The heterogeneity of common variable immunodeficiency (CVID) calls for a classification addressing pathogenic mechanisms as well as clinical relevance. This European multicenter trial was initiated to develop a consensus of 2 existing classification schemes based on flowcytometric B-cell phenotyping and the clinical course. The clinical evaluation of 303 patients with the established diagnosis of CVID demonstrated a significant coincidence of granulomatous disease, autoimmune cytopenia, and splenomegaly. Phenotyping of B-cell subpopulations confirmed a severe reduction of switched memory B cells in most of the patients that was associated with a higher risk for splenomegaly and granulomatous disease. An expansion of CD21low B cells marked patients with splenomegaly. Lymphadenopathy was significantly linked with transitional B-cell expansion. Based on these findings and pathogenic consideration of B-cell differentiation, we suggest an improved classification for CVID (EUROclass), separating patients with nearly absent B cells (less than 1%), severely reduced switched memory B cells (less than 2%), and expansion of transitional (more than 9%) or CD21low B cells (more than 10%). Whereas the first group contains all patients with severe defects of early B-cell differentiation, severely reduced switched memory B cells indicate a defective germinal center development as found in inducible constimulator (ICOS) or CD40L deficiency. The underlying defects of expanded transitional or CD21low B cells remain to be elucidated. This trial is re-gistered at http://www.uniklinik-freiburg.de/zks/live/uklregister/Oeffentlich.html as UKF000308.
Introduction
Common variable immunodeficiency (CVID) is the most common primary immunodeficiency in adults.1 Recurrent bacterial infections of the respiratory tract are the clinical hallmark present in nearly all patients.2 In addition, up to 40% of the patients show gastrointestinal disease, concomitant lymphoproliferative disorders, autoimmune phenomena, or granulomatous inflammation.2 The pathogenic understanding of antibody deficiency in humans has always been hampered by the great heterogeneity of the syndrome.3 In 1966, Rosen and Janeway started to group antibody deficiencies by their mode of inheritance.4 In 1973, Cooper included the clinical course and serum immunoglobulin levels, thereby separating hyper-IgM syndromes and selective IgA deficiency.5 The remaining group of still very heterogeneous antibody deficiencies was termed CVID. Consecutive attempts to subclassify CVID by B-cell function in vitro6,7 failed to reach diagnostic acceptance because of laborious and poorly standardized procedures and a lack of clinical relevance
In 2002, we and others suggested a flow cytometric classification of CVID according to the B-cell phenotype.8,9 The abnormalities of circulating B cells in patients with CVID had already been recognized earlier,10 but only with the ease and the broad availability of flow cytometry was a widespread and systematic analysis of these aberrations possible. The Freiburg classification divided patients into 3 groups by analyzing the expression of IgM, IgD, CD27 and CD21.8 Group 1 was characterized by a severe reduction of switched memory B cells (IgD−IgM−CD27+ less than 0.4% of lymphocytes), while group 2 representing 25% of the analyzed CVID patients exhibited nearly normal numbers of class-switched memory B cells, suggesting a post germinal center defect. Group 1 was subdivided in a group 1a, which showed a markedly increased proportion of B cells with low expression of CD21 (CD21low B cells more than 20%) and a group with normal or slightly elevated numbers of CD21low B cells. The incidence of splenomegaly was significantly increased in group 1a.
The Paris classification by Piqueras et al9 classified patients according to the changes in the memory B-cell phenotype. This proposal distinguished patients with a decrease of total CD27+ B cells under 11% of B cells (MB0), a group MB1 with a selective reduction of class switched memory B cells (more than 11% total CD27+ B cells and reduced IgD−IgM−CD27+ less than 8% of B cells) and a group of patients not fulfilling the criteria of either group (MB2). This classification was also able to cluster patients with splenomegaly in one group (MB0). Besides the inclusion of CD21low B cells in the Freiburg classification and total CD27+ memory B cells in the Paris protocol, the other difference between the 2 schemes resides in the expression of class switched memory B cells as a percentage of total lymphocytes or B cells, respectively. So far only small numbers of patients have been analyzed according to either classification protocol and no direct comparison has been made. Therefore the EUROclass trial, a B-cell phenotypic classification of a large cohort of CVID patients was initiated in 8 European immunodeficiency centers. The aims were to determine the clinical and immunologic phenotype of CVID patients in Europe and to test and possibly improve and unify the current classification schemes.
Methods
Patients
All patients were diagnosed as having CVID based on the European Society for Immunodeficiencies/Pan-American Group for Immunodeficiency (ESID/PAGID) criteria,11 including a marked decrease of IgG (at least 2 standard deviations [SDs] below the mean for age) and a marked decrease in at least one of the isotypes IgM or IgA, the onset of clinical significant immunodeficiency at greater than 2 years of age, and the exclusion of defined causes of hypogammaglobulinemia (see also www.esid.org). Not all patients have been evaluated for absent isohemagglutinins and/or poor response to vaccines. For the final evaluation of B-cell phenotyping, the following exclusion criteria were adopted: patients younger than 6 years of age at the time of flowcytometric evaluation, patients on immunosuppressive treatment, patients suffering currently from malignancies, and patients with less than 1% peripheral B cells. Altogether, 303 patients of originally 370 patients from 8 European centers were included in the study (Freiburg, Germany: 76; Paris, France: 65; Oxford, United Kingdom: 44; Brno, Czech Republic: 33; London, United Kingdom: 29; Hannover, Germany: 27; Barcelona, Spain: 19; and Rotterdam, the Netherlands: 10). Most patients were on regular subcutaneous or intravenous IgG substitution. Table 1 summarizes the epidemiologic and clinical findings. The clinical data comprised splenomegaly (proven by ultrasound or computed tomography [CT] scan according to local criteria); lymphadenopathy (by clinical, ultrasound, or CT examination); granulomatous disease (proven by biopsy); and autoimmune phenomena (autoimmune cytopenias such as autoimmune hemolytic anemia [AIHA] and thrombocytopenia [AITP]), as well as other autoimmune disorders (pernicious anemia, autoimmune enteropathy, thyroiditis, vitiligo, arthritis, connective tissue disease, and a few others). Informed written consent according to the internal ethics review board–approved clinical study protocol (University Hospital Freiburg 239/99) was obtained in accordance with the Declaration of Helsinki from each individual in the contributing centers before participation in the study.
Epidemiologic data (n = 303)
| Characteristics . | Records, no. . | Clinical data . |
|---|---|---|
| Sex | 302 | 169 females, 133 males |
| Year of birth (± SD) | 300 | 1957 (± 17) |
| Age at onset, y (± SD) | 156 | 27 (± 17) |
| Age at diagnosis | 289 | 35 (± 16) |
| Splenomegaly, % | 284 | 40.5 |
| Lymphadenopathy, % | 260 | 26.2 |
| Granulomatous disease, % | 303 | 11.6 |
| Autoimmune phenomena, % | 286 | 20.3 |
| Autoimmune cytopenia, % | 213 | 20.2 |
| Characteristics . | Records, no. . | Clinical data . |
|---|---|---|
| Sex | 302 | 169 females, 133 males |
| Year of birth (± SD) | 300 | 1957 (± 17) |
| Age at onset, y (± SD) | 156 | 27 (± 17) |
| Age at diagnosis | 289 | 35 (± 16) |
| Splenomegaly, % | 284 | 40.5 |
| Lymphadenopathy, % | 260 | 26.2 |
| Granulomatous disease, % | 303 | 11.6 |
| Autoimmune phenomena, % | 286 | 20.3 |
| Autoimmune cytopenia, % | 213 | 20.2 |
Description of the European cohort, including sex, age, age of onset, age of diagnosis, and associated clinical phenomena of the 303 patients included in this trial. Splenomegaly was defined by ultrasound or CT scan to be larger than 4.7 × 11 cm. Lymphadenopathy was defined by clinical examination or ultrasound or CT scan. Granulomatous disease was judged positive only in histologically proven cases. All others were assumed negative. Autoimmune phenomena other than cytopenia included autoimmune thyroiditis, vitiligo, and pernicious anemia. Autoimmune cytopenia refers to AIHA and AITP.
Flow cytometric analysis of peripheral blood lymphocytes
Blood samples from CVID patients were always taken before intravenous Ig substitution and prepared as described previously.8 Some centers used the whole blood method, which had been shown previously to yield comparable results to the flow cytometric analysis of isolated lymphocytes.12 Peripheral blood mononuclear cells (PBMCs) were stained for 20 minutes at 4°C with a mixture of the following antibodies at optimal concentrations: anti–CD27-fluorescein isothiocyanate (FITC; Dako, Glostrup, Denmark), anti–CD38-FITC (BD Biosciences, Heidelberg. Germany), anti–CD21-phycoerythrin (PE; BD Biosciences), anti–IgD-PE (Southern Biotechnology Associates, Birmingham, AL), CD19-PC7 (Beckman-Coulter, Miami, FL), and anti–IgM-Cy5 (Dianova, Hamburg, Germany; for details, see Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). T cells were characterized by staining with anti–CD8-FITC, CD4-PE (both Beckman-Coulter), anti CD45R0-PerCP, and anti–CD3-allophycocyanin (both BD Biosciences). Four-color data acquisition was performed with FACSCalibur (BD Biosciences) or Epics MCL (Beckman-Coulter). Data were analyzed with the help of CellQuest analysis software (BD Biosciences) or Expo 32, version 1.2 Analysis (Beckman-Coulter).
Statistics
Statistical analysis was performed with R (http://www.r-project.org/). All data are expressed as mean plus or minus SD except when indicated otherwise. Correlation comparisons between paired samples were made by Pearson's product moment correlation coefficient. Analysis of variance and Tukey honest significant differences were used by creating a set of confidence intervals on the differences between the means of the levels of a factor with the specified family-wise probability of coverage. Comparisons between the classifications were made by logistic regression models (logit models), which are part of generalized linear models. Kruskal-Wallis rank sum test was used for comparisons between classifications and variables. Mann-Whitney test was used for comparisons between clinical parameters and variables.
Results
Epidemiologic and clinical analysis of the European multicenter cohort of CVID patients
The cross-sectional study was performed on 303 European CVID patients between 10 and 84 years of age (47 ± 17 years). There was no preference in sex (F:M 169 [56%]:133 [44%]). The average age of onset (n = 156), defined by the first pneumonia, significant increase of severity, or frequency of other infections that led to the presentation at an immunodeficiency center, was 27 plus or minus 17 years (median, 25 years; range, 2-71 years), and the average age at diagnosis (n = 289) was 35 years (mean, 35 ± 16 years; median, 33 years; range, 3-74 years). The delay in diagnosis (n = 156) averaged 8 plus or minus 10 years (median, 4 years; range, 0-59 years). Interestingly, the mean age of onset and diagnosis was later for women (29 ± 17 years and 37 ± 16 years) than for men (25 ± 18 years and 32 ± 16 years, P = .2 not significant and P = .008), as has been reported previously for the New York cohort.2
Lymphoproliferative disease.
In 115/284 patients (40.5%), the spleen was enlarged. There was no detectable difference in the incidence between men (55/121 patients, 45%) and women (60/162 patients, 37%). No significant differences were detectable for the age of onset or diagnosis in patients with or without splenomegaly.
Lymphadenopathy was described in 68/260 patients (26.2%) without detectable differences in sex, age of onset, or age of diagnosis between patients with and without lymphadenopathy.
Autoimmune phenomena.
The autoimmune phenomena consisted mostly of autoimmune cytopenias that were present in 43 of 213 patients (20.2%) without significant differences in sex and age of diagnosis between patients with and without autoimmune cytopenia. The age of onset of immunodeficiency seemed to be delayed in patients with autoimmune cytopenia (23 ± 14 years vs 34 ± 18 years), but because of low numbers, the difference did not reach significance. A total of 64% of the patients with autoimmune cytopenia suffered from ITP, 25% from AIHA, and 11% from both.
Other autoimmune phenomena included pernicious anemia (9 patients), autoimmune thyroiditis (5 patients), arthritis (9 pa-tients), psoriasis (2 patients), antinuclear antibody–positive connective tissue disease-like syndromes (5 patients), and others (9 patients). There was no difference between the sexes.
Granulomatous disease.
Granulomatous disease was histologically proven in 35 of 303 patients (11.6%). Because the negative result cannot be definitive, all other patients were assumed to be negative. There were no significant differences between men and women or with regard to age of onset or diagnosis between the groups.
Summary of clinical data.
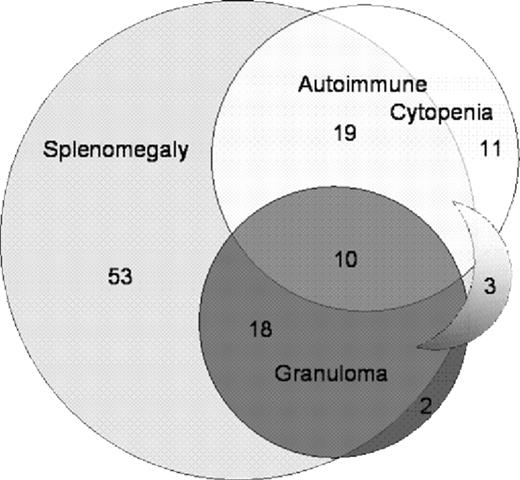
The European cohort demonstrates a strong association among granulomatous inflammation, autoimmune phenomena, and splenomegaly (Figure 1) in patients with CVID. Granulomatous disease is almost exclusively detectable in patients with splenomegaly (28/33 patients, P < .001); however, autoimmune cytopenia (P = .006), other autoimmune phenomena (P = .003), and lymphadenopathy (P = .001) are also significantly associated with splenomegaly. Similarly, there is a significant coincidence of autoimmunity (autoimmune cytopenia [P = .006] and other autoimmune phenomena [P < .001]) with granulomatous disease. Patients with granulomatous disease present more often with concomitant lymphadenopathy (P = .008).
Coincidence of granulomatous disease and autoimmune cytopenia with splenomegaly in patients with CVID. The diagram indicates the coincidence of splenomegaly, granulomatous disease, and autoimmune cytopenia in the European cohort of CVID patients. In 3 patients, granulomatous disease and autoimmune cytopenia were detectable in the absence of splenomegaly (sickle shape).
Coincidence of granulomatous disease and autoimmune cytopenia with splenomegaly in patients with CVID. The diagram indicates the coincidence of splenomegaly, granulomatous disease, and autoimmune cytopenia in the European cohort of CVID patients. In 3 patients, granulomatous disease and autoimmune cytopenia were detectable in the absence of splenomegaly (sickle shape).
Immunologic analysis of the European multicenter cohort of CVID patients
Immunoglobulin serum levels.
Usually in all 3 immunoglobulin classes, but especially IgG and IgA, levels are reduced in patients with CVID. Serum IgG levels before substitution (2.1 ± 1.65 g/L; normal range, 7-16 g/L) were available for analysis in 181 patients. In 62 of 181 patients, serum IgG levels were below 1 g/L (34%) on initial presentation. Serum IgA levels were recorded in 257 patients. One patient had elevated IgA levels, 10 patients (3.9%) had normal IgA levels (0.8-1.9 g/L; normal range, 0.7-4.0 g/L), 62 patients had levels between 0.2 and 0.7, and 129 (50%) had no detectable levels (less than 0.2 g/L; Table S2).
IgM levels were increased (2.7, 2.8, 4.8, and 7.3 g/L, respectively) in 4 patients without a known defect in class switch recombination. Normal levels of IgM (0.4-2.3 g/L) were found in 53/268 patients, whereas 211 of 268 patients (79%) had decreased IgM levels. In 81 of these 211 patients (30%), IgM was not detectable.
Peripheral lymphocyte homeostasis.
In most CVID patients, the percentage of total B cells was within the lower normal range (B cells 9.7 ± 6.8%; normal range, 6%-19%, 144 ± 133/μL, normal range, 100-500 cells/μL). Less than 10% of patients presented with less than 1% of B cells (data not shown). These patients were not included in the additional analysis of B-cell subpopulations.
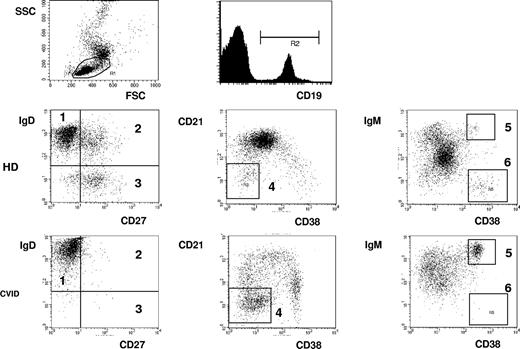
The circulating B-cell pool consists of up to 6 distinct populations (Figure 2), and the distribution of these subpopulations reflects the antigen-independent and -dependent differentiation of B cells in primary and secondary lymphoid tissues. So it is possible to distinguish naive IgM+IgD+CD27− B cells from IgM+IgD+CD27+ marginal zone like B cells and IgD−CD27+switched memory B cells, CD38hiIgMhi tran-sitional B cells from activated CD21lowCD38low B cells and CD38+++IgM− class switched plasmablasts.
Flow cytometric analysis of B-cell subpopulations. PBMCs are analyzed by flow cytometry. After gating on lymphocytes according to forward (FSC)/side scatter (SSC), B cells are characterized by CD19 staining. By staining for CD27 and IgD, naive IgD+IgM+CD27− B cells (1), IgD+IgM+CD27+ marginal zone B cells (2), and IgD−IgM−CD27+ switched memory B cells (3) can be distinguished. The staining for CD21 and CD38 expression allows the additional distinction of CD38lowCD21low B cells, (4) CD38++IgMhigh transitional B cells (5), and CD38+++IgM− plasmablasts (6). Examples of one healthy donor (HD) and one CVID patient (CVID) are demonstrated.
Flow cytometric analysis of B-cell subpopulations. PBMCs are analyzed by flow cytometry. After gating on lymphocytes according to forward (FSC)/side scatter (SSC), B cells are characterized by CD19 staining. By staining for CD27 and IgD, naive IgD+IgM+CD27− B cells (1), IgD+IgM+CD27+ marginal zone B cells (2), and IgD−IgM−CD27+ switched memory B cells (3) can be distinguished. The staining for CD21 and CD38 expression allows the additional distinction of CD38lowCD21low B cells, (4) CD38++IgMhigh transitional B cells (5), and CD38+++IgM− plasmablasts (6). Examples of one healthy donor (HD) and one CVID patient (CVID) are demonstrated.
The most common aberration in CVID is the reduction of IgM−IgD−CD27+switched memory B cells (3.4 ± 5.2% of B cells, normal range 6.5%-29.2%, absolute counts 5 ± 8/μL). Thus, 176 of 303 patients (58%) have less than or equal to 2%, and 10 patients (3.3%) have no detectable switched memory B cells in the peripheral blood. The percentage of circulating switched memory B cells correlates with serum IgA and IgG levels (P = .001 and P = .005).
IgM+IgD+CD27+ marginal zone B cells (14.3 ± 13.6% of B cells, normal range 7.2%-30.8%, absolute counts 23 ± 40/μL) are less consistently affected. A severe reduction to 2% and less is present in only 32 of 303 patients (10.5%). All but 2 of 32 have severely reduced switched memory B cells less than or equal to 2% at the same time. There is no correlation between the percentage or absolute number of marginal zone B cells and serum IgM levels (P = .6 and P = .4, respectively).
The percentage of CD38hiIgMhi transitional B cells (6.1 ± 7.6% of B cells; normal range, 0.6%-3.5%, absolute counts, 8 ± 12/μL) is slightly higher in CVID patients. A strong expansion (at least 9% transitional B cells) is found in approximately 15% (33/211) of patients and is associated with a reduced number of marginal zone B cells (5.5 ± 6.1% vs 15.1 ± 13.1%, P < .001 and 5 ± 7cells/μL vs 24 ± 36 cells/μL, P < .001). There is no correlation between the absolute number of B cells and the percentage of transitional B cells (P = .4) as one would expect in case the immune system attempts to compensate for low B-cell numbers.
In addition, the blood of some CVID patients contains an expanded CD21low B-cell population (13.3 ± 13.4% in 229 of 303 patients, normal range 1.1%-6.9%, absolute counts 21 ± 39/μL), characterized by the low expression of CD21 and CD38 and increased expression of CD1913 (not shown). These B cells are rare in the blood of healthy controls but can be found in patients with autoimmune disease.14
Finally, the percentage of CD38+++IgM− class-switched plasmablasts in the peripheral blood is reduced in most CVID patients compared with normal controls (0.79 ± 1.73% of B cells, normal range 0.4%-3.6%, absolute counts 1 ± 2/μL). The occurrence of these plasmablasts correlated significantly with the presence of switched memory B cells (P < .001).
Further analysis of lymphocyte subpopulations was restricted to total T cells, CD4 and CD8 T cells, as well as natural killer (NK) cells. Most patients have normal T-cell numbers (1204 ± 806/μL, normal range 700-2100/μL), total CD4 (617 ± 357/μL, normal range 300-1400/μL) and CD8 T-cell (537 ± 553/μL, normal range 200-900/μL) and NK-cell numbers (170 ± 134/μL, normal range 90-600/μL; data not shown).
Association of clinical parameters and B-cell homeostasis.
One major goal of the trial was to identify immunologic surrogate markers for clinical events such as splenomegaly, lymphadenopathy, granulomatous disease, and autoimmunity. Splenomegaly is associated with major disturbances of B-cell homeostasis (Figure 3A). Patients with splenomegaly tend to have a lower percentage of total B cells (8.7 ± 6.1% vs 10.5 ± 7.2%, P = .02), MZ B cells (11.8 ± 11.1% vs 14.7 ± 13.2%, P = .05), as well as switched memory B cells (2.4 ± 5.1% vs 4.0 ± 5.3%, P = .01). The decrease of switched memory B cells becomes highly significant when analyzed as percentage of lymphocytes instead of B cells (0.18 ± 0.28% vs 0.42 ± 0.62%, P = .002) as well as absolute counts (3 ± 6/μL vs 6 ± 9/μL, P = .004). However, the best marker for splenomegaly is an increased percentage of CD21low B cells (patients with splenomegaly 17.7 ± 15.3% vs patients without splenomegaly 10.1 ± 11.0% of B cells, P < .001, absolute counts 28 ± 53/μL vs 15 ± 23/μL).
Association of clinical phenomena with dysregulated B-cell subpopulations. The box plots indicate the most significant dysregulations of B-cell subpopulations in patients with splenomegaly (A), lymphadenopathy (B), and granulomatous disease (C). Boxes indicate median, 25th and 75th percentiles. The bars indicate minimum and maximum values, excluding outliers and extreme values. PBL indicates peripheral blood lymphocytes.
Association of clinical phenomena with dysregulated B-cell subpopulations. The box plots indicate the most significant dysregulations of B-cell subpopulations in patients with splenomegaly (A), lymphadenopathy (B), and granulomatous disease (C). Boxes indicate median, 25th and 75th percentiles. The bars indicate minimum and maximum values, excluding outliers and extreme values. PBL indicates peripheral blood lymphocytes.
Lymphadenopathy was associated with a relative and absolute increase of transitional B cells (9.5 ± 10.6% vs 4.8 ± 5.5% of B cells, P = .008; 13 ± 19/μL vs 6 ± 7/μL, P = .005, Figure 3B).
Granulomatous disease was significantly connected with a severe reduction of switched memory B cells (1.2 ± 1.0% vs 3.7 ± 5.4% of B cells, P < .001; 2 ± 3/μL vs 5 ± 8/μL, P < .001; Figure 3C) and to a lesser extent with reduced marginal zone B cells (9.0 ± 9.1% vs 15.0 ± 13.9% of B cells, P < .001; 12 ± 24/μL vs 25 ± 42/μL, P < .02).
The only significant association to autoimmune cytopenia existed with near absent absolute numbers of plasmablasts (0 ± 0.2/μL vs 1 ± 2.1/μL, P < .001), which was not included in further consideration for classification purposes because of the very low numbers in both groups.
Comparison of classification schemes.
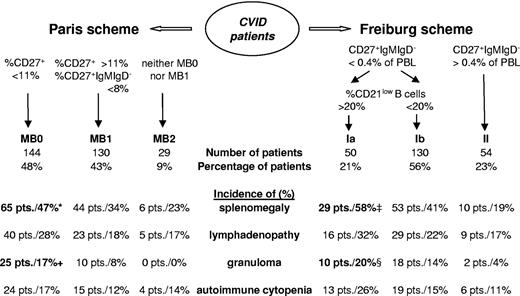
The second major goal of the study was to test and unify the existing classification schemes for patients with CVID. A total of 303 patients were classified according to the Paris classification (Figure 4). Most patients have a reduction of both CD27+ B-cell populations (MB0 = 144 patients, 48%) or at least of switched memory B cells (MB1 = 130 patients 43%), with only a few patients with nearly normal CD27+ B-cell populations (MB2 = 29 patients, 9%). The comparison of the groups revealed a significant (P = .03) increase of splenomegaly in group MB0 (47%) compared with MB2 (23%). Granulomatous disease is more common in group MB0 (17.4%) than group MB1 (7.8%, P = .02) and absent in the 29 patients in group MB2.
Evaluation of the Paris and Freiburg classification scheme. Classification of the European cohort according to the Paris9 and the Freiburg8 classification schemes is shown in the bottom half of the figure. Absolute and relative numbers of patients are indicated. Percentages refer to the prevalence of the indicated clinical phenomenon in the respective subgroup of patients. Significant differences in the prevalence between subgroups are indicated (*P = .03 compared with MB2; + P = .02 compared with MB1; ‡P = .04 compared with group 1b, P < .001 compared with group 2; §P = .02 compared with group 2).
Evaluation of the Paris and Freiburg classification scheme. Classification of the European cohort according to the Paris9 and the Freiburg8 classification schemes is shown in the bottom half of the figure. Absolute and relative numbers of patients are indicated. Percentages refer to the prevalence of the indicated clinical phenomenon in the respective subgroup of patients. Significant differences in the prevalence between subgroups are indicated (*P = .03 compared with MB2; + P = .02 compared with MB1; ‡P = .04 compared with group 1b, P < .001 compared with group 2; §P = .02 compared with group 2).
A total of 234 patients were classified according to the Freiburg classification (Figure 4). Of these patients, 77% (180) fell into group 1, including 50 patients with a high percentage of CD21low B cells (group 1a). Nearly a quarter of the analyzed patients classify as type 2 (54 patients). The incidence of splenomegaly is significantly increased in group 1a (59%) compared with group 1b (42%, P = .04) and group 2 (19%, P < .001). Granulomata are more commonly found in group 1a (20%) than in group 2 (3.7%, P = .02).
Because the analysis of clinical and immunologic parameters had suggested switched memory B cells, transitional B cells, and CD21low B cells as the most relevant B-cell parameters for classification, a new consensus European classification (EUROclass) was based on these parameters. Relevant cutoffs for these parameters were optimized to distinguish patients with and without granulomatous disease, lymphadenopathy, and splenomegaly.
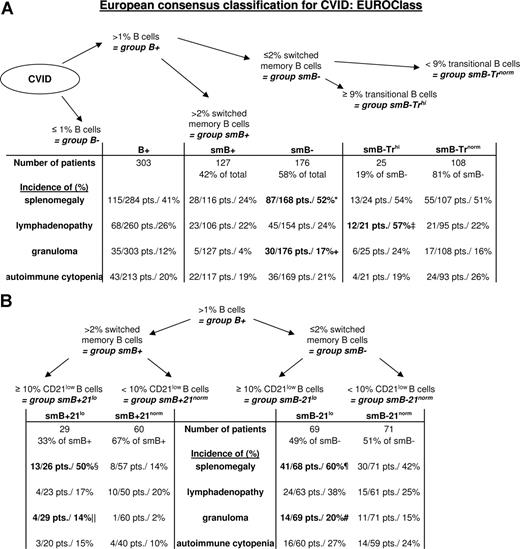
At first, EUROclass distinguishes between CVID patients with less than or equal to 1% B cells (group B−) and those with more than 1% B cells (group B+, Figure 5). To identify patients with a severe defect in the generation of a class-switched memory B-cell compartment group, B+ is separated into group smB− with less than or equal to 2% switched memory B cells (smB−, 176 patients) and group smB+ with more than 2% switched memory B cells (smB+, 127 patients). This severe reduction of class-switched B-cell memory is associated with a significant decrease in serum IgG (1.8 ± 1.6g/L vs 2.5 ± 1.6 g/L, P = .008) and IgA (0.12 ± 0.24g/L vs 0.24 ± 0.58 g/L, P = .04) at the time of diagnosis. Absolute numbers of marginal zone B cells (14 ± 30 cells/μL vs 36 ± 48 cells/μL, P < .001) as well as absolute and relative numbers of plasmablasts (0.6 ± 1 cells/μL vs 1.8 ± 2.9 cells/μL, P = .002; 0.5 ± 0.7% of B cells vs 1.3 ± 2.6% of B cells, P = .002) were severely decreased in patients of group smB−. The incidence of splenomegaly (87/168 patients in smB− vs 28/116 patients in smB+, P < .001) and granulomatous disease (30/176 patients in smB− vs 5/127 patients in smB+, P = .001) is increased in patients of group smB−.
The consensus EUROclass classification scheme. The figure depicts the new consensus classification scheme according to transitional B cells (A) CD21low B cells (B). B indicates total B cells; smB, switched memory B cells; Tr, transitional, and 21, CD21low B cells. Patients in group B− with less than 1% B cells were not examined in this study. The prevalence of the respective clinical phenomena is listed for each subgroup, and significant differences in the prevalence between subgroups are indicated. Because data on transitional cells and CD21low B cells were not available in 43 and 74 patients, respectively, the missing patients were excluded from the subgroup analysis. *P < .001 compared with smB+. +P < .01 compared with smB+. ‡P = .002 compared with smB− Trnorm as well as to smB+. §P = .009 compared with smB+ 21norm. ‖P = .049 compared with smB+ 21norm. ¶P = .03 compared with smB− 21norm and P < .001 compared with smB+ 21norm, #P = .016 compared with smB+ 21norm.
The consensus EUROclass classification scheme. The figure depicts the new consensus classification scheme according to transitional B cells (A) CD21low B cells (B). B indicates total B cells; smB, switched memory B cells; Tr, transitional, and 21, CD21low B cells. Patients in group B− with less than 1% B cells were not examined in this study. The prevalence of the respective clinical phenomena is listed for each subgroup, and significant differences in the prevalence between subgroups are indicated. Because data on transitional cells and CD21low B cells were not available in 43 and 74 patients, respectively, the missing patients were excluded from the subgroup analysis. *P < .001 compared with smB+. +P < .01 compared with smB+. ‡P = .002 compared with smB− Trnorm as well as to smB+. §P = .009 compared with smB+ 21norm. ‖P = .049 compared with smB+ 21norm. ¶P = .03 compared with smB− 21norm and P < .001 compared with smB+ 21norm, #P = .016 compared with smB+ 21norm.
Further analysis of the B-cell phenotype identified a subgroup of group smB− with high relative and absolute numbers of transitional B cells. This group smB− Trhi, with equal to or greater than 9% of transitional B cells, clusters patients with lymphadenopathy (12/21 patients, 57%, P = .002 compared with smB− Trnorm as well as smB+). Interestingly, this group showed a significant reduction of relative as well as absolute numbers of marginal zone B cells (5.0 ± 4.8% of B cells vs 10.8 ± 10.1% [smB− Trnorm] and 21.0 ± 15.1% [smB+] P < .001 for both; 6 ± 7 cells/μL vs 17 ± 34 cells/μL [smB− Trnorm] and 36 ± 48 cells/μL [smB+], P = .006 and P < .001, respectively).
In addition, subgroups with an expansion of CD21low B cells above 10% of B cells are designated “21lo.” This expansion of CD21low B cells is significantly more common in group smB− (69/140 patients) than group smB+ (29/89 patients, P = .01). The incidence of splenomegaly (41/68 patients) was significantly increased in smB− 21lo patients compared with the subgroups without expanded CD21low B cells (smB− 21norm: P = .03, smB+ 21norm: P < .001, Figure 5). This association was independent of the reduction of switched memory B cells because smB+ 21lo patients also presented more commonly with splenomegaly than smB+ 21norm patients (P = .009). Granulomatous disease was also more common in subgroup smB− 21lo (14/69), whereas it was nearly absent in group smB+ 21norm (1/60, P = .016, Figure 5). There is a significant delay in the onset (31 ± 15 years) and therefore diagnosis (36 ± 14 years) of immunodeficiency in this group compared with patients in group smB− 21norm (19 ± 13 years and 28 ± 13 years, P = .006 and P = .02, respectively).
Discussion
The classification of primary antibody deficiency syndromes needs to address clinical as well as pathogenic concepts. Although the increased frequency of respiratory tract infections is common to nearly all patients, the incidence of lymphoproliferative, granulomatous, and autoimmune disease marks distinct clinical subgroups. This study reveals a significant coincidence of these 3 complications and thereby verifies unconfirmed observations of others.15 These patients suffer from a worse outcome,15,16 possibly due to delayed diagnosis (our data and Morimoto et al15 ) and secondary complications rendering the early identification of these patients critical. Although splenomegaly is easily detectable by ultrasound, granulomatous disease and lymphadenopathy may be undiscovered, and a surrogate marker for this clinical pattern is warranted. This subgroup also seems to manifest immunodeficiency later in life, and autoimmune phenomena often precede immunodeficiency.17 In CVID patients with granulomatous disease, an underlying viral infection has been suggested by several groups.18,19 In a small study, human herpes virus 8 (HHV8)19 infection was detectable in 6 of 9 patients with granulomatous and or interstitial lung disease, whereas the virus was found in only 1 CVID patient without apparent granulomatous disease and was undetectable in all controls. Unfortunately, no data are available on the B-cell phenotype of these patients. Lymphadenopathy is associated with splenomegaly in some patients but not in others, as has been observed previously,20 suggesting different pathomechanisms underlying these 2 forms of lymphoproliferation in the latter group of patients. The pathogenesis of human antibody deficiency is largely unknown. To classify patients according to pathogenic aspects, a classification discerning B-cell differentiation by the characterization of circulating B-cell subpopulations becomes an important tool.
In nearly 10% of patients with hypogammaglobulinemia, circulating B cells comprise less than 1% of lymphocytes. Several of these patients suffer from early differentiation defects of the B-cell lineage, whereas the defect in others is not known. Because of the difficulty of analyzing B-cell subpopulations in very low numbers and the current definition of CVID, patients with less than or equal to 1% B cells were labeled as “B−” and excluded from further analysis. However, this group will need to be revisited in future studies.
Of the other 90% of patients with hypogammaglobulinemia (B+), more than 80% of patients show reduced numbers of switched memory B cells, confirming previous results8 indicating a disturbed germinal center function.21 Because germinal centers are critical for T-dependent antibody responses, switched memory B cells were chosen by all classification systems8,9 as the primary classifying parameter. Expressing switched B-cell numbers as percentage of lymphocytes or absolute counts describes more closely the dysregulation of B-cell homeostasis in the presence of splenomegaly (P < .001) than switched B-cell numbers as the percentage of B cells (P = .01). However, for clinical practice, it is easier to use the latter as suggested by the Paris classification because no additional calculation of parameters is necessary. The optimal cutoff for switched memory B cells was determined at 2% of total B cells. CD40L deficiency22 and ICOS deficiency23 represent 2 genetic human defects in which germinal center reactions are abrogated. In both syndromes, switched memory B cells are reduced to less than 2% of B cells,24,25 corroborating the chosen limit. Based on these findings, patients with B-cell counts greater than 1% (B+) and less than or equal to 2% of switched memory B cells evidently suffer from disturbed germinal center development and are grouped into smB− in the new consensus classification. Serum IgA and IgG levels were significantly lower in this group compared with patients in group smB+, with greater than 2% of switched memory B cells (P = .008 and P = .04, respectively). However, this reduction of B-cell memory is not specific for CVID and was originally described for patients with X-linked Hyper-IgM syndrome25 , and since in patients with Wiskott Aldrich syndrome,26 X-linked lymphoproliferative disease (XLP),27 idiopathic CD4 lymphopenia,28 and chronic granulomatous disease.29 Interestingly, all CD19-deficient patients of the Colombian family also fall into the group smB−.30 smB− patients have a higher incidence of splenomegaly and granulomatous disease. It remains speculative whether splenomegaly and granulomatous disease precede the loss of the switched memory B-cell compartment or vice versa. Given the frequently observed sequential order with splenomegaly, granulomatous disease, and autoimmune cytopenia preceding symptoms of the immunodeficiency, this pathogenic consideration is attractive. The fact that the absence of switched memory B cells in CD40L deficiency is rarely associated with these clinical phenomena also makes it unlikely that the absence of memory B-cell differentiation per se predisposes for the manifestation of splenomegaly and granulomatous disease. The longitudinal observation of patients during the development of the immunodeficient state will be instructive.
Transitional B cells were the only B-cell subpopulation associated with lymphadenopathy in the multivariate analysis. Among smB− patients, a subgroup with a strong expansion of transitional B cells could be identified (group smB− Trhi). In these patients, marginal zone B cells were significantly reduced compared with other patients, and more than 50% of the patients presented with lymphadenopathy, suggesting a complex immune dysregulation in this subgroup of patients. Expansions of transitional B cells have been observed in XLP31 and idiopathic CD4 lymphopenia,28 but so far, there is no description of an association with lymphadenopathy. Interestingly, recent data (I. Quinti, R. Carsetti, manuscript submitted) suggest a direct succession of marginal zone B cells from transitional B-cell precursors, rendering smB− Trhi patients with increased transitional B cells and decreased marginal zone B cells candidates for a defective differentiation along this pathway.
The other form of lymphoproliferative disease, splenomegaly, is most significantly associated with the expansion of CD21low B cells (17.7 ± 15.3% vs 10.1 ± 11% of B cells in patients without splenomegaly, P < .001). Besides the reduction of switched memory B cells (P = .009), it is the only population with a significant association with splenomegaly in the multivariate analysis (P = .009), underlining the clinical relevance of these cells. This B-cell population was originally described as CD19hiCD21lo B cells13 , and since has developed into the present characterization CD21lowCD38lowCD19hi to separate this population from other CD21 low–expressing B-cell populations. The consensus classification identifies patients with expansion of these cells to greater than or equal to 10% of B cells (21lo patients). This grouping demonstrates the best discrimination of patients with splenomegaly and granulomatous disease (Figure 5). Patients in group smB− 21lo and smB+ 21lo had a significant later onset and diagnosis than other patients in groups smB− and smB+, whereas there was no difference between smB− 21lo and smB+ 21lo. Recently, Moratto et al32 examined 25 patients according to the Freiburg classification and confirmed the association of expanded CD21low B cells with splenomegaly and autoimmune disease. In addition, these patients suffered from a higher incidence of lower respiratory tract infections and chronic lung disease,32 neither of which was analyzed by the European trial. The immune dysregulation in these patients was extended to a severe reduction of naive CD45RA+ CD4 T cells, also described by Vlkova et al.33 The other findings of reduced total B-cell counts or CD4 T-cell counts in patients with expanded CD21low B cells cannot be confirmed by this study.
The Paris scheme indirectly includes IgM/IgD+CD27+ B cells as well into the classification. These cells originate most likely in the marginal zone.34 They develop in a germinal center–independent manner,34 but their reduction in most patients with ICOS as well as CD40L deficiency suggests a T-cell dependency of this subpopulation.24 In accordance with this, a severe reduction of these B cells (less than 2% of B cells) was almost exclusively found in patients with severely reduced switched memory B cells (30/176 patients in group smB− vs 2/127 patients in group smB+, P < .001). The prominent reduction of circulating IgM/IgD+CD27+ B cells in these few patients possibly implies defects described for murine models with absent marginal zones.24,35 Patients with splenomegaly had significantly decreased absolute numbers of marginal zone B cells (14 ± 26/μL vs 27 ± 43/μL, P = .005), whereas the relative decrease (percentage of B cells) was only weakly significant (11.8 ± 11.1% vs 14.7 ± 13.2%, P = .047) because of the concomitant decrease of switched memory B cells in most of the patients. EUROclass does not include IgM/IgD+CD27+ B cells as a classification parameter because the observed associations with clinical phenotypes were weaker compared with other B-cell subpopulations. However, recent work has demonstrated an important role of IgM/IgD+CD27+ B cells in the defense against capsulated bacteria36 that had not been known at the beginning of this trial. Therefore, further work needs to establish clinically relevant cutoffs for these B cells that then can be integrated into EUROclass.
EUROclass allows assigning 3 of the 4 known genetic defects associated with CVID (ICOS, CD19, and Baff-R) into specific groups. Only transmembrane activator and calcium modulator and cyclophilin ligand–interactor (TACI) deficiency is not linked to a specific B-cell phenotype, corroborating the current understanding that TACI is not involved in B-cell differentiation up to the stage of memory B cells but may be relevant in plasma cell differentiation. In addition, the heterogeneity of the B-cell phenotype in TACI deficiency is mostly likely also because some of the genetic variations in TACI are rather disease modifying more so than causing events in a complex immune dysregulation.
In summary, the evaluation of a large European cohort of patients with CVID confirms B-cell homeostasis as a pathogenic and clinically meaningful parameter for classification. Specific B-cell phenotypes indicate disturbed early B-cell differentiation, marginal zone differentiation, or germinal center development. The new classification is superior to previous models in the differentiation of clinical phenomena such as lymphadenopathy, splenomegaly, and granulomatous disease, whereas neither of the models is able to predict autoimmune phenomena. This classification will serve the definition of more homogeneic subgroups for additional genetic and functional analysis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank R. Draeger (Freiburg, Germany) for her excellent technical support and R. Carsetti for discussion.
This research was funded by the German Research Foundation (DFG) grants SFB620; DFG projects C1 to K.W. and H.H.P.; Ministry of Health, Czech Republic, grant NR/9035-4 to V.T. and J.L.; the Camillo Golgi Foundation, Brescia, to A.P; the Medical Research Fund of Tampere University Hospital to T.K. and M.V.; and EU funding in the 5th and 6th Frameworks for EUROPID QLQ1-CT-2001-01 395 and EUROPOLICY SP23-CT-2005-006411 to B.F., E.B., and H.C.
Authorship
Contribution: C.W. analyzed the data, wrote parts of the manuscript, and designed Figures 2,Figure 3,Figure 4–5. T.K. and M.V. performed the statistical analysis of data. C.S., B.F., T.E., A.D.W., H.C., E.O., and H.H.P. participated in the study design. P.M.v.H., A.P., R.E.S., I.Q., T.E., A.D.W., H.C., E.O., and H.H.P. provided patients and funds in their centers. C.S., B.F., T.W., P.D., M.S., J.L., A.P., V.T., I.Q., M.V., E.O., and H.H.P. critically read the manuscript. M.V., D.D., M.H., P.R.B., G.P., S. Gutenberger, M.S., F.B.-v.d.C., M.L.G., R.J., J.J., and E.B. performed the flow cytometric analysis. T.W., E.E., H.v.B., S. Goldacker, U.B., and J.L. cared for the involved patients and provided clinical data. K.W. designed and organized the study, analyzed the data, and wrote the manuscript.
C.W. and T.K. contributed equally to the project.
A complete list of the members of the EUROclass trial is provided in Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Klaus Warnatz, Division of Rheumatology and Clinical Immunology, Medical Center, University of Freiburg, Hugstetterstr. 55, 79106 Freiburg, Germany; e-mail: klaus.warnatz@uniklinik-freiburg.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal