Lenalidomide is approved for red blood cell (RBC) transfusion-dependent anemia due to low or intermediate-1 (int-1) risk myelodysplastic syndromes (MDSs) associated with a chromosome 5q deletion with or without additional cytogenetic abnormalities. We report results of a multicenter, phase 2 trial evaluating lenalidomide therapy for transfusion-dependent patients with low- or int-1–risk MDS without deletion 5q. Eligible patients had 50 000/mm3 or more platelets and required 2 U or more RBCs within the previous 8 weeks; 214 patients received 10 mg oral lenalidomide daily or 10 mg on days 1 to 21 of a 28-day cycle. The most common grade 3/4 adverse events were neutropenia (30%) and thrombocytopenia (25%). Using an intention-to-treat analysis, 56 (26%) patients achieved transfusion independence (TI) after a median of 4.8 weeks of treatment with a median duration of TI of 41.0 weeks. In patients who achieved TI, the median rise in hemoglobin was 32 g/L (3.2 g/dL; range, 10-98 g/L [1.0-9.8 g/dL]) from baseline. A 50% or greater reduction in transfusion requirement occurred in 37 additional patients, yielding a 43% overall rate of hematologic improvement (TI response +‖≥ 50% reduction in transfusion requirement). Lenalidomide has clinically meaningful activity in transfusion-dependent patients with low- or int-1–risk MDS who lack the deletion 5q karyotypic abnormality. This study is registered at www.clinicaltrials.gov as no. NCT00064974.
Introduction
The myelodysplastic syndromes (MDSs) encompass a spectrum of hematopoietic stem-cell malignancies that are characterized by ineffective hematopoiesis and a propensity to evolve to acute myeloid leukemia.1,2 Patients with MDS are classified by the International Prognostic Scoring System (IPSS) into low-, intermediate-1 (int-1)–, intermediate-2 (int-2)–, and high-risk groups based on the percentage of marrow blasts, number of cytopenias, and bone marrow cytogenetic findings.3 These features offer predictive value for both overall survival and the risk for transformation to acute myeloid leukemia (AML). Among patients with lower-risk (low or int-1) MDS, refractory anemia (RA) is the principal therapeutic challenge. Erythropoietic stimulating agents (ESAs) alone or in combination with myeloid growth factors may be effective in a subset of patients with MDS with limited transfusion burden and with low serum erythropoietin concentration.4,5 However, once anemia is symptomatic, red blood cell (RBC) transfusions supported by iron chelation therapy remain the mainstay of treatment.6
Lenalidomide is a 4-amino-glutarimide analog of thalidomide with potent immune modulatory and antiangiogenic activity.7,,–10 A recent study confirmed that lenalidomide improves erythropoiesis in patients with low- or int-1–risk MDS associated with a chromosome 5q interstitial deletion either alone or with additional chromosomal abnormalities.11 Erythroid response to lenalidomide was associated with meaningful increases in hemoglobin, RBC transfusion independence (TI), and cytogenetic response. Neutropenia and thrombocytopenia were the most common reasons for interrupting treatment or adjusting the lenalidomide dose. These studies led to the US Food and Drug Administration approval of lenalidomide for the treatment of transfusion-dependent anemia in patients with low- or int-1–risk MDS with a chromosome 5q deletion with or without additional cytogenetic abnormalities.
The initial safety and efficacy study of lenalidomide in MDS included patients with diverse karyotypes.12 Whereas erythroid responses occurred in 83% of patients with deletion 5q, responses were also achieved in patients with a normal karyotype (57%) and with other cytogenetic abnormalities (12%).12 Similar findings, albeit with lower frequency of response, were reported for thalidomide without apparent relation to karyotype, suggesting that thalidomide and lenalidomide may improve erythropoiesis through direct effects on the MDS clone.13,14 Based on these results, a multicenter phase 2 study was conducted to investigate the use of lenalidomide in low- or int-1–risk MDS with normal or abnormal karyotypes other than deletion 5q.
Methods
Patients
Eligible patients had a confirmed diagnosis of IPSS low- or int-1–risk MDS3 and transfusion-dependent anemia, which was defined as documentation of 2 units or more RBCs within 8 weeks of the first day of lenalidomide treatment. Study exclusions included a deletion 5q cytogenetic abnormality; severe neutropenia (< 500 polymorphonuclear neutrophils/mm3) or thrombocytopenia (< 50 000 platelets/mm3); inadequate renal or hepatic function (serum creatinine > 221 μM [2.5 mg/dL]; serum glutamic oxaloacetic transaminase/aspartate aminotransferase [SGOT/AST] or serum glutamic pyruvic transaminase/alanine aminotransferase [SGPT/ALT] > 3.0 times upper limit of normal [ULN]; serum direct bilirubin > 34.2 μM [2.0 mg/dL]); prior history of malignancy other than MDS (except basal cell or squamous cell carcinoma or carcinoma in situ of the cervix or breast) unless free of disease for at least 3 years; clinically significant coexisting medical conditions; and prior grade 3 or 4 allergic reaction or hypersensitivity to thalidomide. Hematopoietic growth factor administration was not permitted within 7 days of the first day of lenalidomide treatment. Any experimental or conventional treatment other than blood products (ie, chemotherapeutic, immunosuppressive, or cytoprotective agents) was not allowed within 28 days of study treatment.
Study design
Written informed consent was obtained from all registered patients in accordance with the Declaration of Helsinki, and the study was approved by the Institutional Review Board of each of the 38 participating medical centers. The trial was designed, monitored, and analyzed by the principal investigator (A.F.L.) along with the cytogenetic (G.W.D.) and pathology (J.M.B.) reviewers in consultation with Celgene Corporation. The manuscript was authored by A.F.L. and A.R. with editorial revision by coauthors without sponsor limitation.
Lenalidomide was supplied in 5-mg capsules and was initially administered using a syncopated regimen (10 mg daily for 21 days of every 28-day cycle) based upon early data from other studies. With the availability of additional data, it became evident that daily dosing provided a superior interval to response with no apparent increase in toxicity.12 The study was activated in July 2003, and the treatment schedule was amended to 10 mg daily on September 12, 2003. Patients who had initiated lenalidomide on the syncopated regimen could be switched to the daily dosing regimen at the discretion of the treating physician.
Treatment was interrupted and the dose was modified for grade 3 or greater adverse events. For grade 4 neutropenia or thrombocytopenia, lenalidomide was omitted for the remainder of the cycle and was resumed at a dose of 5 mg/day at the planned start of the next cycle if the toxicity resolved. Subsequent dose reductions were 5 mg every other day, 5 mg 3 times a week, 5 mg 2 times a week, and 5 mg once a week, according to tolerance. Patients who could not tolerate 5 mg lenalidomide once a week every 28 days were discontinued from the study.
Complete blood profiles were obtained weekly for the first 8 weeks, then every 2 weeks for the second 8 weeks, followed by monthly thereafter. Bone marrow aspirate, biopsy, and marrow cytogenetics were repeated after 24 weeks of treatment for response assessment. Responding patients continued lenalidomide until disease progression, treatment failure, or unacceptable toxicity. RBC transfusions were administered according to prestudy clinical indicators with the following guidelines: hematocrit less than 25%, 2 units; hematocrit less than 21%, 3 units; and hematocrit less than 18%, 4 units. Myeloid growth factors were the only cytokines permitted.
Assessment of response and toxicity
The primary endpoint of the study was the frequency of RBC TI. Secondary analyses included the duration of RBC TI, frequency of minor erythroid response, cytogenetic and pathologic response, and the safety of treatment in the study population. Hematologic response was assessed according to International Working Group (IWG) 2000 criteria, in which response required sustained improvement for 8 or more consecutive weeks.15 A more stringent definition of RBC TI was applied in which TI must be accompanied by a peak hemoglobin rise of 10 g/L (1 g/dL) or higher. Minor erythroid response was defined as a 50% or greater reduction in RBC transfusions compared with baseline. Hemoglobin rise in patients achieving RBC TI was calculated as the difference between the maximum hemoglobin response and the minimum pretransfusion hemoglobin concentration in the 8 weeks before the first lenalidomide dose. Because new response criteria were proposed in 2006, the revised criteria for erythroid response were also applied. The IWG 2006 criteria omit minor erythroid responses, thereby accepting only clinically significant or major erythroid improvements. We interpreted the proposal to define an erythroid response as either a 4 units/8 weeks reduction in RBC transfusions triggered by a hemoglobin transfusion threshold of 90 g/L (9 g/dL) or lower among transfusion-dependent patients, or an increase in hemoglobin of 15 g/L (1.5 g/dL) or more for at least 8 weeks in non–transfusion-dependent patients.16 Cytogenetic response was determined by standard metaphase analysis before and after treatment for those patients with 20 or more evaluable metaphases in sequential specimens. Complete cytogenetic response was defined as the absence of metaphases containing an abnormal clone. Partial cytogenetic response was defined as a 50% or greater reduction in the proportion of abnormal metaphases after treatment. Cytogenetic nonresponders included those patients with any outcome other than complete or partial cytogenetic response. Cytogenetic progression was defined as acquisition of a new chromosomal abnormality. Pretreatment and follow-up marrow pathology and karyotypes were reviewed for assignment of French-American-British (FAB) category17 and cytogenetic pattern by J.M.B. and G.D., respectively. Study eligibility was determined according to local institutional pathologic and cytogenetic assessment. Adverse events were graded for severity using the National Cancer Institute Common Toxicity Criteria (NCI CTC) version 2.0. Follow-up ceased at 30 days after permanent withdrawal from protocol treatment.
Statistical analysis
A 1-stage binomial design based on a targeted response rate of 25% statistically required at least 114 patients. Due to favorable efficacy and safety results as assessed throughout the study by the sponsor's internal data monitoring committee, enrollment of 215 patients was permitted in order to provide more substantial safety data and to obtain more precise response estimates. One patient with a lower-risk MDS associated with a deletion 5q cytogenetic abnormality was mistakenly enrolled into this study. This patient's results have been excluded from this analysis. All response and toxicity analyses were performed using the intention-to-treat principle and included all of the remaining 214 registered patients and reflect data available as of July 5, 2006.
The time to achievement of RBC TI represents the number of days from initiation of study treatment until the day after the last RBC transfusion that proceeded the first 8-week response period. Duration of RBC TI was calculated from the day after the date of the last RBC transfusion to the day prior to the date of the resumption of RBC transfusions. Median duration of RBC TI was estimated using the Kaplan-Meier method.18 Univariate comparisons were performed by Fisher exact test, a 2-sample independent t test, or a Wilcoxon rank-sum test. All reported P values are 2-sided. Summary statistics (N, mean, standard deviation, median, and minimum/maximum) are reported as appropriate. Multivariate stepwise logistic regression techniques were used to investigate correlation of prognostic variables with response. Efficacy end-point frequency estimates were accompanied by exact 95% confidence intervals (CIs).
Results
All 214 patients analyzed received lenalidomide treatment. A total of 114 patients initiated treatment on the 21-day schedule, and 100 patients received continuous daily dosing. Low- or int-1–risk IPSS scores (Table 1) were confirmed in 168 (79%) patients. A total of 8 (4%) patients had int-2 or high-risk IPSS scores, and for 38 (18%) patients, an IPSS category could not be assigned because of inadequate pathology material to confirm the percentage of marrow blasts, inadequate metaphases to determine karyotype complexity, or a diagnosis other than MDS (1 AML and 1 atypical chronic myeloid leukemia). Assignment to FAB subtype by central review19 was as follows: RA (22%); RA with ringed sideroblasts (RARS; 40%); RA with excess blasts (RAEB; 11%); RAEB in transformation (RAEB-t; 2%); and chronic myelomonocytic leukemia (CMML; 9%). The World Health Organization (WHO) classification was also applied.20 RA with limitation of dysplasia to the erythroid lineage was found in only 5% of patients, while RA with multilineage dysplasia was present in 16% of patients. Few patients with RARS had single-lineage dysplasia (3%) whereas RARS with multilineage dysplasia represented 40% of patients. Most patients with RAEB (89%) were classified as RAEB-1 (5% to 9% marrow blasts), and all of the patients with CMML had fewer than 10% blasts (ie, CMML-1).
Clinical and hematologic features
| Characteristic . | Quantity . |
|---|---|
| Patients, no. | 214 |
| Median age, y (range) | 72 (27-94) |
| Male:female ratio (%) | 138:76 (65) |
| Median MDS duration, y (range) | 2.2 (0.0-12.9) |
| Median RBC units/8 wk (range) | 4.0 (1-24) |
| Patients receiving 2 or more RBC units/mo | 139 (65) |
| IPSS risk category | |
| Low | 92 (43) |
| Intermediate-1 | 76 (36) |
| Intermediate-2/high | 8 (4) |
| Unclassified | 38 (18) |
| FAB type | |
| RA | 47 (22) |
| RARS | 86 (40) |
| RAEB | 24 (11) |
| CMML | 20 (9) |
| RAEB-t | 5 (2) |
| AML | 1 (1) |
| Inadequate specimen | 31 (14) |
| Neutropenia (< 1.5×109/L) | 57 (27) |
| Thrombocytopenia (< 100×109/L) | 41 (20) |
| Characteristic . | Quantity . |
|---|---|
| Patients, no. | 214 |
| Median age, y (range) | 72 (27-94) |
| Male:female ratio (%) | 138:76 (65) |
| Median MDS duration, y (range) | 2.2 (0.0-12.9) |
| Median RBC units/8 wk (range) | 4.0 (1-24) |
| Patients receiving 2 or more RBC units/mo | 139 (65) |
| IPSS risk category | |
| Low | 92 (43) |
| Intermediate-1 | 76 (36) |
| Intermediate-2/high | 8 (4) |
| Unclassified | 38 (18) |
| FAB type | |
| RA | 47 (22) |
| RARS | 86 (40) |
| RAEB | 24 (11) |
| CMML | 20 (9) |
| RAEB-t | 5 (2) |
| AML | 1 (1) |
| Inadequate specimen | 31 (14) |
| Neutropenia (< 1.5×109/L) | 57 (27) |
| Thrombocytopenia (< 100×109/L) | 41 (20) |
Data are numbers of patients (%) unless otherwise indicated.
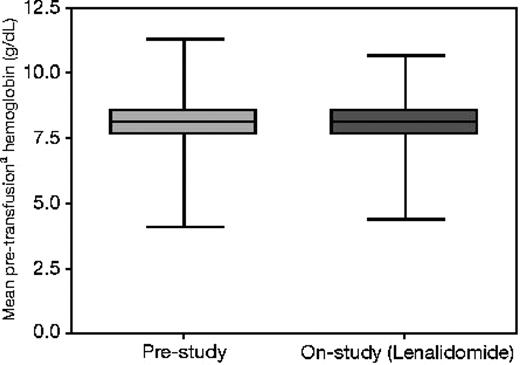
The median number of RBC transfusions administered in the 8 weeks before the study was 4 (range, 1 to 24 transfusions). Prestudy RBC transfusions could not be documented for 2 patients; therefore, these patients were included in the analysis as nonresponders. A total of 175 (82%) patients received at least 2 units RBCs in the month before study entry (Table 2). The distribution of hemoglobin values triggering RBC transfusions was similar before and during study treatment, indicating that the transfusion thresholds did not differ from baseline (Figure 1). Moderate to severe neutropenia and thrombocytopenia were present in 57 (27%) and 41 (20%) patients, respectively (Table 1). Most patients (83%) had a normal or favorable IPSS karyotype (Table 3). Overall, only 47 (22%) participants had a clonal cytogenetic abnormality.
Number of RBC units transfused during weeks 0 to 4, 5 to 8, and 0 to 8 before start of lenalidomide dosing
| RBC units transfused . | Week 0 to 4 before study, no. (%) . | Week 5 to 8 before study, no. (%) . | Week 0 to 8 before study (eligibility), no. (%) . |
|---|---|---|---|
| 0 | 32 (15) | 48 (22) | 2 (0.9)* |
| 1 | 7 (3) | 2 (1) | 0 |
| 2 | 101 (47) | 89 (42) | 63 (29) |
| 3 | 15 (7) | 21 (10) | 10 (5) |
| 4 | 32 (15) | 39 (18) | 44 (21) |
| 5 | 5 (2) | 3 (1) | 10 (5) |
| 6 | 15 (7) | 9 (4) | 42 (20) |
| 7 | 0 | 0 | 6 (3) |
| 8 | 3 (1) | 3 (1) | 12 (6) |
| 9 | 2 (1) | 0 | 5 (2) |
| 10 | 0 | 0 | 9 (4) |
| 11 | 1 (0.5) | 0 | 3 (1) |
| 12 | 0 | 0 | 2 (0.9) |
| 13 | 0 | 0 | 1 (0.5) |
| 14 | 0 | 0 | 3 (1) |
| 15 | 0 | 0 | 1 (0.5) |
| 16 | 1 (0.5) | 0 | 0 |
| 24 | 0 | 0 | 1 (0.5) |
| RBC units transfused . | Week 0 to 4 before study, no. (%) . | Week 5 to 8 before study, no. (%) . | Week 0 to 8 before study (eligibility), no. (%) . |
|---|---|---|---|
| 0 | 32 (15) | 48 (22) | 2 (0.9)* |
| 1 | 7 (3) | 2 (1) | 0 |
| 2 | 101 (47) | 89 (42) | 63 (29) |
| 3 | 15 (7) | 21 (10) | 10 (5) |
| 4 | 32 (15) | 39 (18) | 44 (21) |
| 5 | 5 (2) | 3 (1) | 10 (5) |
| 6 | 15 (7) | 9 (4) | 42 (20) |
| 7 | 0 | 0 | 6 (3) |
| 8 | 3 (1) | 3 (1) | 12 (6) |
| 9 | 2 (1) | 0 | 5 (2) |
| 10 | 0 | 0 | 9 (4) |
| 11 | 1 (0.5) | 0 | 3 (1) |
| 12 | 0 | 0 | 2 (0.9) |
| 13 | 0 | 0 | 1 (0.5) |
| 14 | 0 | 0 | 3 (1) |
| 15 | 0 | 0 | 1 (0.5) |
| 16 | 1 (0.5) | 0 | 0 |
| 24 | 0 | 0 | 1 (0.5) |
Transfusion needs were not documented in 2 patients. These patients were counted as nonresponders in the efficacy analysis.
Distribution of hemoglobin transfusion thresholds before and during study treatment (N = 211). Boxes represent the interquartile range (25th to 75th percentiles), and the brackets extend to the minimum and maximum values. Hemoglobin on or within the 3 days preceding a transfusion (transfusion trigger).
Distribution of hemoglobin transfusion thresholds before and during study treatment (N = 211). Boxes represent the interquartile range (25th to 75th percentiles), and the brackets extend to the minimum and maximum values. Hemoglobin on or within the 3 days preceding a transfusion (transfusion trigger).
Cytogenetic features at baseline
| IPSS prognostic group . | No. (%) . |
|---|---|
| No. patients | 214 (100) |
| Good | 177 (83) |
| Normal karyotype | 160 |
| −Y | 10 |
| Deletion 20q | 6 |
| +X | 1 |
| Intermediate | 27 (13) |
| Trisomy 8 | 11 |
| Deletion 13q | 3 |
| Trisomy 19 | 2 |
| Deletion 11q | 2 |
| Trisomy 15 | 1 |
| Deletion 17p | 1 |
| i14q | 1 |
| Add 11q | 1 |
| Deletion 20q and add 20p | 1 |
| t(1;12) | 1 |
| t(2;8) | 1 |
| t(2;3) | 1 |
| t(2;11) | 1 |
| Poor | 3 (1) |
| Deletion 7q | 2 |
| Complex | 1 |
| Missing | 7 (3) |
| IPSS prognostic group . | No. (%) . |
|---|---|
| No. patients | 214 (100) |
| Good | 177 (83) |
| Normal karyotype | 160 |
| −Y | 10 |
| Deletion 20q | 6 |
| +X | 1 |
| Intermediate | 27 (13) |
| Trisomy 8 | 11 |
| Deletion 13q | 3 |
| Trisomy 19 | 2 |
| Deletion 11q | 2 |
| Trisomy 15 | 1 |
| Deletion 17p | 1 |
| i14q | 1 |
| Add 11q | 1 |
| Deletion 20q and add 20p | 1 |
| t(1;12) | 1 |
| t(2;8) | 1 |
| t(2;3) | 1 |
| t(2;11) | 1 |
| Poor | 3 (1) |
| Deletion 7q | 2 |
| Complex | 1 |
| Missing | 7 (3) |
Hematologic response
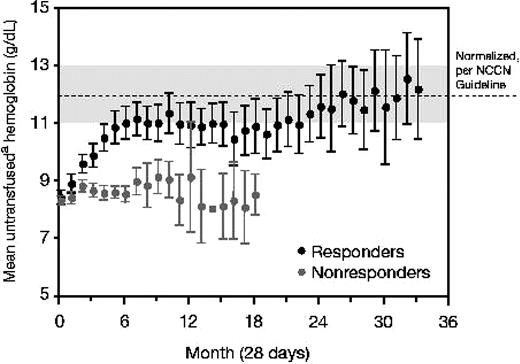
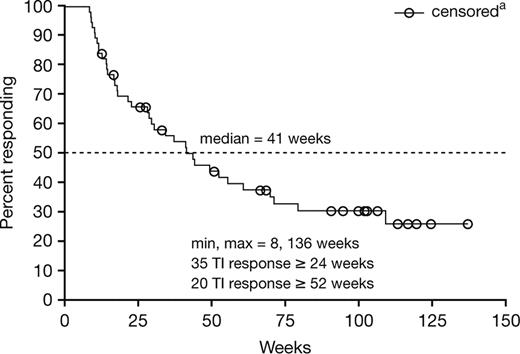
A total of 93 patients (43%) responded to lenalidomide treatment according to the modified IWG 2000 criteria (Table 4). A total of 56 (26%) patients achieved RBC TI with a concurrent 10 g/L (1 g/dL) or higher peak rise in hemoglobin; 37 (17%) patients had a 50% or greater reduction in transfusions. There was no significant difference in response rate according to assigned treatment schedule (P = 0.876). Among the 56 patients who achieved RBC TI, the mean untransfused hemoglobin concentration increased to within the normal or near normal range (Figure 2), and the median peak increase in hemoglobin compared with baseline was 32 g/L (3.2 g/dL; range, 10-98 g/L [1.0-9.8 g/dL]). A total of 25 (45%) responding patients reached a hemoglobin concentration of 120 g/L (12 g/dL) or greater, corresponding to a median hemoglobin of 133 g/L (13.3 g/dL; range, 120-180 g/L [12-18 g/dL]). The median interval to the beginning of the RBC transfusion–independent period was 4.8 weeks and ranged from 1 to 39 weeks. Among patients who achieved TI, 90% of patients began the transfusion-independent period by 16.9 weeks, 95% by 26 weeks, and 100% by 39 weeks. After a median follow-up of 76 weeks (range, 16 to 173 weeks) for the 56 transfusion-independent responders, the median duration of transfusion independence was 41 weeks (Figure 3). RBC TI continued for at least 6 months in 35 (63%) of 56 responders, and in 20 (36%) responders, the duration of RBC TI exceeded 1 year. Multivariate analysis correlating baseline and other prognostic variables (including age [≤ 70 vs > 70 years], sex [male vs female], Eastern Cooperative Oncology Group [ECOG] performance status [0 vs 1 or 2], IPSS [low- vs int-1], FAB [RA/RARS vs other], baseline neutropenia [≤ 1500 vs > 1500 × 109/L], baseline thrombocytopenia [≤ 150 000 vs > 150 000 × 109/L], RBC transfusion requirements [< 4 vs ≥ 4 units/8 weeks], lactate dehydrogenase [≤ ULN vs > ULN], blast percentage [≤ 5% vs > 5%], duration of MDS [≤ 2 vs > 2 years], and maximum percentage drop in platelet and absolute neutrophil counts [ANCs] in the first 16 weeks [continuous]) to the achievement of TI indicated that transfusion burden less than 4 units RBC (P < .001; odds ratio [OR] = 4.00), baseline platelet count of 150 000 mm3 or higher (P = .033; OR = 2.66), shorter duration of MDS (P = .005; OR = 3.03), and lactate dehydrogenase less than or equal to the ULN (P = .051; OR = 3.29) were associated with higher rates of TI response. There were no significant differences in TI response rate with respect to age, sex, FAB type, IPSS category, cytogenetic pattern, or cytopenias in the first 16 weeks (Table 5). Major neutrophil and platelet responses (IWG 2000 criteria) were achieved in 3 patients each, respectively.
Modified IWG 2000 erythroid response to lenalidomide
| Variable . | Continuous daily dosing, n = 100* . | 21-day dosing, n = 114* . | All patients, n = 214 . |
|---|---|---|---|
| Erythroid response, no (%) [95% CI] | |||
| Transfusion independence and 10 g/L Hb or more increase | 27 (27) | 29 (25) | 56 (26) [20-33] |
| 50% or greater decrease in no. of transfusions | 15 (15) | 22 (19) | 37 (17) [12-23] |
| Total transfusion response | 42 (42) | 51 (45) | 93 (43) [37-50] |
| Median time to transfusion independence, wk (range) | 7.4 (1-24) | 4.1 (1-39) | 4.8 (1-39) |
| Hemoglobin, g/L | |||
| Baseline, median (range)† | 79 (62-97) | 81 (61-106) | 80 (61-106) |
| Response, median (range)‡ | 116 (91-168) | 110 (73-180) | 116 (73-180) |
| Increase, median (range) | 33 (15-92) | 31 (10-98) | 32 (10-98) |
| Variable . | Continuous daily dosing, n = 100* . | 21-day dosing, n = 114* . | All patients, n = 214 . |
|---|---|---|---|
| Erythroid response, no (%) [95% CI] | |||
| Transfusion independence and 10 g/L Hb or more increase | 27 (27) | 29 (25) | 56 (26) [20-33] |
| 50% or greater decrease in no. of transfusions | 15 (15) | 22 (19) | 37 (17) [12-23] |
| Total transfusion response | 42 (42) | 51 (45) | 93 (43) [37-50] |
| Median time to transfusion independence, wk (range) | 7.4 (1-24) | 4.1 (1-39) | 4.8 (1-39) |
| Hemoglobin, g/L | |||
| Baseline, median (range)† | 79 (62-97) | 81 (61-106) | 80 (61-106) |
| Response, median (range)‡ | 116 (91-168) | 110 (73-180) | 116 (73-180) |
| Increase, median (range) | 33 (15-92) | 31 (10-98) | 32 (10-98) |
Hb indicates hemoglobin.
Daily dose was 10 mg.
Baseline hemoglobin concentration was the minimum value during the baseline period.
Response hemoglobin concentration was the maximum value during the transfusion-independent response period.
Mean monthly untransfused hemoglobin levels in TI responders and nonresponders (N = 214). 95% confidence intervals are provided when n is greater than 3. NCCN indicates National Comprehensive Cancer Network. Hemoglobin values in the 7 days following a transfusion were excluded, unless the values were on or within 3 days preceding a subsequent transfusion.
Mean monthly untransfused hemoglobin levels in TI responders and nonresponders (N = 214). 95% confidence intervals are provided when n is greater than 3. NCCN indicates National Comprehensive Cancer Network. Hemoglobin values in the 7 days following a transfusion were excluded, unless the values were on or within 3 days preceding a subsequent transfusion.
Duration of TI among responders in the intent-to-treat study population (N = 56). Patients who remain transfusion independent at the time of data cutoff or at time of study discontinuation.
Duration of TI among responders in the intent-to-treat study population (N = 56). Patients who remain transfusion independent at the time of data cutoff or at time of study discontinuation.
TI response by baseline FAB type, IPSS risk category, cytogenetic findings, and transfusion burden
| Characteristic . | No. . | Patients with TI response, no. (%) . |
|---|---|---|
| FAB type | ||
| RA | 47 | 16 (34) |
| RARS | 86 | 30 (35) |
| RAEB | 24 | 6 (25) |
| CMML | 20 | 3 (15) |
| RAEB-t | 5 | 0 |
| AML | 1 | 0 |
| Missing | 31 | 1 (3) |
| IPSS risk category | ||
| Low | 92 | 31 (34) |
| Intermediate-1 | 76 | 23 (30) |
| Intermediate-2/high | 8 | 0 |
| Missing | 38 | 2 (5) |
| Cytogenetic findings | ||
| Normal karyotype | 160 | 42 (26) |
| Abnormal karyotype | 47 | 13 (28) |
| Missing | 7 | 1 (14) |
| Transfusion burden per 8 wk | ||
| Fewer than 4 units pRBC | 75 | 33 (44) |
| Four or more units pRBC | 139 | 23 (17) |
| Characteristic . | No. . | Patients with TI response, no. (%) . |
|---|---|---|
| FAB type | ||
| RA | 47 | 16 (34) |
| RARS | 86 | 30 (35) |
| RAEB | 24 | 6 (25) |
| CMML | 20 | 3 (15) |
| RAEB-t | 5 | 0 |
| AML | 1 | 0 |
| Missing | 31 | 1 (3) |
| IPSS risk category | ||
| Low | 92 | 31 (34) |
| Intermediate-1 | 76 | 23 (30) |
| Intermediate-2/high | 8 | 0 |
| Missing | 38 | 2 (5) |
| Cytogenetic findings | ||
| Normal karyotype | 160 | 42 (26) |
| Abnormal karyotype | 47 | 13 (28) |
| Missing | 7 | 1 (14) |
| Transfusion burden per 8 wk | ||
| Fewer than 4 units pRBC | 75 | 33 (44) |
| Four or more units pRBC | 139 | 23 (17) |
pRBC indicates packed RBCs.
Using the IWG 2006 erythroid response criteria, 133 of the 214 study patients received 4 units or more RBC for hemoglobin values below the transfusion threshold of 90 g/L (9 g/dL) or less in the 8 weeks preceding study treatment. Among these patients eligible for transfusion response (TR), TI sustained 8 weeks or longer was achieved by 23 patients, and an additional 17 patients had a 4 units or greater decrease from baseline in RBC transfusion requirement for an overall TR rate of 30% (40 of 133 patients). Among the 81 patients not eligible for TR, 30 achieved a hemoglobin response (HbR) defined as a 15 g/L (1.5 g/dL) or greater rise in hemoglobin maintained for at least 8 weeks. Thus, erythroid hematologic improvement (TR or HbR) was achieved by 70 of 214 patients for an overall erythroid response rate of 33% (Table 6).
Erythroid hematologic improvement according to IWG 2006 criteria
| HI-E . | No. patients/N (%) . |
|---|---|
| Baseline transfusion dependence* | 133/214 (62) |
| TR† | 40/133 (30) |
| TI‡ | 23/214 (17) |
| HbR§ | 30/81 (37) |
| Overall HI-E response (TR or HbR) | 70/214 (33) |
| HI-E . | No. patients/N (%) . |
|---|---|
| Baseline transfusion dependence* | 133/214 (62) |
| TR† | 40/133 (30) |
| TI‡ | 23/214 (17) |
| HbR§ | 30/81 (37) |
| Overall HI-E response (TR or HbR) | 70/214 (33) |
HI-E indicates hematologic improvement–erythroid.
Transfusion dependence criteria: 4 units or more of RBC transfusions triggered by Hb values of 90 g/L or less during the 8 weeks immediately preceding first dose of lenalidomide.
TR: decrease from baseline 8-wk RBC transfusion requirement of 4 units or more during any 8-wk period after first dose of lenalidomide.
TI: absence of RBC transfusions during any 8-wk period after first dose of lenalidomide.
HbR: Hb increase of 15 g/L or more from baseline sustained for 8 wk or more.
Cytogenetic and pathologic findings
Favorable IPSS cytogenetic abnormalities or a normal karyotype were found in 177 (83%) patients, while intermediate and poor prognostic karyotypes were found in 27 (13%) and 3 (1%) patients, respectively (Table 3). A total of 75 percent of the patients had a normal karyotype. Among the 56 patients who achieved RBC TI, 45 (80%) patients had a favorable karyotype (42 normal; 3 lacked only a Y chromosome [−Y]) at baseline, 10 (18%) patients had an intermediate prognostic karyotype (trisomy 8 [n = 3], chromosome 11q abnormalities [n = 3], deletion 20q plus add 20p [n = 1], deletion 13q [n = 1], deletion 17p [n = 1], and t(2;8) [n = 1]), and in 1 (2%) patient, the karyotype at baseline was unknown. None of the 3 patients with a poor prognostic karyotype achieved TI. Among patients with favorable and intermediate karyotypes, 25% and 37%, respectively, achieved TI.
Overall, only 47 patients had a clonal cytogenetic abnormality at baseline. Among these, 9 (19%) were cytogenetic responders (4 complete, 5 partial responses). The responding karyotypes included trisomy 8 (n = 3), −Y (n = 3), deletion 11q (n = 2), and deletion 17p (n = 1). All 4 complete cytogenetic responses were associated with achievement of RBC TI, and 3 of 5 partial cytogenetic responders also attained RBC TI. A total of 4 patients experienced cytogenetic progression. Among these, 3 patients had a normal karyotype at baseline, and 1 patient had a clonal deletion 20q plus add 20p marker. A total of 3 of these patients had achieved RBC TI, and cytogenetic progression was associated with a relapse to transfusion-dependent anemia and bone marrow histologic progression. In 1 patient, cytogenetic progression was marked by the appearance of a deletion 7q marker that was associated with an increase in marrow blasts from 2% at baseline to 6%. The second patient acquired a dicentric (9,20) cytogenetic marker upon evolution to AML. The third patient with chromosome 20q deletion acquired an additional 12p deletion and developed pure red cell aplasia.
Bone marrow aspirate specimens obtained at baseline and at least once during follow-up were considered adequate for central review for 105 patients. During lenalidomide treatment, a more than 50% reduction in marrow blasts was observed in 2 of the 27 patients with 5% or greater blasts, resulting in a change in FAB subtype from CMML (n = 1) or RAEB (n = 1) to RA. Both of these patients achieved RBC TI. A decrease in ringed sideroblasts to less than 15% occurred in 14 (16%) of 86 patient with RARS during lenalidomide therapy. Of these 14 patients, 1 achieved a complete marrow response with resolution of dysplastic features, and 13 became RBC trans-fusion independent. Over the 3-year duration of this study, 10 (5%) patients experienced progressive disease marked by a 50% or greater increase in marrow blasts and a worsening of FAB subtype and/or disease transformation to acute leukemia (n = 9; 4%). The baseline FAB types of the 9 patients experiencing leukemia transformation included RAEB (n = 4), CMML (n = 3), and RARS (n = 2). One of the 3 patients with CMML with AML progression had a chromosome 7q deletion at baseline. The duration of lenalidomide treatment ranged from 3 to 85 weeks for these patients prior to transformation.
Adverse events
Neutropenia, thrombocytopenia, rash, and pruritis were the most common lenalidomide-associated adverse events (Table 7). NCI CTC grade 3 or 4 neutropenia (< 1000/μL) and thrombocytopenia (< 50 000/μL) were reported in 30% and 25% of patients, respectively, and were the most common reasons for dose adjustment. Little difference was observed in the frequency of grade 3 or higher neutropenia and thrombocytopenia between patients receiving the continuous and 21-day schedules. Myelosuppression often occurred early in the treatment course, with 43% of grade 3 or 4 hematologic adverse events occurring within the first 8 weeks of lenalidomide therapy. Most other adverse events were of low or moderate severity and included rash, pruritis, constipation, diarrhea, fatigue, peripheral edema, and nausea. Only 2 (1%) patients had a deep vein thrombosis, and no cases of pulmonary embolism were reported. In addition, 6 patients developed autoimmune hemolytic anemia (AIHA) after a median of 29 days of study treatment (range, 8-153 days) necessitating lenalidomide withdrawal in 3 patients. Patients with reported AIHA were either nonresponders (n = 5) or a minor responder (n = 1). A warm reactive autoantibody was confirmed in only 2 patients, whereas none of the patients had demonstrable changes in serum lactate dehydrogenase. A total of 5 patients received a trial of prednisone without demonstrable change in transfusion requirement.
Treatment-associated adverse events (≥ 10%)
| Adverse event . | Lenalidomide dose . | |||||
|---|---|---|---|---|---|---|
| Continuous daily dosing (n=100) . | 21-day dosing (n = 114) . | All patients (n = 214) . | ||||
| Any grade . | Grade 3 or 4 . | Any grade . | Grade 3 or 4 . | Any grade . | Grade 3 or 4 . | |
| Neutropenia | 30 | 27 | 26 | 23 | 28 | 25 |
| Thrombocytopenia | 31 | 22 | 22 | 18 | 26 | 20 |
| Rash | 24 | 2 | 20 | 6 | 22 | 4 |
| Pruritus | 21 | 1 | 21 | 1 | 21 | 1 |
| Constipation | 17 | 0 | 13 | 1 | 15 | 1 |
| Diarrhea | 16 | 1 | 13 | 1 | 15 | 1 |
| Fatigue | 18 | 4 | 13 | 4 | 15 | 4 |
| Peripheral edema | 3 | 0 | 16 | 1 | 10 | 1 |
| Nausea | 11 | 2 | 6 | 0 | 8 | 1 |
| Adverse event . | Lenalidomide dose . | |||||
|---|---|---|---|---|---|---|
| Continuous daily dosing (n=100) . | 21-day dosing (n = 114) . | All patients (n = 214) . | ||||
| Any grade . | Grade 3 or 4 . | Any grade . | Grade 3 or 4 . | Any grade . | Grade 3 or 4 . | |
| Neutropenia | 30 | 27 | 26 | 23 | 28 | 25 |
| Thrombocytopenia | 31 | 22 | 22 | 18 | 26 | 20 |
| Rash | 24 | 2 | 20 | 6 | 22 | 4 |
| Pruritus | 21 | 1 | 21 | 1 | 21 | 1 |
| Constipation | 17 | 0 | 13 | 1 | 15 | 1 |
| Diarrhea | 16 | 1 | 13 | 1 | 15 | 1 |
| Fatigue | 18 | 4 | 13 | 4 | 15 | 4 |
| Peripheral edema | 3 | 0 | 16 | 1 | 10 | 1 |
| Nausea | 11 | 2 | 6 | 0 | 8 | 1 |
Data are percentages.
A total of 117 (54.7%) patients required dose adjustment during the lenalidomide treatment course, including 56 patients receiving the continuous schedule and 61 patients receiving the 21-day schedule. The median time to dose adjustment was 7 weeks (range, < 1-80 weeks).
From July 2003 to July 2006, 21 deaths occurred either during the study or within 30 days of the last clinic visit. One death attributed to urosepsis/septic shock and another death attributed to respiratory failure were judged to be possibly treatment related by the treating investigators. Other deaths were due to disease progression (n = 6), cardiac arrest (n = 4), pneumonia (n = 2), renal failure (n = 2), multiorgan failure (n = 1), hepatic failure (n = 1), respiratory failure (n = 1), septic shock (n = 1), and unknown (n = 1).
Discussion
Lenalidomide is highly active in transfusion-dependent patients with lower-risk MDS with a chromosome 5q deletion. In this study, we confirm our initial observations that a significant proportion of transfusion-dependent patients with low- or int-1–risk, non–deletion 5q MDS benefit from lenalidomide therapy.12 The participants in this study had a long-standing diagnosis of MDS (median, 2.2 years) and substantial RBC transfusion requirements (median, 4 units/8 weeks). Although the number of patients that had failed prior treatment with ESA was not recorded, 65% of the study patients averaged 2 units or more per month during the screening period, indicating that a high proportion of patients had a poor ESA response profile. A total of 43% of patients treated with lenalidomide in this study experienced a reduction in transfusion needs and 26% became transfusion independent, with a median hemoglobin rise at the time of maximal improvement of 32 g/L (3.2 g/dL). Indeed, the frequency of transfusion response is comparable with that reported in the initial pilot study (48%) in patients who previously failed ESA treatment.12 Response to treatment was relatively rapid, with a median interval to onset of transfusion independence of 4.8 weeks (range, 1-39 weeks). Although the duration of TI does not approach that for deletion 5q MDS responders (median, > 2 years), major erythroid responses were clinically meaningful with 36% of responders remaining transfusion free for at least 1 year. Variables associated with a higher rate of TI response included lower baseline transfusion requirement (P < .001), normal platelet count (P = .033), shorter duration of MDS (P = .005), and normal serum lactate dehydrogenase (P = .051). We found no significant differences in TI response rate according to age, sex, FAB type, IPSS category, cytogenetic pattern, or early cytopenias.
To our knowledge, this is the first study to attempt to apply the 2006 IWG response criteria.16 Application of the criteria was challenging due to ambiguity in the erythroid improvement response definition and the recommendation to exclude transfusions administered above a hemoglobin threshold of 90 g/L (9 g/dL), which might not be considered clinically indicated. Although the latter was intended to minimize investigator bias and nonmeaningful extensions in transfusion intervals with the exclusion of minor responses, the requirement for a minimum rise in hemoglobin for the 8-week transfusion-free period that we applied may obviate the need for such scrutiny if transfusion patterns for symptomatic anemia are consistent with prestudy practice. As directed by the protocol, we found that transfusion thresholds applied prior to and during study treatment did not differ (Figure 1). We interpreted the revised IWG criteria to define an erythroid response as either a greater than or equal to 4 units/8-week reduction in RBC transfusions triggered by a hemoglobin transfusion threshold lower than 90 g/L (9 g/dL) (thereby defining transfusion dependence as 4 units or more per 8 weeks); or an increase in hemoglobin of 15 g/L (1.5 g/dL) or more for at least 8 weeks in non–transfusion-dependent patients. Using these criteria, we found a higher rate of erythroid response (33%) compared with our modified IWG 2000 major response criteria (26%). These differences relate largely to the inclusion of 17 patients (43% of transfusion responders) who achieved a 4 units RBC or greater reduction as erythroid responders using the IWG 2006 criteria, who otherwise did not achieve TI.
Myelosuppression occurs commonly with lenalidomide treatment in patients with deletion 5q MDS, which is consistent with the drug's action to suppress the malignant clone. In the non–deletion 5q patients in this study, moderate to severe neutropenia and thrombocytopenia developed in 30% and 25% of patients, respectively, and generally occurred early in the course of treatment. Infectious or hemorrhagic complications were infrequent. Although one patient succumbed to neutropenic infection early in the trial, the institution of weekly laboratory monitoring during the initial weeks and allowance for myeloid growth factors may mitigate serious neutropenic events.
Experience from the dose finding study (MDS-001) and the deletion 5q registration trial (MDS-003) suggested that lenalidomide's mechanism of action in MDS is karyotype dependent.11,12 Indeed, cytogenetic response was associated with a high frequency of complete histologic response in deletion 5q patients, whereas in patients with alternate karyotypes in this study, evidence for clonal suppression was uncommon, with rare histologic remissions. Overall, 41 patients achieved TI with minimal change in bone marrow morphology, suggesting that erythroid response in non–deletion 5q arises from effects of lenalidomide on the existing MDS clone that restore erythropoietic potential. Because lenalidomide has pleotropic immunologic and biologic properties that extend to cytokine modulation, T-cell costimulation, and angiogenesis inhibition,7,8,14,21,,,–25 effects on both the MDS clone and the microenvironment likely contribute to the drug's action.
Our finding that lenalidomide has erythropoietic activity in patients with a heavy transfusion burden (2 units/month or more) indicates that lenalidomide may offer an alternate therapeutic strategy for patients with MDS who do not benefit from ESA treatment. Delineation of features predictive for lenalidomide response would optimize candidate selection, analogous to the application of growth factor response profiles.26,27 Given that lenalidomide's erythropoietic action in non–deletion 5q MDS is restorative rather than suppressive, combination strategies with ESAs might improve activity. The ECOG is initiating an intergroup phase 3 trial (E2905) that will evaluate erythroid response to treatment with either lenalidomide alone or in combination with ESA in patients that have either failed ESA treatment or have a poor ESA response profile. Because the median time to onset of transfusion independence was relatively short, we recommend considering alternate treatment strategies in nonresponders after 4 months of treatment.
We conclude that lenalidomide has meaningful activity in the treatment of transfusion-dependent, low- or int-1–risk, non–deletion 5q MDS, and that further investigation is warranted.
The online version of this article contains a data supplement.
Presented in abstract form at the 48th annual meeting of the American Society of Hematology, Orlando, FL, December 9-12, 2006.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Other than the authors, investigators in the MDS-002 Study Group are listed in Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article.
This work was supported in part by research funding from Celgene Corporation (Summit, NJ).
Authorship
Contribution: A.R., J.A.R., E.J.F., H.J.D., L.D., C.A.S., R.M.S., P.L.G., P.T.C., V.M.K., J.M.S., D.T., and A.F.L. contributed to the paper as clinical research investigators and helped write and edit the paper. G.W.D. reviewed the cytogenetic data and helped write and edit the paper. J.M.B. reviewed the pathology data and helped write and edit the paper. J.B.Z. helped design the trial, analyze the data, and write and edit the paper. R.D.K. functioned as Medical Monitor for the trial and helped design the trial and edit the paper. M.S. functioned as a Clinical Research Scientist for the trial. K.W. functioned as lead statistician for the study, and performed and provided safety and efficacy analyses supporting the paper.
Conflict-of-interest disclosure: The Institution received money from Celgene for the clinical trial MDS-002, on which this manuscript is based, as well as clinical trial MDS-003. A.R. is on the Speakers Bureau for Celgene, and her annual honorarium is greater than $10 000. E.J.F. is on the Speakers Bureau for Celgene and has received more than $10 000 in compensation. J.A.R. owns stock in Celgene. J.D. is a Celgene consultant. C.A.S. is Celgene consultant and Celgene stock owner. R.D.K. is a Celgene employee. J.B.Z. is a Celgene employee and stockholder, with a financial interest greater than $10 000 in Celgene; he is also a patent holder for the use of lenalidomide and related compounds to treat a variety of hematologic conditions. A.L. discloses research/grant support from Scios; is a consultant with Scios, Celgene, and Pharmion; is on advisory boards for Kanisa and Schering; and is on speakers bureaus for Pharmion and Celgene. J.M.S. is on the Celgene Speakers Bureau, is a consultant for Celgene, and has received research support from Celgene. M.S. is a Celgene employee. K.W. is a Celgene employee and stockholder. G.W.D. is a cytogeneticist at Mayo Clinic. J.M.B. is consultant and on the speakers bureau for Celgene. R.M.S. received money for the clinical trials MDS 002 and MDS 003 from Celgene, and is on the Speakers Bureau for Celgene and Pharmion; his annual honorarium from each is less than $10 000. P.L.G. is a consultant for Celgene and a member of the Celgene Advisory Board, and has received research funds from Celgene for the clinical trials MDS-002 and MDS-003. D.A.T. has received research funds from Celgene for the clinical trials MDS-002 and MDS-003.
Correspondence: Alan F. List, H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Dr, Tampa, FL 33612; e-mail: alan.list@moffitt.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal