columns (Qiagen, Valencia, CA). Human U133 Plus 2.0 GeneChip oligonucleotide arrays (Affymetrix) were used in this study. Complementary DNA and cRNA synthesis, labeling, hybridization, washing, staining, and scanning were conducted at NRPGM Microarray and Gene Expression Analysis Core Facility. Background correlation, normalization, and log2 transformation of the array data were carried out using the Robust Multi-Array Average method with their default settings within the BioConductor suite for R software package (http://www.bioconductor.org). Probe sets were identified as having statistically significant differences if they satisfied a false discovery rate threshold of q < 0.05 after a Limma t-statistic test according to a pipeline described previously.32 Functional annotation and classification of genes of interest, based on gene ontology, were identified using the Ingenuity Pathways Analysis software (Ingenuity Systems, Redwood City, CA). A heat map of differential gene expression was created by exporting the selected RMA data to the dChip microarray analysis package. Microarray data are available at http://www.ncbi.nlm.nih.gov/geo/33 as GSE10856.
Immunohistochemical analysis
Immunohistochemical analysis was carried out using the Ventana Benchmark automated staining system (Ventana Medical Systems, Tucson, AZ). Immunostaining of DcR3 was performed using the ultra- View Universal DAB detection kit (Ventana Medical Systems) and assessed in a blinded manner. The extent of DcR3 expression was determined by immunostaining, using a semiquantitative scoring method, as follows: samples without positive cells were scored as 0, those with less than 10% of cells staining positive were scored as 1+, those with between 10% and 50% of positive cells were scored as 2+, and those with more than 50% positive cells were scored as 3+. CD68 and HLA-DR double staining was performed using the Ventana iVIEW DAB detection kit and the Enhanced V-Red detection kit (Ventana Medical Systems). The intensity of HLA-DR expression on TAMs was scored as “high” when this was equal to or higher than that seen for small lymphocytes (the circled cells in Figure 3C) and scored as “low” when expression was lower than that seen for small lymphocytes.
Immunofluorescent staining
The tumor mass was embedded in Tissue-Tek OCT compound (Sakura Finetek, Torrance, CA), and snap frozen in liquid nitrogen. Cryostat sections (5-μm thickness) were prepared and placed on Silane-coated glass slides (Dako). After fixation and blocking, sections were incubated with Alexa Fluor 488–conjugated mouse antimouse I-Ab mAb (BioLegend) and Alexa Fluor 647–conjugated rat antimouse CD11b mAb (BioLegend) for 3 hours at room temperature, followed by washing with PBS 3 times. Specimens were analyzed using a confocal microscope (LSM510META; Carl Zeiss, Heidelberg, Germany) with a narrow bandpass filter. Confocal images were obtained using a 63× oil-immersion lens with a resolution of 1024 × 1024 pixels.
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) assays were performed according to the manufacturer's instructions (Upstate Biotechnology, Bar Harbor, ME). In brief, cells were treated with 1% (vol/vol) formaldehyde, followed by sonication. The sheared DNA was immunoprecipitated with anti-AcH3 Abs (Upstate Biotechnology), and the recovered protein-DNA complexes were dissociated using 0.4 M NaCl and the purified DNA fragments were amplified by polymerase chain reaction (PCR; using the primers listed in Table S2, available on the Blood website; see the Supplemental Materials link at the top of the online article) and were fractionated on 2% (wt/vol) agarose gels. Immunoprecipitation with normal rabbit serum served as a negative control, and amplification of the proximal promoter of the human GAPDH gene was used as a positive control.
RT-PCR
Total RNA was extracted from macrophages using Trizol according to the supplier's instructions, and a 2-μg aliquot was reverse-transcribed using RevertAid First Strand cDNA Synthesis Kit (Fermentas, Burlington, ON) was used as template for PCR amplification. Ethidium bromide–stained PCR products were separated on 2% (wt/vol) agarose gels. The primers used for each gene validated are shown in Table S2.
Quantitative real-time PCR
Real-time reverse-transcription (RT)–PCR was performed on the LightCycler thermal cycler system (Roche Diagnostics, Mannheim, Germany) using TaqMan probes from the Universal Probe System (Roche Diagnostics) as described by the manufacturer. Briefly, 50 ng cDNA was placed into a 20-μL reaction volume containing 0.2 μM of each primer, 0.1 μM Universal ProbeLibrary probes, and 1× TaqMan Master. cDNA was amplified by denaturing for 10 minutes at 95°C, followed by 45 cycles of denaturation at 95°C for 10 seconds and annealing-extension at 60°C for 30 seconds. Data were processed using the LightCycler Relative Quantification Software using the 2−ΔΔCT methods with GAPDH as the reference gene. All real-time results were expressed as fold changes in mRNA expression with respect to the control cells. For each replicate in each experiment, RNA from macrophages of different donors was used. The primers and probes used for each gene validated are shown in Table S2.
Tumor samples
Between October 1997 and 2004, tissue specimens and clinicopathologic data were collected from 68 consecutive patients with newly diagnosed ductal adenocarcinoma of pancreas. All the patients were treated with Whipple operation at Chang Gung Memorial Hospital, Lin-Kou following the institutional guideline. None of the patients had distant metastasis at initial presentation, and all the samples were collected from the primary lesion when all the 68 patients underwent curative surgery as the primary treatment. None of the specimens were from the recurrent tumor samples. Age, sex, tumor differentiation, and pathological stage are shown in Table 1. Tumor staging was defined according to the “6th Edition Pancreatic Cancer Staging System”34 of the American Joint Committee on Cancer. Approval for the study was obtained from the Chang Gung hospital institutional review board. Informed consent was obtained in accordance with the Declaration of Helsinki.
Tumor transplantation in mice
The promoter used for the generation of DcR3-transgenic mice was as described previously,35,36 and mice were maintained on a C57BL/6 background. CT26 cells (a colon adenocarcinoma cell line derived from BALB/c mice) were inoculated subcutaneously (105 cells) into the dorsal tissue of the F1 generation crossed between BALB/c and DcR3-transgenic mice (6-8 weeks old). Tumor growth was measured 2 to 3 times per week using calipers with a Vernier scale. Tumor size was calculated based on 3 perpendicular measurements. Tumor-bearing mice were killed when the tumor volume reached 3000 mm3. All animal experiments were conducted in specific pathogen-free conditions and in accordance with the guidelines approved by the Animal Care and Usage Committee of National Yang-Ming University.
Statistical evaluation
Data are expressed as the means plus or minus SD of at least 3 experiments. The Student t test from the GraphPad Prism software package (San Diego, CA) was used to assess the statistical significance of differences observed, with P values less than .05 considered statistically significant. DcR3 expression levels, in relation to pathological stage, histologic type, and cancer cell differentiation, were analyzed for significance using the χ2 test. Overall survival periods were calculated by the Kaplan-Meier method, and the difference in survival between subgroups was analyzed with the long-rank test (SPSS software; SPSS, Chicago, IL). Multivariate analysis was performed using the Cox proportional hazards regression model with the SPSS software. P values less than .05 were considered statistically significant.
Results
DcR3-mediated MHC class II suppression
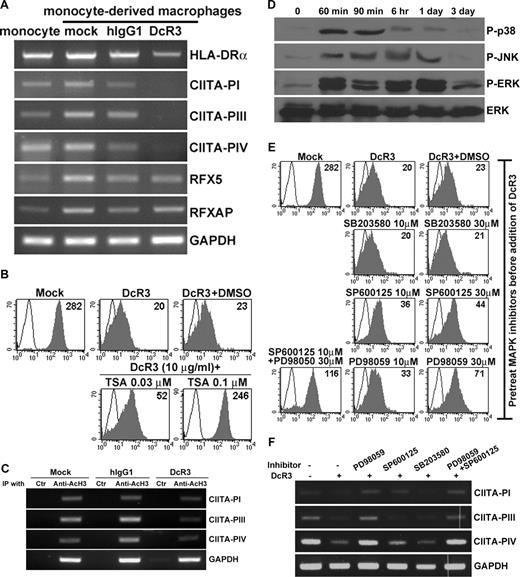
To identify genes modulated by DcR3, human monocytes were treated with recombinant DcR3 fusion protein or human IgG1 (hIgG1) (as control) for 48 hours, and the gene profiling was analyzed using the Affymetrix oligonucleotide chips. The experiment was performed in duplicate and followed by setting a q value of 0.05 to control for multiple testing errors of P values. Based on these criteria, 1103 probe sets of a total of 47 000 probe sets were identified with significant change (Table S1). Among the 1103 targets modulated by DcR3, we found that genes characteristically expressed by TAM/M2 macrophages,9 such as chemokine (C-C motif) ligand 22 (CCL22), matrix metallopeptidase 7 (MMP7), interleukin-1 receptor antagonist (IL1RN), and interleukin-1 receptor, type II (IL1R2), were up-regulated, while genes involved in antimicrobial responses (eg, toll-like receptor 5 [TLR5], TLR7, azurocidin 1 [Azu1]) were down-regulated (Table S1). These observations were further confirmed by real-time RT-PCR (Figure 1A). The most striking feature of the transcriptional profile was the down-regulation of genes that encode proteins with essential roles in MHC-II–mediated antigen presentation, including CIITA, the classical MHC-II molecules (HLA-DR, -DP, and -DQ), and those involved in intracellular trafficking and peptide loading (invariant chain, HLA-DM, and HLA-DO) (Figure 1B). These observations were confirmed by flow cytometry analysis with monoclonal antibodies specific for HLA-DR (Figure 1C top panel) and the pan-MHC-II molecules (Figure 1C bottom panel).
Modulation of M1/M2 markers and down-regulation of MHC-II molecules by DcR3. (A) Validation of microarray analysis. Quantitative real-time reverse transcription–polymerase chain reaction (RT-PCR) was used to determine gene expression. The mean fold change was calculated by comparing the expression level of each gene, relative to that of GAPDH, in hIgG1- or DcR3-treated MDMs. (B) Heat map of genes involved in MHC-II–mediated antigen presentation pathway. Down-regulated genes are shown in blue, while up-regulated genes are shown in red. (C) Expression of MHC-II molecules in MDMs. DcR3-treated or control MDMs were stained with FITC-conjugated anti–HLA-ABC mAb in conjugation with allophycocyanin-conjugated anti–HLA-DR mAb (top panel) or PE-conjugated anti–HLA-DR, -DP, and -DQ mAbs (bottom panel), followed by flow cytometric analysis. (D) Down-regulation of HLA-DR expression in MDMs after cocultivation with SW480 cells. At 24 hours after siRNA transfection, SW480 cells were cocultured with PKH67-labeled monocytes for 5 days, followed by gating PKH67-positive MDMs to determine surface expression of HLA-DR by FACS Calibur system (top panel). Open histograms correspond to isotype control and shaded histograms to specific staining. The concentrations of DcR3 in SW480 culture media were determined by ELISA (bottom panel). (E) Impaired T-cell stimulatory ability of DcR3-treated MDMs. Allogeneic CD4+ T cells were cocultured with γ-irradiated macrophages for 4 days at different ratios, followed by addition of 1 μCi (0.037 MBq)/well [3H]-thymidine for another 16 hours. The incorporation of [3H]-thymidine was measured using a β-counter. P values for the coupled differences, compared with control, were determined using the Student t test: *P < .05; **P < .01 (A,E). Error bars in panels A, D, and E represent SD.
Modulation of M1/M2 markers and down-regulation of MHC-II molecules by DcR3. (A) Validation of microarray analysis. Quantitative real-time reverse transcription–polymerase chain reaction (RT-PCR) was used to determine gene expression. The mean fold change was calculated by comparing the expression level of each gene, relative to that of GAPDH, in hIgG1- or DcR3-treated MDMs. (B) Heat map of genes involved in MHC-II–mediated antigen presentation pathway. Down-regulated genes are shown in blue, while up-regulated genes are shown in red. (C) Expression of MHC-II molecules in MDMs. DcR3-treated or control MDMs were stained with FITC-conjugated anti–HLA-ABC mAb in conjugation with allophycocyanin-conjugated anti–HLA-DR mAb (top panel) or PE-conjugated anti–HLA-DR, -DP, and -DQ mAbs (bottom panel), followed by flow cytometric analysis. (D) Down-regulation of HLA-DR expression in MDMs after cocultivation with SW480 cells. At 24 hours after siRNA transfection, SW480 cells were cocultured with PKH67-labeled monocytes for 5 days, followed by gating PKH67-positive MDMs to determine surface expression of HLA-DR by FACS Calibur system (top panel). Open histograms correspond to isotype control and shaded histograms to specific staining. The concentrations of DcR3 in SW480 culture media were determined by ELISA (bottom panel). (E) Impaired T-cell stimulatory ability of DcR3-treated MDMs. Allogeneic CD4+ T cells were cocultured with γ-irradiated macrophages for 4 days at different ratios, followed by addition of 1 μCi (0.037 MBq)/well [3H]-thymidine for another 16 hours. The incorporation of [3H]-thymidine was measured using a β-counter. P values for the coupled differences, compared with control, were determined using the Student t test: *P < .05; **P < .01 (A,E). Error bars in panels A, D, and E represent SD.
To determine whether endogenous DcR3 secreted by tumor cells has similar effects as recombinant DVR3 on MHC-II down-regulation (Figure 1 B,C), monocytes were cocultured with colorectal adenocarcinoma SW480 cells, which have been reported to express high levels of DcR3, to address this question.23 After 5 days of coincubation, the expression of HLA-DR on DcR3-treated monocyte-derived macrophages (MDMs) was down-regulated (Figure 1D top panel), while knockdown of endogenous DcR3 expression (Figure 1D bottom panel) partially restored HLA-DR expression (Figure 1D top panel). The down-regulation of MHC-II was consistent with the impaired stimulatory effects of DcR3-treated MDMs toward CD4+ T cells in allogeneic mixed lymphocyte reactions (Figure 1E).
Histone deacetylation of CIITA promoters by DcR3 occurs via activation of JNK and ERK
Four key trans-acting factors critical for the transcriptional regulation of MHC-II genes have been identified, namely, regulatory factor X5 (RFX5), RFX-associated protein (RFXAP), RFX-associated ankyrin-containing protein (RFXANK), and the non-DNA binding master regulator CIITA.17 To determine whether the down-regulation of MHC-II expression related to changes in the transcription of these trans-acting factors, we examined the expression of RFX5, RFXAP, and CIITA by RT-PCR analysis. Levels of RFX5 and RFXAP were unaffected, while all 3 isoforms of CIITA (CIITA-PI, -PIII, and -PIV) were suppressed by DcR3 (Figure 2A). Although DNA hypermethylation is one of the chief mechanisms for silencing CIITA expression in various malignant tumor cells,37 DNA methylation does not appear to be a factor in CIITA silencing by DcR3 in TAMs (Figure S1). We therefore investigated the acetylation status of histones on nucleosomes, which is thought to define the chromatin structure and thereby regulate gene transcription.38 The histone deacetylase (HDAC) inhibitor, trichostatin A (TSA), was incubated with MDMs in the presence of DcR3 for 6 days. As shown in Figure 2B, TSA rescued DcR3-mediated HLA-DR suppression in a dose-dependent manner, suggesting that histone deacetylation contributes to DcR3-mediated CIITA silencing. The histone acetylation status was further analyzed by chromatin immunoprecipitation (ChIP) assay, using antibodies specific for the acetylated form of histone H3 (AcH3). AcH3 was found on the CIITA PI, PIII, and PIV promoters in control groups, but was substantially suppressed in DcR3-treated MDMs (Figure 2C).
Histone deacetylation of CIITA promoters by DcR3 occurs via activation of JNK and ERK. (A) Expression of RFX factors and CIITA in MDMs. cDNAs were prepared from MDMs treated with either mock (medium only), hIgG1, or DcR3 for 6 days and the expression of mRNAs corresponding to HLA-DR, RFX5, RFXAP, and the 3 CIITA isoforms was examined by RT-PCR analysis. (B) Effects of trichostatin A (TSA) on the expression of HLA-DR. MDMs were pretreated with TSA for 30 minutes, followed by DcR3 treatment for 6 days. Expression of HLA-DR was examined by flow cytometry with allophycocyanin-conjugated anti–HLA-DR mAb. The open histogram represents isotype control, and the shaded histogram corresponds to specific staining. (C) Effect of DcR3 on the acetylation of H3 associated with CIITA promoters. ChIP was performed with antibodies specific for acetylated histone H3 (AcH3). The immunoprecipitates were analyzed by PCR to determine the presence of the regulatory region of the CIITA genes. The proximal promoter of the GAPDH was amplified as an internal control. (D) Kinetics of MAPK activation after DcR3 treatment. Cell lysates were collected at the indicated time points after DcR3 treatment to examine the activation status of ERK, JNK, and p38 using antibodies specific for the phosphorylated form of each MAPK. (E) Effects of MAPK inhibitors on the expression of HLA-DR. Macrophages were pretreated with SP600125, SB203580, and PD98059 for 30 minutes, followed by DcR3 treatment for 6 days. Expression of HLA-DR in MDMs was determined by flow cytometry with allophycocyanin-conjugated anti–HLA-DR mAb. Open histograms represent isotype control and shaded histograms correspond to specific staining. (F) Effects of MAPK inhibitors on the acetylation of H3 associated with CIITA promoters. MDMs were prepared as in panel E, and chromatin was immunoprecipitated with antibodies specific for AcH3; this was followed by PCR to determine the presence of the CIITA regulatory regions. The proximal promoter of the GAPDH was amplified as an internal control.
Histone deacetylation of CIITA promoters by DcR3 occurs via activation of JNK and ERK. (A) Expression of RFX factors and CIITA in MDMs. cDNAs were prepared from MDMs treated with either mock (medium only), hIgG1, or DcR3 for 6 days and the expression of mRNAs corresponding to HLA-DR, RFX5, RFXAP, and the 3 CIITA isoforms was examined by RT-PCR analysis. (B) Effects of trichostatin A (TSA) on the expression of HLA-DR. MDMs were pretreated with TSA for 30 minutes, followed by DcR3 treatment for 6 days. Expression of HLA-DR was examined by flow cytometry with allophycocyanin-conjugated anti–HLA-DR mAb. The open histogram represents isotype control, and the shaded histogram corresponds to specific staining. (C) Effect of DcR3 on the acetylation of H3 associated with CIITA promoters. ChIP was performed with antibodies specific for acetylated histone H3 (AcH3). The immunoprecipitates were analyzed by PCR to determine the presence of the regulatory region of the CIITA genes. The proximal promoter of the GAPDH was amplified as an internal control. (D) Kinetics of MAPK activation after DcR3 treatment. Cell lysates were collected at the indicated time points after DcR3 treatment to examine the activation status of ERK, JNK, and p38 using antibodies specific for the phosphorylated form of each MAPK. (E) Effects of MAPK inhibitors on the expression of HLA-DR. Macrophages were pretreated with SP600125, SB203580, and PD98059 for 30 minutes, followed by DcR3 treatment for 6 days. Expression of HLA-DR in MDMs was determined by flow cytometry with allophycocyanin-conjugated anti–HLA-DR mAb. Open histograms represent isotype control and shaded histograms correspond to specific staining. (F) Effects of MAPK inhibitors on the acetylation of H3 associated with CIITA promoters. MDMs were prepared as in panel E, and chromatin was immunoprecipitated with antibodies specific for AcH3; this was followed by PCR to determine the presence of the CIITA regulatory regions. The proximal promoter of the GAPDH was amplified as an internal control.
It has been reported that down-regulation of HLA-DR expression by LPS occurs via the activation of ERK and p38 MAPKs, where these induce histone deacetylation of the CIITA locus.39 We therefore investigated whether DcR3 might down-regulate CIITA expression via the activation of MAPKs. In response to DcR3 treatment, ERK was phosphorylated and sustained for 72 hours, while JNK and p38 MAPKs were phosphorylated to much smaller extents and remained active for a shorter time (24 hours; Figure 2D). In contrast to the LPS-mediated suppression of HLA-DR,39 the p38 MAPK inhibitor SB203580 could not restore HLA-DR expression following DcR3 treatment (Figure 2E). However, DcR3-mediated suppression of HLA-DR was rescued by PD98059 (an ERK inhibitor) and SP600125 (a JNK inhibitor). Moreover, the combination of PD98059 and SP600125 synergistically abolished DcR3-mediated HLA-DR suppression (Figure 2E). These data indicate that DcR3 inhibits HLA-DR expression via activation of both ERK and JNK. Quantification of AcH3 on the CIITA promoters revealed that DcR3 inhibits histone acetylation on all CIITA isoforms, while PD98058 and SP600125, but not SB203580, restored histone acetylation in all cases (Figure 2F). Thus, we concluded that DcR3-mediated HLA-DR suppression results from the deacetylation of histones associated with CIITA promoters and that this is mediated via the activation of ERK and JNK.
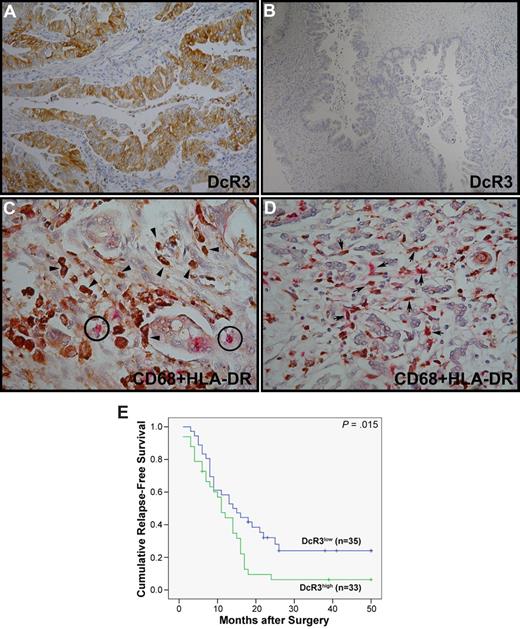
Expression of HLA-DR by TAMs reversely correlates with DcR3 expression on human pancreatic ductal adenocarcinomas
To determine whether DcR3 down-regulates HLA-DR expression in vivo, we scored the expression of HLA-DR by TAMs isolated from pancreatic cancers into 4 categories (3+, 2+, 1+, and 0) as described in “Immunohistochemical analysis.” In pancreatic cancers with high DcR3 expression (Figure 3A; 10 cases of DcR3high with scores of 3+ and 2+), HLA-DR was either down-regulated or undetectable in CD68+ TAMs (Figure 3C). In contrast, TAMs from specimens with low DcR3 expression (Figure 3B; 10 cases of DcR3low with scores of 1+ and 0) showed high HLA-DR expression (Figure 3D; χ2 test, P = .002). Therefore, there is a negative correlation between the expression of HLA-DR on TAMs and the production of DcR3 by pancreatic ductal adenocarcinomas. Although the ages and sexes of the patients and the characteristics of the tumors did not differ significantly between the DcR3high and DcR3low groups (Table 1), the survival rate of DcR3high pancreatic cancer patients was significantly lower than that of the DcR3low group, when followed for up to 50 months, as revealed by Kaplan-Meier analysis (P = .015; Figure 3E). Furthermore, multivariate analysis revealed that DcR3 is an independent risk factor for survival time (Table 2).
Expression of MHC-II molecules in TAMs from pancreatic cancers. DcR3 expression in human pancreatic cancers was visualized by immunohistochemistry (IHC) staining using anti-DcR3 mAb and the ultra-View Universal DAB detection kit (A,B). Double staining of CD68 (brown) and HLA-DR (pink) was performed with the Ventana iVIEW DAB and the Enhanced V-Red detection kit, respectively (C-D). Circled cells correspond to small lymphocytes as an HLA-DR–positive control, arrowheads indicate HLA-DR–negative TAMs (strong brown color) in DcR3high sample (C), and arrows point to HLA-DR–positive TAMs (weak brown color interdispersed with pink color) in DcR3low sample (D) (magnification 10 × 60). (E) Effects of DcR3 expression on the relapse-free survival of pancreatic cancer patients. Kaplan-Meier survival curves are shown for patients from DcR3high (3+ and 2+) and DcR3low (1+ and 0) groups in green and blue, respectively.
Expression of MHC-II molecules in TAMs from pancreatic cancers. DcR3 expression in human pancreatic cancers was visualized by immunohistochemistry (IHC) staining using anti-DcR3 mAb and the ultra-View Universal DAB detection kit (A,B). Double staining of CD68 (brown) and HLA-DR (pink) was performed with the Ventana iVIEW DAB and the Enhanced V-Red detection kit, respectively (C-D). Circled cells correspond to small lymphocytes as an HLA-DR–positive control, arrowheads indicate HLA-DR–negative TAMs (strong brown color) in DcR3high sample (C), and arrows point to HLA-DR–positive TAMs (weak brown color interdispersed with pink color) in DcR3low sample (D) (magnification 10 × 60). (E) Effects of DcR3 expression on the relapse-free survival of pancreatic cancer patients. Kaplan-Meier survival curves are shown for patients from DcR3high (3+ and 2+) and DcR3low (1+ and 0) groups in green and blue, respectively.
Summary of clinicopathologic features of patients and tumors
| . | No. of patients . | Percentage of DcR3-positive tumor cells . | P* . | |
|---|---|---|---|---|
| Less than 10% . | 10% or more . | |||
| Age | .88 | |||
| Younger than 60 y | 18 | 9 | 9 | |
| 60 y or older | 50 | 26 | 24 | |
| Sex | .49 | |||
| Female | 28 | 13 | 15 | |
| Male | 40 | 22 | 18 | |
| Histologic grade | .53 | |||
| Well differentiated | 24 | 12 | 12 | |
| Moderately differentiated | 37 | 18 | 19 | |
| Poorly differentiated | 7 | 5 | 2 | |
| Tumor size | .67 | |||
| 1 | 15 | 7 | 8 | |
| 2 to 4 | 53 | 28 | 25 | |
| Lymph node | .42 | |||
| N0 | 26 | 15 | 11 | |
| N1 | 42 | 20 | 22 | |
| Distant metastasis, M0 | 68 | 35 | 33 | |
| Stage | .28 | |||
| S1 to S2 | 25 | 15 | 10 | |
| S3 to S4 | 43 | 20 | 23 | |
| . | No. of patients . | Percentage of DcR3-positive tumor cells . | P* . | |
|---|---|---|---|---|
| Less than 10% . | 10% or more . | |||
| Age | .88 | |||
| Younger than 60 y | 18 | 9 | 9 | |
| 60 y or older | 50 | 26 | 24 | |
| Sex | .49 | |||
| Female | 28 | 13 | 15 | |
| Male | 40 | 22 | 18 | |
| Histologic grade | .53 | |||
| Well differentiated | 24 | 12 | 12 | |
| Moderately differentiated | 37 | 18 | 19 | |
| Poorly differentiated | 7 | 5 | 2 | |
| Tumor size | .67 | |||
| 1 | 15 | 7 | 8 | |
| 2 to 4 | 53 | 28 | 25 | |
| Lymph node | .42 | |||
| N0 | 26 | 15 | 11 | |
| N1 | 42 | 20 | 22 | |
| Distant metastasis, M0 | 68 | 35 | 33 | |
| Stage | .28 | |||
| S1 to S2 | 25 | 15 | 10 | |
| S3 to S4 | 43 | 20 | 23 | |
Test for chi-square.
Multivariate Cox proportional hazard analysis
| Prognostic factors . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|
| Age, younger than 60 y or 60 y or older | 1.494 | 0.73-3.08 | .28 |
| Sex, male or female | 0.441 | 0.23-0.86 | < .05 |
| Nodal status, negative or positive | 1.005 | 0.51-1.98 | .99 |
| Tumor status, T1 or T2 to T4 | 0.447 | 0.21-0.94 | < .01 |
| DcR3 expression, less than 10% or 10% or more | 2.589 | 1.36-4.94 | < .01 |
| Prognostic factors . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|
| Age, younger than 60 y or 60 y or older | 1.494 | 0.73-3.08 | .28 |
| Sex, male or female | 0.441 | 0.23-0.86 | < .05 |
| Nodal status, negative or positive | 1.005 | 0.51-1.98 | .99 |
| Tumor status, T1 or T2 to T4 | 0.447 | 0.21-0.94 | < .01 |
| DcR3 expression, less than 10% or 10% or more | 2.589 | 1.36-4.94 | < .01 |
CI indicates confidence interval.
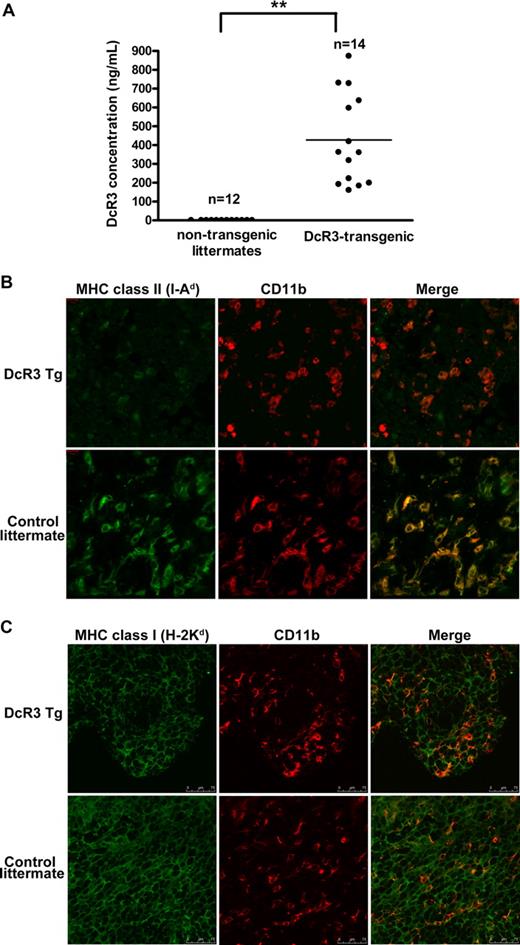
Expression of MHC class II in TAMs from DcR3-transgenic mice
Transgenic mice overexpressing DcR3 were generated to test the hypothesis that DcR3 can mediate MHC-II suppression in vivo. The serum levels of DcR3 in these animals ranged from 150 to 850 ng/mL (Figure 4A), which is comparable with those observed in cancer patients.40 Furthermore, expression of arginase-1 (a key marker expressed by mouse M2 macrophages) was up-regulated in TAMs from DcR3-transgenic mice (Figure S2). Moreover, increased MMP-7 expression and macrophage invasiveness was observed in DcR3-treated MDMs (Figure S3). The level of I-Ad (MHC-II) expression on TAMs (CD11b+ cells) infiltrating implanted CT26 tumors was much lower in DcR3-transgenic mice than in nontransgenic littermates (Figure 4B left panel). Moreover, residually expressed I-Ad in DcR3-transgenic mice was distributed in a dotlike pattern that was clearly different from the even surface distribution seen in TAMs derived from control mice. In contrast, H-2Kd (MHC-I) was expressed at similar levels on TAMs from DcR3-transgenic animals and nontransgenic littermates (Figure 4C left panel). This suggests that DcR3 has a specific role in the down-regulation of MHC-II expression in vivo.
DcR3 down-regulates MHC-II expression in vivo. (A) Serum levels of DcR3 in DcR3-transgenic mice. The concentrations of DcR3 in the sera of DcR3-transgenic mice and controls were determined by ELISA. P value of the coupled differences, compared with controls, was determined using the Student t test: **P < .01. (B,C) Expression of MHC-I and MHC-II in TAMs from DcR3-transgenic mice. CT26 tumor masses were removed from DcR3-transgenic mice or nontransgenic littermates. The expression of I-Ad (B) and H-2Dd (C) by TAMs (ie, CD11b+ cells stained in red) was determined by confocal microscopy (magnification 10 × 63).
DcR3 down-regulates MHC-II expression in vivo. (A) Serum levels of DcR3 in DcR3-transgenic mice. The concentrations of DcR3 in the sera of DcR3-transgenic mice and controls were determined by ELISA. P value of the coupled differences, compared with controls, was determined using the Student t test: **P < .01. (B,C) Expression of MHC-I and MHC-II in TAMs from DcR3-transgenic mice. CT26 tumor masses were removed from DcR3-transgenic mice or nontransgenic littermates. The expression of I-Ad (B) and H-2Dd (C) by TAMs (ie, CD11b+ cells stained in red) was determined by confocal microscopy (magnification 10 × 63).
Discussion
The association of DcR3 expression and tumor progression has been well documented,25,40 although the underlying mechanisms are still unclear. We recently demonstrated that DcR3 can induce dendritic cell apoptosis27 and modulate macrophages differentiation.28 In addition, osteoclast formation and neoangiogenesis were also induced by DcR3.26,41 All these findings suggest that DcR3 is involved in modulating the immune system and in promoting tumor growth.
In this study, we further demonstrated that genes characteristically expressed by TAM/M2 macrophages were up-regulated by DcR3, while genes involved in M1 macrophage polarization and antimicrobial responses were down-regulated. Moreover, DcR3 substantially suppressed genes encoding proteins involved in MHC-II–dependent antigen presentation together with the master regulator CIITA. As shown in Figure 1B, CIITA and genes encoding both classical (HLA-DR, -DP, and -DQ) and nonclassical (invariant chain, HLA-DM and HLA-DO) MHC-II molecules were suppressed in DcR3-treated MDMs. Since CIITA is essential for the transcription of genes involved in MHC-II–dependent antigen presentation, DcR3-mediated transcriptional silencing of CIITA would have significant impact on host immunity.
Macrophages conventionally exert their antitumor effects by secreting cytotoxic molecules or by orienting the adaptive immune response by presenting MHC class II–bound peptides to T cells.42 Poor antigen-presenting capacity is a common characteristic of TAMs, and abundant evidence indicates that T cells undergo inhibitory regulation and become anergic in tumor-bearing hosts.13,43,44 Several mechanisms, including induction of structural abnormalities of T-cell receptor complex,45,46 production of immunosuppressive cytokines,8 or reduced expression of MHC-II molecules,14,47,48 have been proposed to account for T-cell unresponsiveness. However, the molecular mechanism leading to MHC-II down-regulation on TAMs has never been reported before.
Herein we find that de novo MHC-II synthesis is suppressed significantly in DcR3-treated MDMs as a result of transcriptional inactivation of the CIITA gene via histone deacetylation (Figure 2). Although several immunosuppressive cytokines, such as transforming growth factor-β, interleukin-10, and prostaglandin E, have been demonstrated to antagonize IFN-γ–induced MHC-II expression via down-regulation of CIITA mRNA expression or functional activity,49-51 none of these cytokines showed a broad effect on all CIITA promoters as seen with DcR3. Epigenetic modifications play an important role in the transcriptional regulation of CIITA expression. It has been shown that silencing of MHC-II expression during dendritic cell maturation and plasma cell differentiation is mediated by global histone deacetylation of CIITA promoters.52,53 Here we show that DcR3-mediated MHC-II suppression also occurs via histone deacetylation on CIITA promoters. Thus, the global chromatin remodeling of the CIITA upstream regulatory regions by histone deacetylation might be a common mechanism for MHC-II silencing in antigen-presenting cells. The decreased acetylation of histones on CIITA promoters may result from altered expression or enzymatic activity of HDACs or histone acetyl transferases (HATs) after DcR3 treatment. However, DcR3 did not affect the total enzymatic activity of HDACs and HATs (Figure S4). Moreover the expression of CBP/p300 and HDAC1 and HDAC2 was similar in control and DcR3-treated MDMs. In addition, DcR3 also did not affect the mRNA expression of HDAC1, HDAC2, HDAC6, and HDAC9 (Figure S4). These results indicate that DcR3 inhibits CIITA expression via histone deacetylation on CIITA promoters, although it is still unclear whether DcR3-mediated histone deacetylation occurs through regulation of other HDACs and HATs than those already tested, or by suppressing the recruitment of HDACs and HATs to CIITA promoters.
Even though DcR3 has been shown to be a pleiotropic immune modulator in previous studies,28,29,54,55 it is still a challenge to identify the receptors responsible for DcR3-mediated effects. Recently, we have shown that DcR3 is able to interact with heparan sulfate proteoglycans (HSPGs) on cell surface, and DcR3-mediated effects are inhibited by heparin/heparan sulfate. Further study shows that DcR3-mediated signal cascades is abolished by knockdown of syndecan 2 and CD44 on monocytic cell lines.31 Moreover, a stretch of amino acids with positive charge located on amino acid 256 to 259 (the heparin binding domain, HBD) is shown to be responsible for DcR3-mediated immunosuppression.27,31 This suggests that targeting HSPGs may become a novel strategy to subvert DcR3-mediated immunosuppression in the future.
The functional heterogeneity of macrophage depends on expressing distinct transcriptional programs in response to different environmental signals. Recent observations also inferred that tumor microenvironmental factors were involved in TAM polarization.56-58 Human macrophages cocultured with ovarian cancer cells showed up-regulation of mannose receptor, scavenger receptor, CCL22, MMP7, MMP9, and TNF-α, and down-regulation of several members of chemokine including CXCL2.57 It is interesting to note that DcR3-treated MDMs also showed prominent up-regulation of mannose receptor,28 CCL22, and MMP7, and down-regulation of CXCL2 (Tables S1,S3). Therefore, the phenotype of TAMs overlapped with several genes modulated by DcR3, and this suggests that DcR3 is an important molecule to “educate” monocytes to differentiate into TAMs.
Unlike the low-density array used to address ovarian cancer cell–induced TAM polarization,57 Martinez et al identified the gene expression profile differentially expressed in human M1 (by LPS/IFN-γ) and M2 (by IL-4) macrophages using Affymetrix microarray.59 Genes modulated by both DcR3 and IL-4 included the up-regulation of mannose receptor and M160 (scavenger receptor cysteine-rich type 1 protein), and the down-regulation of IL-6 (Table 3). Compared with ovarian cancer–induced TAMs, IL-4 seems less potent than DcR3 to skew monocyte differentiation into TAMs. However, the genes modulated by DcR3 were selected at 48 hours after treatment; while genes modulated by IL-4 were identified at 18 hours after stimulation, we cannot rule out the possibility that genes modulated by IL-4 between 18 to 48 hours might be missing in IL-4–treated MDMs.
Comparison of TAM/M2 gene expression profile resulting from different systems
| . | DcR3-treated MDMs . | Generally M2 phenotype (Mantovani et al8 ) . | IL-4 polarized M2 (Martinez et al58 ) . | TAMs by ovarian cancer (Hagemann et al56 ) . |
|---|---|---|---|---|
| Membrane receptor | ||||
| Mannose receptor | Increased | Increased | Increased | Increased |
| M160 | Increased | Not mentioned | Increased | Not mentioned |
| Fcγ-Rs:CD16/32/64 | Decreased | Decreased | Not identified | Not mentioned |
| CD80 | Decreased | Decreased | Not identified | Not mentioned |
| Cytokine and receptor | ||||
| TNF-α | Decreased | Decreased | Not identified | Increased |
| IL-1 | Decreased | Decreased | Not identified | Not mentioned |
| IL-6 | Decreased | Decreased | Decreased | Not mentioned |
| IL1RN | Increased | Increased | Not identified | Not mentioned |
| IL1R2 | Increased | Increased | Not identified | Not mentioned |
| Chemokine and chemokine receptor | ||||
| CCL22 | Increased | Increased | Not identified | Increased |
| CXCL2 | Decreased | Not mentioned | Not identified | Decreased |
| Effector molecule | ||||
| iNOS | Decreased | Decreased | Not identified | Not mentioned |
| Arginase-1 | Increased* | Increased | Not identified | Not mentioned |
| MMP7 | Increased | Increased | Not identified | Increased |
| MMP9 | Increased | Increased | Not identified | Increased |
| . | DcR3-treated MDMs . | Generally M2 phenotype (Mantovani et al8 ) . | IL-4 polarized M2 (Martinez et al58 ) . | TAMs by ovarian cancer (Hagemann et al56 ) . |
|---|---|---|---|---|
| Membrane receptor | ||||
| Mannose receptor | Increased | Increased | Increased | Increased |
| M160 | Increased | Not mentioned | Increased | Not mentioned |
| Fcγ-Rs:CD16/32/64 | Decreased | Decreased | Not identified | Not mentioned |
| CD80 | Decreased | Decreased | Not identified | Not mentioned |
| Cytokine and receptor | ||||
| TNF-α | Decreased | Decreased | Not identified | Increased |
| IL-1 | Decreased | Decreased | Not identified | Not mentioned |
| IL-6 | Decreased | Decreased | Decreased | Not mentioned |
| IL1RN | Increased | Increased | Not identified | Not mentioned |
| IL1R2 | Increased | Increased | Not identified | Not mentioned |
| Chemokine and chemokine receptor | ||||
| CCL22 | Increased | Increased | Not identified | Increased |
| CXCL2 | Decreased | Not mentioned | Not identified | Decreased |
| Effector molecule | ||||
| iNOS | Decreased | Decreased | Not identified | Not mentioned |
| Arginase-1 | Increased* | Increased | Not identified | Not mentioned |
| MMP7 | Increased | Increased | Not identified | Increased |
| MMP9 | Increased | Increased | Not identified | Increased |
Arginase-1 up-regulation was observed on TAMs from DcR3 transgenic mice but not found in DcR3-treated human MDMs.
Compared with IL-4–treated human M2, the phenotype of murine M28 is more similar to DcR3-treated MDMs. Up-regulation of mannose receptor, IL1RN, IL1R2, CCL22, arginase-1, and MMP7, as well as down-regulation of FcγRs, CD80, TNF-α, IL-1, and iNOS, were observed (Table 3). Based on the observations shown in Figure 1 and Table 3, DcR3 seems to be a potent tumor-secreted factor that skews macrophage polarization toward TAM/M2.
In summary, our study demonstrates that DcR3 is able to down-regulate genes involved in MHC-II–dependent antigen presentation via histone deacetylation of the CIITA promoters, where CIITA is the master regulator of MHC-II expression and DcR3 is an important tumor-secreted factor that “educates” macrophage differentiation toward the TAM/M2 phenotype. DcR3 is detectable in a diversity of cancer cells, where the neutralization of this protein in vivo represents an important target for enhancing the efficacy of anticancer therapy in the future.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Ren-In You for technical assistance and Dr. Ming-Zong Lai for providing essential materials.
This work was supported mainly by grant 95-2320-B-010-040-MY3 from the National Sciences Council, grant V96S5-001 from Taipei Veterans General Hospital, and grants 94M002-1 and AS-97-FP-L06-5 from Academia Sinica (S.-L.H.). Y.-C.C. was supported by a postdoctoral fellowship program (95-2811-B-010-011 and 96-2811-B-010-009).
Authorship
Contribution: Y.-C.C. designed, performed, and analyzed experiments, and wrote the paper; T.-C.C. performed experiments and contributed to writing the paper; C.-T.L. and C.-Y. Y. performed experiments; H.-W.W. performed microarray analysis; C.-C.W. performed statistical analysis; S.-L.H. designed and analyzed experiments, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shie-Liang Hsieh, Department and Institute of Microbiology and Immunology, National Yang-Ming University, Taipei 11221, Taiwan; e-mail: slhsieh@ym.edu.tw or slhsieh@gate.sinica.edu.tw.

![Figure 1. Modulation of M1/M2 markers and down-regulation of MHC-II molecules by DcR3. (A) Validation of microarray analysis. Quantitative real-time reverse transcription–polymerase chain reaction (RT-PCR) was used to determine gene expression. The mean fold change was calculated by comparing the expression level of each gene, relative to that of GAPDH, in hIgG1- or DcR3-treated MDMs. (B) Heat map of genes involved in MHC-II–mediated antigen presentation pathway. Down-regulated genes are shown in blue, while up-regulated genes are shown in red. (C) Expression of MHC-II molecules in MDMs. DcR3-treated or control MDMs were stained with FITC-conjugated anti–HLA-ABC mAb in conjugation with allophycocyanin-conjugated anti–HLA-DR mAb (top panel) or PE-conjugated anti–HLA-DR, -DP, and -DQ mAbs (bottom panel), followed by flow cytometric analysis. (D) Down-regulation of HLA-DR expression in MDMs after cocultivation with SW480 cells. At 24 hours after siRNA transfection, SW480 cells were cocultured with PKH67-labeled monocytes for 5 days, followed by gating PKH67-positive MDMs to determine surface expression of HLA-DR by FACS Calibur system (top panel). Open histograms correspond to isotype control and shaded histograms to specific staining. The concentrations of DcR3 in SW480 culture media were determined by ELISA (bottom panel). (E) Impaired T-cell stimulatory ability of DcR3-treated MDMs. Allogeneic CD4+ T cells were cocultured with γ-irradiated macrophages for 4 days at different ratios, followed by addition of 1 μCi (0.037 MBq)/well [3H]-thymidine for another 16 hours. The incorporation of [3H]-thymidine was measured using a β-counter. P values for the coupled differences, compared with control, were determined using the Student t test: *P < .05; **P < .01 (A,E). Error bars in panels A, D, and E represent SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/10/10.1182_blood-2007-12-130609/6/m_zh80110819950001.jpeg?Expires=1769095596&Signature=1ZDEKAGwGKskQXsZVrx59-~lB5chhqj0OPXgQxz8NlsuAkJbVwL4naSRn-8P6-4U00y~bR0jjSQnX3rKaK39Go8ZHBb~~p4-bvAE9hc~spZ5cVo3PSh~ZoJ0NPNoEmmE4sz3iWa98HLuH4EJ2QHlZLZmxgLWlSscS~n1Ni6jM2ie8qQSr4tkR7MVeE7KC6Cvk5NmnPA~TuSjZ-DuYH7UvVlyNoDt2wejw~-ET0cMyKoZNKaM0KYJV3XujPh5dQcvthW3yhpcpLAUfMPpZIskmbd7t2vrT3B537x9BzxRN5zgGBp-XiAaEGMFXr2DSeGsc2aU9A0tE4l80Hwl0w3o9Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)