Human cytomegalovirus (CMV) infection has been linked to inflammatory diseases, including vascular disease and chronic transplant rejection, that involve vascular endothelial damage. We have previously shown that the host CD4+ T-cell response to CMV antigen can produce IFNγ and TNFα at levels sufficient to drive induction of fractalkine, a key marker of inflammation in endothelial cells. We have also observed a major pathogenic effect in which endothelial cell damage and loss follow the induction of frac-talkine and up-regulation of cell adhesion markers in the presence of peripheral blood mononuclear cells (PBMCs) from donors with a high CMV-specific T-cell frequency. In this report, we show that the fractalkine-CX3CR1 interaction resulting in recruitment of natural killer (NK) cells and monocyte-macrophages plays an important role in mediating this endothelial damage. Supportive evidence for frac-talkine's key role is shown by the ability of specific antibody to CX3CR1 to reduce significantly CX3CR1+-bearing cell chemoattraction and to protect against endothelial damage. These findings support CMV as a member of a class of persistent pathogens in which a high T-cell response and chemokine-mediated effects are a risk factor for development of chronic inflammation and endothelial cell injury.
Introduction
Human cytomegalovirus (CMV), like all herpesviruses, establishes a latent infection for the life of the host and can periodically reactivate in the immunologically normal host.1,2 As an opportunistic pathogen, CMV infection causes significant rates of morbidity and mortality in immunocompromised populations, including transplant recipients, the developing fetus, and HIV-infected persons. There is also increasing evidence to associate CMV infection with inflammatory-related diseases.3,,,,,–9 The mechanisms through which CMV affects the pathogenesis of these inflammatory diseases are for the most part unknown.10
Vascular complications and transplant loss have been linked with CMV infection. Specific examples of disorders in which CMV may play a role include coronary artery disease, restenosis after angioplasty procedures, transplant vascular sclerosis (TVS) in chronic graft rejection, and CMV-associated systemic sclerosis.7,–9,11,,,,–16 Prior infection with CMV has been shown to be a strong independent risk factor for restenosis.7,8 CMV has also been found in atherosclerotic lesions and encodes several gene products to modulate the immune cell responses and vascular cell activities.4,17,,–20
Endothelial cell inflammation and damage play a major role in the development of vascular disease and involve the interaction between immune cells and key effector molecules, including chemokines. Of these, the fractalkine (CX3CL1)–fractalkine receptor (CX3CR1) interaction is a critical mediator in the host inflammatory response leading to vascular injury (reviewed in Hansson,4 Umehara et al,10 Feng et al,21 Imai et al,22 and Umehara and Imai23 ). The expression of fractalkine on activated endothelial cells contributes to leukocyte adhesion and can be secreted to form a chemoattractant gradient to induce migration of natural killer (NK) cells, monocytes, and specific CD8+ populations.23,,,–27 Frac-talkine expression is up-regulated on endothelial cells in cases of human atherosclerosis and TVS.4,28,,–31 In addition, cytomegalovirus-mediated up-regulation of chemokine expression, including frac-talkine, correlates with the acceleration of chronic rejection in a rat heart transplantation model.17,32
In our research, we have hypothesized that CMV-associated chronic endothelial cell inflammation and damage are mediated through induction of chemokines produced by activated endothelium, whereby endothelial cells are activated by the cytokine response from CMV antigen-specific T-cell stimulation. We have reported that the host CD4+ T-cell response to CMV antigen can produce IFNγ and TNFα at levels sufficient to drive induction of fractalkine, a key marker of inflammation in endothelial cells.10,27,33 In many seropositive persons, a relatively high percentage of the host T-cell response is invested in recognition of CMV antigens, emphasizing the potential of the host immune system to respond aggressively to CMV and contribute to a process of vascular inflammation leading to endothelial damage.34,–36 We have also observed a major pathogenic effect whereby endothelial cell damage and loss follow the induction of fractalkine and up-regulation of cell adhesion markers in the presence of peripheral blood mononuclear cells (PBMCs) from donors with relatively higher frequencies of CMV-specific T cells.37 These previous observations support the hypothesis that the endothelial damage is the result of a chemokine-mediated immunopathogenic effect. The current research was designed to test the role of chemokine interactions in mediating this damage process and to identify the primary immune effector cells responsible for endothelial damage. Our results show that interference with the fractalkine-CX3CR1 interaction protects the endothelial cells from damage by CX3CR1-bearing monocyte-macrophage and NK populations. This also highlights the important role of the fractalkine-CX3CR1 interaction during the course of the inflammatory cascade events initiated by the host T-cell response to CMV antigen in the endothelial setting.
Methods
Blood was obtained from volunteer donors through the University of California San Diego Center for AIDS Research Virology Core, and informed consent was obtained in accordance with the Declaration of Helsinki. This protocol was approved by the University of California San Diego Human Research Protocol Program Project 050307.
Cells
Human PBMCs were isolated from heparinized blood of healthy donors using density centrifugation over Ficoll-Hypaque (Pharmacia-Biotech, Piscataway, NJ). Donor CMV serostatus was determined by latex agglutination (Becton Dickinson, Sparks, MD). CD4+ T cells, CD8+ T cells, CD14+ cells, and NK cells were isolated from CMV seropositive donor PBMCs according to the manufacturer's instructions by using a negative selection MidiMACs system and LS+ immunomagnetic columns (Miltenyi Biotech, Cologne, Germany).
Primary human aortic endothelial cells (AECs) and culture medium were purchased from Clonetics (San Diego, CA); cells were used at low passage. All experiments were repeated with at least 2 different donors of AECs.
Cocultures of PBMCs or subset populations with AEC monolayers
Cocultures were established with resting confluent primary AEC monolayers, overlaid with either 106 PBMCs or CD4+ cells, except when specified. Cocultured cells were stimulated with heat-inactivated CMV strain AD169 and incubated in a humidified incubator for 72 hours at 37°C in 5% CO2. Cocultures were maintained in a 50:50 mix of RPMI/10% FCS and EGM-2 media/2% FCS (Clonetics) to support both cell types.
Transwell migration assays and specific antibody neutralization assays
The transwell inserts were 6.5-mm diameter, 5-μm pore size polycarbonate membranes, in 24-well polystyrene plates available from CoStar/Corning (Corning, NY). Coculture supernatants or control media samples were loaded into lower well chambers. Supernatants were prepared from either CMV seropositive or seronegative donor PBMCs cocultured with endothelial monolayers and stimulated with CMV antigen. For transwell migration assays, 4 × 106 PBMCs in a 150-μL volume of control media were loaded into the upper chamber well inserts of 24-well transwell migration assay plates and incubated for 3 hours. For each assay, 3 replicate wells were set up, and aliquots of the transmigrated populations were counted from each well independently and reported as the mean of the migrated cell number per milliliter (± SD).
Blocking assays
The following antibodies were used to block each chemokine or chemokine receptor: rabbit polyclonal IgG anti–human CX3CR1 (Torrey Pines BioLabs, Houston, TX), mouse monoclonal anti–human RANTES (R&D Systems, Minneapolis, MN), rabbit IgG (R&D Systems), mouse monoclonal anti–human CCR5 (R&D Systems), mouse monoclonal IgG2B (R&D Systems), and mouse monoclonal IgG (R&D Systems).
Endothelial damage assays and specific antibody-blocking assays
A 2-step assay for endothelial damage was used. First, PBMCs were set up in transwell migration assays as described in “Transwell migration assays and specific antibody neutralization monolayers” by using coculture supernatants previously collected from endothelial monolayers activated by CD4+ T-cell response to CMV antigen. In a parallel series of assays, PBMCs were treated with specific blocking antibody directed against CX3CR1 or RANTES, compared with untreated controls. PBMC samples were then placed in transwell chambers, over coculture supernatants or control media. In the second step, the transmigrated cell populations were collected and placed onto previously activated endothelial cell monolayers and induced for fractalkine. Endothelial cell damage and destruction were monitored over time, and the results were compared using the different antibodies to block specific chemokine receptor-ligand interactions.
Identification of PBMC subsets associated with endothelial damage
For initial demonstration of endothelial damage by CMV seropositive donor PBMCs, co-cultures were stimulated with CMV antigen and monitored for endothelial loss on day 5. Cells were fixed in BD Cytofix (BD Biosciences) on day 5. Samples were viewed using a Leica DMIL inverted microscope with Leica C PLAN lens at 20×/0.30 NA (Leica Microsystems, Bannockburn, IL), and 1× PBS as imaging solution. Images were acquired with a Nikon digital microscopy system consisting of a Nikon digital camera head with 5-megapixel CCD model DS-5M and a DS-L1 control unit (Nikon, Melville, NY). Images were processed with the control unit DS-L1 version 2.2 software (Nikon) and Microsoft Office PowerPoint version 2003 (Microsoft, Redmond, WA). For identification of PBMC subsets associated with endothelial damage, PBMCs or subset populations of CD14+, CD16+/56+, CD4+ T cells, and CD8+ T cells were cocultured with confluent AEC monolayers in 48-well plates over 5 days. PBMCs or subsets were overlaid at a ratio of 20:1 cells per AEC. The cocultures were then stimulated with CMV antigen and monitored for endothelial loss on day 5. Cells were fixed in BD Cytofix (BD Biosciences) on day 5. Samples were viewed using a Nikon Diaphot inverted phase-contrast microscope (Nikon) with Nikon CFDL lens at 20×/0.40 NA, and 1× PBS as imaging solution. Images were acquired using a Nikon digital microscopy system and processed as described for demonstration of endothelial damage by CMV antigen-stimulated PBMCs. Results were quantitated using cell counts on fixed samples with remaining adherent endothelial cells per field of view (FOV), using the same Nikon microscope and lens as described for sample viewing. Negative controls included untreated AEC wells and AECs with the same ratio of PBMCs, but without viral antigen stimulation. Three FOVs were counted per sample, and counts were expressed as mean of cell counts or mean percentage of negative control untreated sample plus or minus SD.
ELISA
Soluble fractalkine (CX3CL1) was detected in culture supernatants with an enzyme-linked immunoabsorbent assay (ELISA) detection kit (no. DY365 DuoSet System for human fractalkine/CX3CL1; R&D Systems). For detection of RANTES and MIP-1β, human cytokine kits were obtained from R&D Systems, and assays were performed in duplicate as per protocol.
Flow cytometry
Cells were surface labeled using antibodies specific for the following: CD45+ (CyChrome conjugated; BD Biosciences), CD16+/CD56+ (phycoerythrin [PE] conjugated; BD Biosciences), CD14+ (antigen-presenting cell [APC] conjugated; BD Biosciences), CD3+ (PE conjugated; BD Biosciences), CD8+ (PE conjugated; BD Biosciences), CD4+ (APC conjugated; BD Biosciences).
Results
Endothelial cell damage is associated with fractalkine induction in the presence of CMV-seropositive donor PBMCs treated with CMV antigen
Previously, we had shown that the CD4+ T-cell response to CMV antigen can drive induction of fractalkine in endothelial cells and that TNFα and IFNγ produced by the T-cell response are critical to fractalkine induction.33 In these studies, CD4+ T cells from CMV seronegative donors consistently failed to induce fractalkine in endothelial cells.33 We had also shown that endothelial damage and loss follow induction of fractalkine in the presence of PBMCs from CMV-seropositive donors stimulated with CMV antigen.37 Increased cell migration together with up-regulation of cell adhesion markers indicated that this damage occurred through a chemokine-mediated process.37 Because the endothelial damage occurred consistently with donors having a high CMV-specific T-cell response, we decided to focus further experiments on using these donors to study the chemokine and immune effector cells involved in endothelial damage.
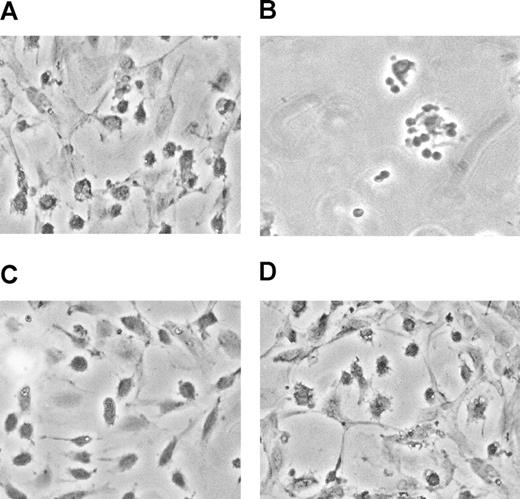
For these experiments, PBMCs were collected from CMV-seropositive donors with a high T-cell frequency to CMV and were added as suspension cell cultures to primary AEC monolayer cultures. High CMV-specific T-cell response donors were defined as those donor PBMCs that produced high levels of fractalkine and other chemokines in culture.37 The cultures were treated with heat-inactivated CMV to stimulate CMV antigen-specific CD4+ T cells. By day 3 of coculture, the fractalkine levels secreted into the culture supernatants had reached peak levels (data not shown). An example of this is shown on day 5, when extensive damage and loss of the endothelial monolayers was observed, with only isolated cell debris and PBMC clusters remaining (Figure 1B). This is in contrast to the intact endothelial monolayers (Figure 1A) in which the same donor PBMCs have been maintained in culture with endothelial cells, but in the absence of CMV antigen. No damage or fractalkine induction was detected in the endothelial cells when there was no antigen source to stimulate the CD4+ T cells and produce IFNγ and TNFα or with PBMCs obtained from CMV-seronegative donors exposed to CMV antigen (Figure 1D).
CMV-seropositive donor PBMCs exposed to CMV antigen results in endothelial cell damage in cocultures. (A) PBMCs isolated from a CMV-seropositive donor and cocultured with endothelial cells after 5 days are shown. (B) The same donor PBMCs cocultured on endothelial monolayers for 5 days are shown, in the presence of CMV antigen. (C) Control in which endothelial monolayers alone were maintained for 5 days. (D) CMV-seronegative donor PBMC exposed to the same conditions had no effect on endothelial cell monolayers and are indistinguishable from panel C. Results are representative of 6 different CMV-seropositive and -seronegative donor pairs.
CMV-seropositive donor PBMCs exposed to CMV antigen results in endothelial cell damage in cocultures. (A) PBMCs isolated from a CMV-seropositive donor and cocultured with endothelial cells after 5 days are shown. (B) The same donor PBMCs cocultured on endothelial monolayers for 5 days are shown, in the presence of CMV antigen. (C) Control in which endothelial monolayers alone were maintained for 5 days. (D) CMV-seronegative donor PBMC exposed to the same conditions had no effect on endothelial cell monolayers and are indistinguishable from panel C. Results are representative of 6 different CMV-seropositive and -seronegative donor pairs.
Increased chemoattraction is associated with factors present in supernatants from antigen-stimulated CMV-seropositive donor PBMCs
Previous results had shown that factors present in supernatants from antigen-stimulated CMV-seropositive donor PBMCs support increased chemoattraction of PBMCs.37 In the following assays, we extensively examined whether background factors could account for the increased chemoattraction associated with CMV antigen stimulation. Transwell migration assays were set up to compare the levels of chemoattraction between coculture supernatants generated from CMV-seropositive donors versus -seronegative donors.
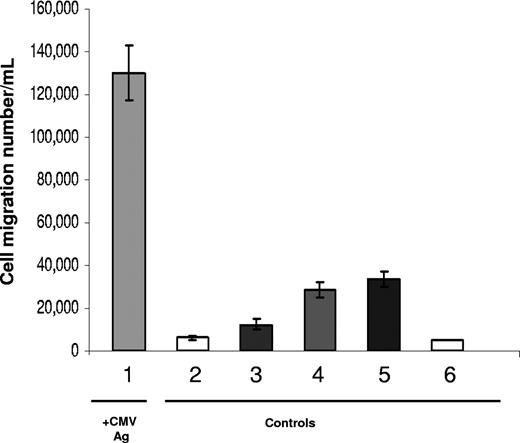
The highest numbers of transmigrated cells (1.3 × 105 ±1.25 × 104 cells/mL) were observed with supernatants from endothelial cell/CMV-seropositive PBMCs stimulated with CMV antigen (Figure 2). In contrast to these results, an approximate 20-fold decrease in the numbers of transmigrated cells (6.25 × 103 ± 1.7 × 103 cells/mL) were observed with supernatants prepared from cocultures of CMV-seropositive PBMCs without CMV antigen stimulation. Transmigration results with cocultures prepared from CMV-seronegative PBMCs with CMV antigen were also 4.5-fold lower (1.25 × 104 ± 2.5 × 103 cells/mL) and 10-fold lower without CMV antigen present (2.8 × 104 ± 3.7 ×103 cells/mL). The chemoattraction by resting AEC culture supernatants was not due to media factors only, because background migration rates tested with chemokine-negative control media resulted in the lowest levels of transmigrated cells at 5 × 103 (± 2.5 × 102) cells/mL.
Factors present in supernatants from antigen-stimulated CMV-seropositive donor PBMCs support chemoattraction of PBMCs. Results are shown for transmigrated populations from cell migration assays with coculture supernatants representative of a CMV-seropositive donor and -seronegative donor. PBMCs were loaded into the upper chambers of transwells to test migration into coculture supernatants as described in “Transwell migration assays and specific antibody neutralization assays.” Sample 1 was CMV-seropositive donor PBMCs + AECs + CMV antigen, 2 was CMV-seropositive donor PBMCs + AECs, 3 was CMV-seronegative donor PBMCs + AECs + CMV antigen, 4 was CMV-seronegative donor PBMCs + AECs, 5 was AEC cultures, resting, and sample 6 was RPMI/1% FCS. For each sample, 3 transmigration wells were set up, and the transmigrated populations were counted from each well independently. Results are shown as the mean (± SD) in cells per milliliter and are representative of 3 independent assays with different CMV-seropositive and -seronegative donor pairs compared in each.
Factors present in supernatants from antigen-stimulated CMV-seropositive donor PBMCs support chemoattraction of PBMCs. Results are shown for transmigrated populations from cell migration assays with coculture supernatants representative of a CMV-seropositive donor and -seronegative donor. PBMCs were loaded into the upper chambers of transwells to test migration into coculture supernatants as described in “Transwell migration assays and specific antibody neutralization assays.” Sample 1 was CMV-seropositive donor PBMCs + AECs + CMV antigen, 2 was CMV-seropositive donor PBMCs + AECs, 3 was CMV-seronegative donor PBMCs + AECs + CMV antigen, 4 was CMV-seronegative donor PBMCs + AECs, 5 was AEC cultures, resting, and sample 6 was RPMI/1% FCS. For each sample, 3 transmigration wells were set up, and the transmigrated populations were counted from each well independently. Results are shown as the mean (± SD) in cells per milliliter and are representative of 3 independent assays with different CMV-seropositive and -seronegative donor pairs compared in each.
Fractalkine is the dominant chemoattractant of PBMCs present within coculture supernatants from CMV-seropositive donors
The next set of experiments was designed to determine whether the types and concentrations of chemokines present in the coculture supernatants were sufficient to support chemoattraction of PBMCs. Antigen-stimulated endothelial coculture supernatants prepared from high T-cell response CMV-seropositive donor PBMCs contained several chemokines, including frac-talkine, RANTES, and MIP-1β.37 Of the elevated chemokines, fractalkine was detected at the highest levels in antigen-stimulated cultures (mean of 210 ng/mL), whereas it was undetectable in control cultures. The mean induction levels (by day 3 in coculture for these high T-cell response donors) of MIP-1β was 5500 pg/mL and 28 000 pg/mL for RANTES; both were undetectable in control cultures.37 The high level of induction of fractalkine between antigen-stimulated and unstimulated conditions, together with the endothelial damage, suggested that fractalkine might play a critical role in coordinating the events associated with the endothelial cell destruction. RANTES was also selected for further study because it was induced to high levels, and many of the cell types that respond to fractalkine also have receptors for RANTES.
Specific blocking antibody assays were used in transwell assays to determine the relative contribution of fractalkine and RANTES in attracting PBMCs. Because only the fractalkine-induced cultures from CMV-seropositive donors were associated with endothelial damage, we focused on using those culture supernatants for the endothelial damage assays. Results from the transwell migration assays show that chemokines present in the coculture supernatants can support chemoattraction of PBMCs (Figure 3). The highest numbers of transmigrated cells (9 × 105 ± 9.89 × 104 cells/mL) were observed when there was no antibody treatment to interfere with chemokine functions (Figure 3). In contrast, lower numbers of transmigrated cells were observed in the chemokine-negative control media, at 2.9 × 105 (± 6 × 104) cells/mL (data not shown).
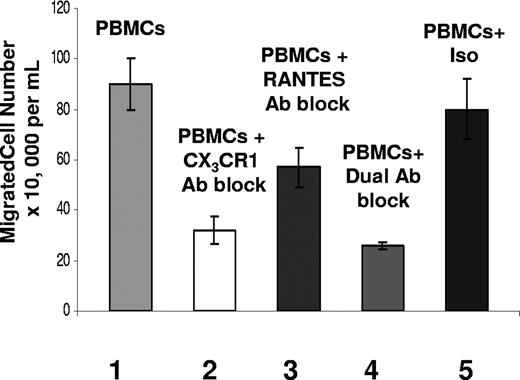
Fractalkine has the greatest effect on chemoattraction. Transwell migration assay results after 3 hours, showing total cell numbers of PBMC populations migrating in response to chemokines present in coculture supernatants from the CMV antigen-stimulated CD4+ T cells with endothelial monolayer samples. Sample sets on x-axis are as follows. Sample set 1 was total cell numbers of PBMC populations transmigrating in response to untreated coculture supernatants; sample set 2 was PBMCs treated with anti–human CX3CR1 antibody transmigrating in response to coculture supernatants; sample set 3 was PBMCs transmigrating in response to coculture supernatants treated with anti–human RANTES antibody; sample set 4 was PBMCs treated with anti–human CX3CR1 antibody and anti–human RANTES antibody; and sample set 5 was PBMCs treated with isotype antibodies rabbit IgG and mouse IgG. Each sample represents the results from 3 replicate transwell migrations in which the transmigrated populations were counted from each well independently. Results are shown as the mean (± SD) and are representative of 5 assays with different seropositive donors.
Fractalkine has the greatest effect on chemoattraction. Transwell migration assay results after 3 hours, showing total cell numbers of PBMC populations migrating in response to chemokines present in coculture supernatants from the CMV antigen-stimulated CD4+ T cells with endothelial monolayer samples. Sample sets on x-axis are as follows. Sample set 1 was total cell numbers of PBMC populations transmigrating in response to untreated coculture supernatants; sample set 2 was PBMCs treated with anti–human CX3CR1 antibody transmigrating in response to coculture supernatants; sample set 3 was PBMCs transmigrating in response to coculture supernatants treated with anti–human RANTES antibody; sample set 4 was PBMCs treated with anti–human CX3CR1 antibody and anti–human RANTES antibody; and sample set 5 was PBMCs treated with isotype antibodies rabbit IgG and mouse IgG. Each sample represents the results from 3 replicate transwell migrations in which the transmigrated populations were counted from each well independently. Results are shown as the mean (± SD) and are representative of 5 assays with different seropositive donors.
Using blocking antibody targeting CX3CR1 present on PBMCs, a 65% decrease was seen in the total number of transmigrated cells for the CX3CR1-blocked sample (Figure 3). Although there was a decline in migrated cells, the overall contribution of RANTES to chemoattraction of PBMCs in coculture was consistently less than that of fractalkine. This is emphasized again using a combination of antibodies against CX3CR1 and RANTES whereby the total number of transmigrated cell populations was decreased by a mean of 71%, compared with a mean of 65% for blocking CX3CR1 alone (Figure 3). Results using an anti-CCR5 block were consistent with those using the anti-RANTES block (data not shown). These findings indicate that the fractalkine-CX3CR1 interaction plays a central role in chemoattraction of PBMC populations, whereas RANTES plays less of a role.
Blocking fractalkine-CX3CR1 binding has the greatest effect on migration of CD14+ cells
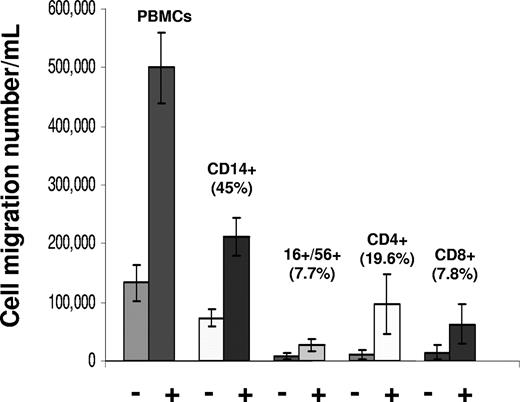
Previously, we had shown by flow cytometry that the main cell populations responding to chemokines present in the antigen-stimulated CMV-seropositive donor PBMC coculture supernatants include CD14+, CD56+/CD16+, CD8+, and CD4+ cells.37 Among the transmigrated cell types, CD14+ cells composed a mean of 45% of the transmigrated cells, CD4+ cells 19.6%, CD8+ cells 7.8%, and CD16+/CD56+ cells 7.7% (Figure 4). When compared with background migration of cells observed using control media, there was a 2.9-fold increase for CD14+ cells, 8.8-fold increase for CD4+ cells, a 4.5-fold increase for CD8+ cells, and a 3.1-fold increase for CD16+/CD56+ cells.
Migration of different PBMC cell types in response to chemokine gradients in coculture supernatants. Results are shown as mean (± SD) of transmigrated populations from cell migration assays of 3 CMV-seropositive donors. PBMCs from each donor were prepared for transwell migration assays, as described in “Transwell migration assays and specific antibody neutralization assays.” PBMCs were loaded into the upper chambers of transwells to test migration into coculture supernatants prepared previously from the same donor, or negative control media. The “+” and “−” symbols refer to migration results using coculture supernatants or negative control media, respectively. Culture supernatants from fractalkine-induced cultures were used for the endothelial damage assays. Percentage values with each cell type represent the mean number of cells migrating of each cell type (ie, mean cells migrated in lower chamber/mean cell initially in upper chamber × 100).
Migration of different PBMC cell types in response to chemokine gradients in coculture supernatants. Results are shown as mean (± SD) of transmigrated populations from cell migration assays of 3 CMV-seropositive donors. PBMCs from each donor were prepared for transwell migration assays, as described in “Transwell migration assays and specific antibody neutralization assays.” PBMCs were loaded into the upper chambers of transwells to test migration into coculture supernatants prepared previously from the same donor, or negative control media. The “+” and “−” symbols refer to migration results using coculture supernatants or negative control media, respectively. Culture supernatants from fractalkine-induced cultures were used for the endothelial damage assays. Percentage values with each cell type represent the mean number of cells migrating of each cell type (ie, mean cells migrated in lower chamber/mean cell initially in upper chamber × 100).
Starting PBMCs and transmigrated populations were also screened for CD14+/CD16+ cells (data not shown). Because all the donors were normal, healthy persons, these cell types were only present as a trace percentage in the PBMCs, even when results were compared from several different donors.38 Although there was some increase in percentage in the transmigrated populations, they constituted no more than 2% of the total transmigrated populations (data not shown). Such a minor percentage of cells was considered unlikely to make a major contribution to endothelial cell damage.
The next set of experiments was designed to test the role of fractalkine or RANTES on migration for each PBMC subset. Briefly, flow cytometry analysis was used to compare the migration results of cell types and percentages between PBMCs and coculture supernatants using CX3CR1 and RANTES interactions blocked by specific antibody. The results are shown as percentages of migrated cell numbers treated with specific antibody blocks compared with total migrated PBMC numbers in the untreated control sample with CMV antigen-stimulated supernatants.
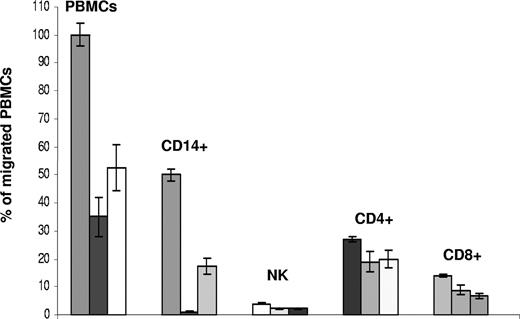
Among the different subsets of PBMCs, the CX3CR1 block had the greatest effect on CD14+ cells with a 45-fold decrease in cell migration numbers (2.85 × 105 cells/mL down to 0.06 × 105 cells/mL) (Figure 5). The RANTES block had less of an effect on cell migration, with a 2.9-fold decrease in migration rates of CD14+ cells (2.85 × 105 cells/mL down to 0.99 × 105 cells/mL). For NK (CD16+/CD56+) cells, the effects of the CX3CR1 or RANTES blocks were similar but lower at 1.8-fold reduction in cell migration (2.28 × 104 cells/mL down to 1.3 × 104 cells/mL relative to total transmigrated cell numbers). For CD8+ T cells, the mean effect was less with a 38% (± 7.6%) decrease in cell migration with the anti-CX3CR1 block and 48R (± 7.7%) with the anti-RANTES block. With CD4+ T cells, the mean effects were a 30% (± 6.0%) decrease with the CX3CR1 block and a 26% (± 3.9%) decrease with the anti-RANTES block.
CX3CR1 block has greatest inhibition effect on migration of CD14+ cells compared with other PBMC subsets. Untreated PBMCs, CX3CR1 blocked, or RANTES blocked cells or supernatants were compared for migration into antigen-stimulated coculture supernatants. Migrated cells were then collected, stained, and analyzed by flow cytometry to identify migrated subsets of CD14+, NK (CD16+/CD56+), CD4+ T cells, and CD8+ T cells. Treated samples for each analysis are grouped together for PBMCs or each subset as no antibody treatment, anti–CX3CR1-neutralizing antibody treatment, and anti–RANTES-neutralizing antibody. Results are shown as mean (± SD) percentage of migrated cell numbers relative to untreated PBMC migrations for PBMCs, CD14+ cells, NK cells, CD4+ T cells, and CD8+ T cells.
CX3CR1 block has greatest inhibition effect on migration of CD14+ cells compared with other PBMC subsets. Untreated PBMCs, CX3CR1 blocked, or RANTES blocked cells or supernatants were compared for migration into antigen-stimulated coculture supernatants. Migrated cells were then collected, stained, and analyzed by flow cytometry to identify migrated subsets of CD14+, NK (CD16+/CD56+), CD4+ T cells, and CD8+ T cells. Treated samples for each analysis are grouped together for PBMCs or each subset as no antibody treatment, anti–CX3CR1-neutralizing antibody treatment, and anti–RANTES-neutralizing antibody. Results are shown as mean (± SD) percentage of migrated cell numbers relative to untreated PBMC migrations for PBMCs, CD14+ cells, NK cells, CD4+ T cells, and CD8+ T cells.
The CX3CR1-specific CD14+ cells were examined for cell migration response effects using a CX3CR1 block to check for consistency of the CX3CR1 response. CX3CR1+/CD14+ migration levels were reduced by 125-fold from 3.23 × 105 cells/mL to 3.8 × 102 cells/mL (0.8%) of the untreated dual-positive cell migration (data not shown).
Endothelial damage is associated with CD14+ or NK-cell populations
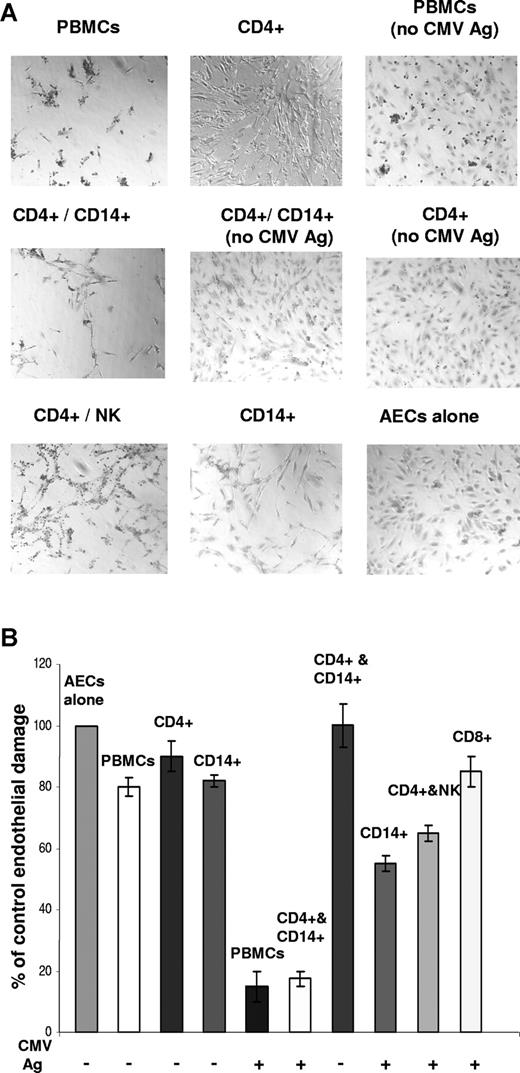
The next experiments were designed to determine whether the cell types mobilized in response to fractalkine, RANTES, or other factors present in the activated endothelial coculture supernatants associated with endothelial cell damage. The PBMC subsets were screened for the ability to cause endothelial damage, by comparing levels of damage between isolated subset populations cocultured with CMV antigen and AEC monolayers by day 5. Negative controls included untreated AEC wells and AECs with the same ratio of PBMCs or subset cells but without viral antigen stimulation. CD4+ T cells had previously been shown to provide the cytokine response with CMV-specific antigen stimulation and to initiate endothelial activation and chemokine induction.33 CD4+ T cells were included in combination with the other subsets to reproduce the factors involved in the endothelial damage process and were not associated with endothelial damage when tested as cocultures (Figure 6A).
Endothelial damage is associated with CD14+ or NK populations. (A) Endothelial damage by CMV antigen–stimulated PBMC subset populations shows NK and CD14+ monocyte-macrophage populations associated with damage after 5 days of coculture. Negative controls without CMV antigen stimulation are indicated. (B) Endothelial damage assays per subset populations in which results were quantitated by counting remaining endothelial cells per field of view (FOV) under the microscope (20× objective). Results are representative of 3 independent assays, each with different CMV-seropositive donors. Error bars represent SD.
Endothelial damage is associated with CD14+ or NK populations. (A) Endothelial damage by CMV antigen–stimulated PBMC subset populations shows NK and CD14+ monocyte-macrophage populations associated with damage after 5 days of coculture. Negative controls without CMV antigen stimulation are indicated. (B) Endothelial damage assays per subset populations in which results were quantitated by counting remaining endothelial cells per field of view (FOV) under the microscope (20× objective). Results are representative of 3 independent assays, each with different CMV-seropositive donors. Error bars represent SD.
Endothelial damage was associated to the greatest extent in CMV antigen–stimulated cocultures with CD14+ or NK-cell populations (Figure 6A). Results were quantitated by counting remaining endothelial cells per field of view under the microscope, where endothelial loss was 82.5% (± 3%) for CD14+ cells cocultured with CMV antigen–stimulated CD4+ T cells and AEC monolayers (Figure 6B). This was similar to the levels of endothelial loss observed with antigen-stimulated PBMC cocultures, at 85% (± 5%). For NK cells cocultured with antigen-stimulated CD4+ T cells and AECs, the endothelial loss was 35% (± 5%). In control cocultures without antigen stimulation, the loss was minimal, ranging from 10% to 20% below control cultures, or not detected.
Fractalkine plays a key role in recruitment and mobilization of PBMCs involved in endothelial cell damage
The flow cytometry analyses identified that most of the cells transmigrating in response to the chemokine gradients were CD14+ monocyte-macrophage cells carrying receptors for fractalkine or RANTES, indicating that these ligand-receptor interactions have a major role in mobilizing the PBMCs involved in the endothelial cell damage and destruction process. We next determined whether the PBMC populations that responded to fractalkine, RANTES, or other factors present in the activated endothelial coculture supernatants could mediate endothelial cell damage and destruction.
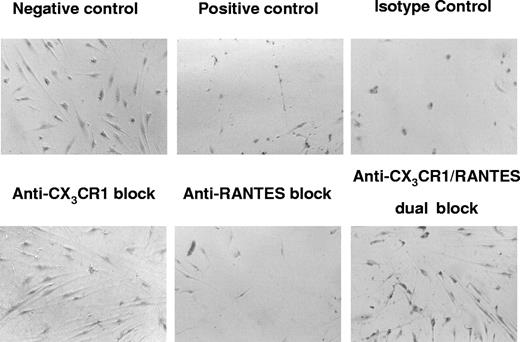
Using a 2-step assay for endothelial damage, transmigrated PBMC populations were collected and tested for the ability to mediate endothelial cell damage. In a parallel series of assays, PBMCs were treated with specific antibody directed against CX3CR1, RANTES, or CX3CR1 plus RANTES as a dual block, compared with untreated controls. Extensive damage and loss of the endothelial cell monolayers were observed in the positive control samples (Figure 7). In contrast, protection against endothelial damage was observed in the presence of the anti-CX3CR1 blocked samples in which the endothelial monolayers were intact, similar to the untreated negative control. In the anti-RANTES block treatment, only partial protection was observed; cell destruction and loss were present albeit to a lesser extent than what was observed in untreated control wells. The dual-blocking treatment against CX3CR1 and RANTES was protective against endothelial cell damage. The isotype control–treated sample was similar to the positive untreated control, indicating that nonspecific antibody-binding effects could be ruled out.
CMV-seropositive donor PBMCs exposed to CMV antigen results in endothelial damage in cocultures that is mediated predominantly by the fractalkine-CX3CR1 interaction. Each micrograph panel shows the resulting effects of specific neutralizing antibody blocks and controls on CMV-induced endothelial damage in PBMC-AEC cocultures at day 6. Negative control was transmigrated PBMC populations using negative control media lacking chemokines; positive control was transmigrated PBMCs using untreated coculture supernatants; isotype control was PBMCs treated with isotype control antibodies for CX3CR1-specific and RANTES-specific neutralizing antibodies, followed by transmigration into coculture supernatants; anti-CX3CR1 block was PBMCs treated with neutralizing antibody specific for CX3CR1, followed by transmigration into coculture supernatants; anti-RANTES block was PBMCs treated with neutralizing antibody specific for RANTES, followed by transmigration into coculture supernatants; dual block was PBMCs treated with both neutralizing antibody specific for CX3CR1 and neutralizing antibody specific for RANTES, followed by transmigration into coculture supernatants. Extensive endothelial damage and loss are seen in positive control, isotype control, and anti-RANTES block. In contrast, protection against endothelial damage is observed in the presence of the anti–CX3CR1-blocked samples.
CMV-seropositive donor PBMCs exposed to CMV antigen results in endothelial damage in cocultures that is mediated predominantly by the fractalkine-CX3CR1 interaction. Each micrograph panel shows the resulting effects of specific neutralizing antibody blocks and controls on CMV-induced endothelial damage in PBMC-AEC cocultures at day 6. Negative control was transmigrated PBMC populations using negative control media lacking chemokines; positive control was transmigrated PBMCs using untreated coculture supernatants; isotype control was PBMCs treated with isotype control antibodies for CX3CR1-specific and RANTES-specific neutralizing antibodies, followed by transmigration into coculture supernatants; anti-CX3CR1 block was PBMCs treated with neutralizing antibody specific for CX3CR1, followed by transmigration into coculture supernatants; anti-RANTES block was PBMCs treated with neutralizing antibody specific for RANTES, followed by transmigration into coculture supernatants; dual block was PBMCs treated with both neutralizing antibody specific for CX3CR1 and neutralizing antibody specific for RANTES, followed by transmigration into coculture supernatants. Extensive endothelial damage and loss are seen in positive control, isotype control, and anti-RANTES block. In contrast, protection against endothelial damage is observed in the presence of the anti–CX3CR1-blocked samples.
Discussion
We have previously reported a major pathogenic effect in which endothelial cell damage and loss follow the induction of fractalkine and up-regulation of cell adhesion markers in the presence of PBMCs from donors with a high T-cell response to CMV.33,37 In this report, we show that the fractalkine-CX3CR1 interaction plays an important role in mediating this endothelial damage together through recruitment of NK cells and monocyte-macrophages. The key role of fractalkine is emphasized by our blocking data, whereby the ability of specific antibody to CX3CR1 greatly reduced CX3CR1+-bearing cell chemoattraction and provided the greatest protection against endothelial cell damage.
Chemokine-mediated damage to endothelium may result from combined effects of chemoattracted cell populations responding to chemokine gradients together with up-regulated adherence factors, including endothelial cell surface–associated and secreted fractal-kine. Binding of fractalkine to its receptor can play a role in activation of immune effector cells involved in cell and tissue damage such as NK cells.11,16 In our studies, PBMC subpopulations expressing CX3CR1 were attracted to activated fractalkine-expressing endothelial cells, in contrast to control cocultures with endothelial monolayers that were in a resting state and null for fractalkine expression. Flow cytometry analysis identified the main subpopulations of migrated PBMC populations as CD14+-, CD56+/CD16+-, CD4+-, and CD8+-expressing cells, all cell types associated with expression of CX3CR1. The highest percentage of cell types responding by migration to fractalkine were CD14+ cells, at a mean of 45% of the total transmigrated PBMC population and an approximate 3-fold increase over percentages of CD14+ cells with negative control media. NK (CD16+/CD56+) cells comprised a mean of 7.7% of the transmigrated cell populations and also an approximate 3-fold increase over background. Results from specific blocking antibody assays showed that disrupting the fractal-kine ligand–receptor interaction had the strongest inhibitory effect on cell migration. This provides support for the importance of fractalkine in the inflammatory response.
After migration along fractalkine gradients, adherence and anchoring of NK and monocyte-macrophage cells to activated endothelial cell surfaces can be initiated through the binding interaction of CX3CR1 to endothelial cell surface–bound fractal-kine. This initial tethering effect promotes adherence of mobilized cells to activated endothelium and prevents the cells from being swept away under the conditions associated with circulating blood.11,16,39 In our observations, up-regulated cell adhesion markers (ICAM-1, VCAM) together with cell-associated fractalkine indicate a contributing role for cell adhesion in the observed endothelial damage process.33,37 We also observed that NK and CD14+ cells were associated with the ability to induce endothelial cell damage. The endothelial damage was associated with chemoattracted populations of NK and CD14+ cells that adhered to endothelial cultures. Protection against endothelial damage was observed in the presence of anti–CX3CR1-specific antibody.
The activation state of CD14+ cells and NK cells may also play a role in endothelial damage. CD14+ cells cocultured without CMV antigen over AECs resulted in no detectable loss of endothelial cells, but in the presence of CMV antigen there was a 45% (± 5%) loss of endothelial cells. Binding of virion structural material to Toll-like receptors or scavenger receptors present on monocyte-macrophage cells can promote an activated state leading to endothelial damage.31,40,41 Consistent with this is the additional observation that MCSF can be produced by the activated endothelial cells at levels capable of inducing this monocyte-macrophage differentiation (data not shown). NK cells can also be activated by binding of fractalkine to their CX3CR1 receptors and can promote perforin-granzyme cell killing.16
The findings from this research are consistent with the hypothesis that endothelial cell damage may result from a chemokine-mediated process and provide support for the following model.11,27 The process can begin as CMV infection of endothelial cells with the release of infectious virus, noninfectious virions, and viral proteins. The latter 2 have the clear potential to be processed and presented as antigen by other adjacent endothelial cells to CMV antigen–specific CD4+ T cells circulating in the peripheral blood and patrolling the vascular tissue system. IFNγ and TNFα are released by antigen-stimulated CD4+ T cells and then activate adjacent endothelial cells. This results in the induction of several chemokine factors, including fractalkine, together with up-regulation of several adhesion molecules and receptors on the activated endothelial surface, including ICAM-1 and VCAM.11,23,27 This localized concentration of fractalkine on the apical surface of endothelial cells, together with the release of soluble fractalkine, results in a chemokine gradient. Fractalkine and, to a lesser extent, RANTES function as chemoattractants to mobilize and recruit inflammatory cells, including monocytes and NK cells that bind to cell adhesion markers and also membrane-bound fractalkine on the endothelial cell surface by CX3CR1.
These findings emphasize the potential of the host immune response to respond aggressively to CMV and to initiate a series of events resulting in endothelial damage. The risk for this to occur may be greater with higher host CMV-specific T-cell frequencies or with increased CMV virus present in the host. Our results indicate that endothelial cells can be protected from chronic inflammation and damage by blocking the inflammatory process at 2 stages, the IFNγ and TNFα response during T-cell activation or targeting the fractalkine ligand-receptor interaction.33,37 These findings further support that CMV may represent one member of a class of pathogens in which viral persistence in the host drives a continuous antigen-specific T-cell response. This continuous cycle may promote a state of chronic immune activation and contribute to chronic inflammation and chemokine-mediated endothelial cell injury.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the National Institute of Allergy and Infectious Diseases (grant AI-36214; Virology Core, University of California, San Diego, Center for AIDS Research).
Authorship
Contributions: C.A.B-F. shared equally in design of project, designed, performed, and analyzed experiments, collected and reviewed data, and prepared the manuscript; S.A.S. shared equally in design of project, reviewed and analyzed data and experiments, and prepared and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stephen A. Spector, Department of Pediatrics, University of California, San Diego, 9500 Gilman Dr, La Jolla, CA 92093-0672; e-mail: saspector@ucsd.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal