The critical importance of plasmacytoid dendritic cells (pDCs) in viral infection, autoimmunity, and tolerance has focused major attention on these cells that are rare in blood and immune organs of humans and mice. The recent development of an Flt-3 ligand (FL) culture system of bone marrow cells has led to the simple generation of large numbers of pDCs that resemble their in vivo steady-state counterparts. The FL system has allowed unforeseen insight into the biology of pDCs, and it is assumed that FL is the crucial growth factor for these cells. Surprisingly we have found that a cell type with high capacity for interferon-α (IFN-α) production in response to CpG-containing oligonucleotides, a feature of pDCs, develop within macrophage–colony-stimulating factor (M-CSF)–generated bone marrow cultures. Analysis of this phenomenon revealed that M-CSF is able to drive pDCs as well as conventional DCs (cDCs) from BM precursor cells in vitro. Furthermore, application of M-CSF to mice was able to drive pDCs and cDCs development in vivo. It is noteworthy that using mice deficient in FL indicated that the M-CSF-driven generation of pDCs and cDCs in vitro and in vivo was independent of endogenous FL.
Introduction
Dendritic cells (DCs) are a rare population of bone marrow-derived cells that can be separated by phenotype and function into multiple subsets.1 Since the discovery of plasmacytoid DCs (pDCs) in human and mouse, DC can be divided into 2 major subgroups: the pDCs and the conventional DCs (cDCs).2 pDCs and cDCs both develop from hematopoietic precursor cells under the influence of growth factors. Among these are only 2 factors that solely are able to drive DC development. The first, granulocyte macrophage–colony-stimulating factor (GM-CSF), was shown to drive hematopoietic precursor cells, but also monocytes, to DC development (GM-DC).3,–5 These GM-DC seem not to represent the majority of steady-state DC subsets in lymphoid organs, because mice deficient for either GM-CSF or the GM-CSF receptor are hardly impaired in vivo.6 Furthermore, GM-CSF actually blocks the generation of pDCs in vitro.7 The second growth factor able to drive DC generation is the ligand of the fms-like tyrosine kinase 3 (FL), which is able to promote the generation of DC subsets, including pDCs, upon addition to bone marrow (BM) precursor cells.8,9 In fact, FL is the only factor known to drive pDC development. In addition to in vitro generation of pDCs and cDCs with FL (FL-DC), FL is a potent inducer of pDCs and cDCs in vivo in human and mice.10,,–13 Moreover, mice deficient in FL (FLKO) have drastically reduced numbers of pDCs9 and cDCs14 in several lymphoid organs
Ex vivo isolated or FL generated pDCs have the unique ability to respond to direct stimulation via Toll like receptors (TLR) 7 and 9, with their respective ligands RNA and DNA, with high level production of interferon-α (IFN-α). Other cell types, including cDCs and M-CSF–generated macrophages, can be induced to produce IFN-α in response to viruses or transfected DNA or RNA, but this IFN-α production is mediated via TLR7 and TLR9 independent pathways including via PKR, RIG-I, MDA5, TLR-3 and as yet unidentified cytoplasmic DNA-recognition complexes.15,–17 Thus, pDCs are the sole cells able to use TLR7 and 9 ligation for high level production of IFN-α. Certain CpG-motif containing oligonucleotides (CpG-ODN A-type) induce extremely high levels of IFN-α solely in pDCs18 and thus IFN-α production in response to A-type CpG-ODN can be used as a functional test for the presence of pDCs, even in mixed cell populations.17
While performing analyses of pDCs developmental kinetics in FL BM cultures we assayed aliquots of CpG-stimulated cultures in parallel with aliquots of M-CSF–stimulated BM cultures, a putative negative control. Unexpectedly, M-CSF– driven BM cultures also promoted cells that produced IFN-α in response to CpG. Our analysis of this phenomenon has revealed that upon culturing BM cells with M-CSF, suspension cells, normally thrown away in the production of adherent M-CSF macrophage cultures, also developed. Our extensive studies demonstrate that among these M-CSF–generated suspension cells are cells that by phenotype and function resemble pDCs. cDCs were also generated in this process.
The receptors for FL and M-CSF, Flt3 and c-fms, respectively, are receptor tyrosine kinases. With a pharmacologic inhibitor specific for c-fms, we provide evidence that M-CSF was able to generate pDC and cDC subsets, via its designated receptor c-fms and not via potential cross-reactivity with the receptor for FL, Flt3. Furthermore, both pDC and cDC populations developed in M-CSF BM cultures from FL knockout (FLKO) mice, ruling out indirect involvement of endogenous FL in the generation of M-CSF–driven pDCs and cDCs. Application of M-CSF to wild-type mice or FLKO mice promoted pDC and cDC numbers in vivo. Investigation into the progenitor cells that generated M-CSF–dependent DCs revealed that common lymphoid progenitors (CLPs) were efficient producers of pDCs. Common myeloid progenitors (CMPs) generated pDCs 10-fold less efficiently but they were more efficient producers of cDCs. Thus, we present for the first time that the macrophage growth factor M-CSF promotes the development of pDCs and cDCs in vitro and in vivo, independently of FL.
Methods
Mice
C57BL/6 mice were obtained from Harlan Winkelmann (Borchen, Germany) and used at 6 to 10 weeks of age. FLKO mice, described by McKenna et al,14 were bred at the Institute of Labortierkunde (University of Zurich, Zurich, Switzerland). Mice expressing green fluorescent protein (GFP) under the ubiquitin promoter [C57BL/6-Tg(UBC-GFP)30Scha/J, referred to hereafter as UBC-GFP mice] were purchased from Charles River Laboratories (Sulzfeld, Germany). Animal experiments were carried out with approval and under the guidelines of the local government animal ethics authorities.
Antibodies and reagents
Recombinant (rec) flag-tagged murine (mu) FL was expressed in Chinese hamster ovary cells and purified in house as described previously.10 We obtained recmuGM-CSF, recmuM-CSF and rec human (hu) M-CSF from Tebu-Bio (Frankfurt, Germany) and rechuM-CSF from R&D Systems (Wiesbaden, Germany). c-fms receptor tyrosine kinase inhibitor and Flt3 inhibitor II were obtained from EMD Biosciences (Darmstadt, Germany).
Oligonucleotides containing CpG motifs (CpG-2216 and CpG-1668) were synthesized by TIB MOLBIOL (Berlin, Germany) according to published sequences.19 Imiquimod (R837) and palmitoyl-3-cysteine-serine-lysine-4 (Pam-3-Cys) were purchased from InvivoGen (San Diego, CA). Poly(cytidylic-inosinic) acid [poly(I:C)] and lipopolysaccharide (LPS) were purchased from Sigma-Aldrich (Taufkirchen, Germany). Antibodies were obtained from Becton Dickinson (Heidelberg, Germany) with the following exceptions: purified and fluorescein isothiocyanate (FITC)-conjugated anti-CD11c (rat clone 223H7) and anti-Ly49Q were obtained from Biozol Diagnostica Vertrieb (Eching, Germany), anti-mPDCA-1 from Miltenyi Biotec (Bergisch Gladbach, Germany), and antibodies anti-F4/80, anti-CD117-PeCy7 (clone 2B8), anti-CD34-Pe (clone RAM34) and anti-CD115 (clone AFS98, and an isotype matched Rat IgG2a control) were obtained from eBiosciences (San Diego, CA). It should be noted that another M-CSFR monoclonal antibody (mAb) (clone 604B5 2E11; Serotec, Dusseldorf, Germany) was also used, but staining with this mAb, unlike that with clone AFS98, was extremely low on total BM, and M-CSFR expression was undetectable on CLPs using this clone. Hybridomas, the supernatants of which were used in the depletion cocktail for ex vivo DC purification20 and progenitor isolation,21 were kindly provided by Prof Ken Shortman (Walter and Eliza Hall Institute [WEHI] of Medical Research, Melbourne, Australia).
M-CSF and FL BM cultures
BM cells were flushed from femurs and tibiae of mice and depleted of red blood cells with red cell lysis buffer (Sigma-Aldrich, St Louis, MO). BM cells were then either cultured directly or after “depletion.” For depletion, BM cells were incubated for 30 minutes with rat antibodies to CD11c and CD45R (clone RA36B2) followed by 30-minute incubation with goat anti-rat magnetic beads (QIAGEN, Hilden, Germany). The depletion procedure routinely removed 65% to 80% of total BM cells. It should be noted that depletion with the beads only, in the absence of the rat antibodies, also depleted approximately 50% of the BM cells, presumably via FcR/Ig interactions. Total or depleted BM cells were cultured at 1.5 × 106 cells/mL in RPMI-1640 media supplemented with 10% fetal calf serum (FCS), 50 μmol/L β-mercaptoethanol, 100 IU/mL penicillin/streptomycin (complete media), and either 20 ng/mL recmuM-CSF/rechuM-CSF or 35 ng/mL recmuFL for 6 to 8 days at 37°C in a humidified atmosphere containing 5% CO2. The M-CSF cultures were fed with fresh M-CSF every 3 days, without media change.
Surface staining of M-CSF or FL BM cultures
Harvested cells were washed in phosphate-buffered saline (PBS) containing 2% FCS and 2 mmol/L ethylenediaminetetraacetic acid (EDTA) (fluorescence-activated cell sorting [FACS] buffer). FcR binding was blocked by incubation with 1 mg/mL purified anti-CD16/32 mAb (clone 2.4G2) for 20 minutes on ice. An equal volume of 2× concentrated antibody (Ab) stain was then added to the cells and incubated for a further 20 minutes. Cells were washed in FACS buffer and resuspended in FACS buffer containing 1 μg/mL propidium iodide. For analysis of DC populations within the cultures, cells were first gated on nondead, nonautofluorescent cells by gating on propidium iodide-negative cells.
Activation of DC subsets and analysis of cytokine production
Nonsorted M-CSF or FL BM cultures, or DC sorted with a FACS-ARIA (BD Biosciences) (0.25-0.5 × 106 cells/mL) were stimulated for 18 to 24 hours in complete media with or without added stimulus. The stimuli used were as follows; 10 ng/mL GM-CSF, 1 μg/mL Pam-3-Cys, 100 μg/mL poly(I:C), 1 μg/mL LPS, 1 μg/mL R837, 0.5 μmol/L CpG-2216, and 0.5 μmol/L CpG-1668. Culture supernatants were assayed for IFN-α by 2-site enzyme-linked immunosorbent assay (ELISA) as described previously.20 Other cytokines (interleukin [IL]-12 p70, IL-6, tumor necrosis factor [TNF]-α, monocyte chemotactic protein-1 [MCP-1] and IFN-γ) were measured using the Cytometric Bead Array, Mouse Inflammation Kit (BD Biosciences).
Stimulated DC were blocked as stated above and stained with antibodies directed to CD8α, CD40, CD69, CD80, and CD86.
In vivo M-CSF treatment
Wild-type and FLKO mice were treated i.p. with 10 μg of M-CSF in 0.01% bovine serum albumin (BSA) (100-μl volume) or with vehicle alone for 5 consecutive days. At the end of 5 days, mice were killed. The peritoneum was flushed 3 times with complete media, and organs were collected for DC purification.
Ex vivo DC purification
DC were purified from spleens of M-CSF or vehicle-treated mice essentially as described previously,20 using FACS buffer, RPMI and 1.077A Nycodenz (Progen Biotechnik, Heidelberg, Germany) that was adjusted to mouse osmolarity (308 mOsmol/L).
Culturing of progenitor cells
CMP (Lin−Sca-1−ckit+CD34+FcRint), CLP (Lin−Sca-1+c-kitintIL-7R+Thy-1−), or GM progenitors (GMP, Lin−Sca-1−c-kit+CD34+FcRhi) were isolated from C57BL/6 BM as described previously21 and sorted to greater than 95% purity on a FACS-ARIA instrument. Twenty-five hundred cells and serial 2-fold dilutions thereof were added to a 1-mL suspension of UBC-GFP BM cells in a 24-well plate. Final UBC-GFP BM cell concentration was 1.0 × 106 cells/mL. M-CSF or FL cultures were carried out in these wells as described above. After 6 days, samples were enumerated, analyzed by FACS, and progeny arising from the C57BL/6 progenitors were gated as GFPneg cells. To analyze M-CSFR (CD115) and Flt3 (CD135) expression on CLP (Lin−Sca-1+c-kitintIL-7R+Thy-1−), lineage depleted cells were first stained with Sca-1-FITC, and Thy-1-phycoerythrin (PE) antibodies and the Sca-1+Thy-1− cells were sorted. These pre-enriched cells (> 95% purity when reanalyzed) were then stained with CD117-PE-CY7, IL-7R-APC, and either CD115-PE or CD135-PE.
Results
Total BM cells cultured with M-CSF were potent producers of IFN-α in response to CpG-oligodeoxynucleotides
Culture of mouse BM cells with FL for 8 to 10 days leads to the generation of millions of highly pure pDCs and cDCs that closely resemble the DC populations of steady-state mouse spleen.22 To routinely test the kinetics of pDC development within these FL cultures, we analyzed the IFN-α–producing capacity of total BM cells incubated with FL in a multiwell format over a time course of 0 to 7 days. As a negative control, we included a parallel culture of total BM cells incubated with M-CSF.
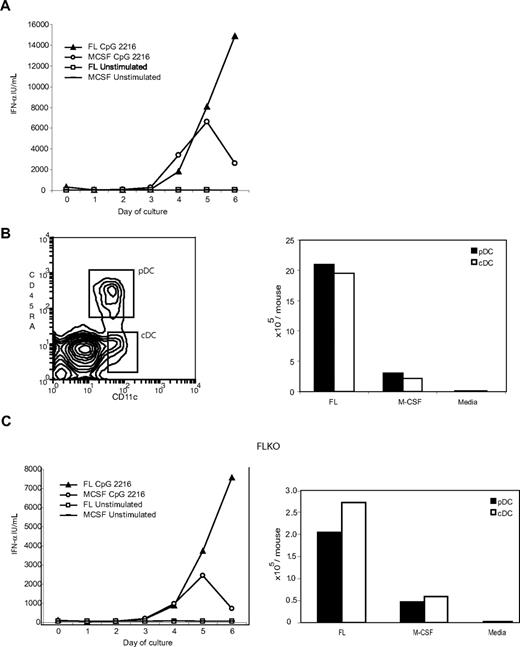
M-CSF is routinely used to generate macrophages from BM cells, and our usual previous protocol involves replacing media and M-CSF every few days, eliminating suspension cells, leaving the adherent cells at the end of a culture period of 7 days or longer.17 Here, however, we have instead treated the M-CSF cultures exactly like the FL cultures and analyzed wells of total M-CSF cultures (including adherent and nonadherent cells) for IFN-α production in response to CpG-2216. To our surprise, IFN-α was induced to high levels in the M-CSF cultures. Moreover, the IFN-α produced in response to CpG-2216 increased with culture time, implying that IFN-α producing cells were being generated in the course of the M-CSF culture (Figure 1A).
M-CSF drives pDC and cDC development from BM cells, even in the absence of FL. (A) Replicate wells of C57BL/6 BM cells depleted of B220+ and CD11c+ cells were incubated for 6 days with M-CSF (20 ng/mL) added at day 0 and again at day 3 or with FL (35 ng/mL) added at day 0 only. On each of days 0 to 6, separate wells were stimulated overnight with CpG-2216 or left unstimulated, and the supernatants were assayed for IFN-α. (B) C57BL/6 BM cells depleted of B220+ and CD11c+ cells were incubated for 6 days with M-CSF (20 ng/mL) added at day 0 and again at day 3. On day 6, the cells were harvested and stained with antibodies to detect CD11c and CD45RA expression. Cells with the phenotype of pDC and cDC populations are shown boxed in the left panel. The number of cells in each of the pDCs and cDCs populations are shown in the right panel and compared with numbers obtained from day 6 FL-generated DC or media only also using BM cells depleted of B220+ and CD11c+ cells. (C) BM cells from mice lacking FL were similarly depleted of B220+ and CD11c+ cells and incubated in replicate wells for 0 to 6 days with FL, of M-CSF (with additional feeding at day 3). On each of days 0 to 6, separate wells were stimulated overnight with CpG-2216 or left unstimulated and the supernatants were assayed for IFN-α (left panel). The pDC and cDC populations present at day 6 in the FLKO cultures are shown in right panel. Data shown are from one experiment representative of 3 similar experiments for the multiple timepoints in panel A: one experiment that was carried out for the multiple timepoints and 3 additional experiments for day 6 and 0 timepoints in panel C left: one experiment representative of 3 experiments of day 6 FL cultures and more than 5 experiments of day 6 M-CSF cultures (B), and one experiment that is representative of 4 experiments (C right panel). In media-only control cultures (B,C right panels), no M-DC were detectable.
M-CSF drives pDC and cDC development from BM cells, even in the absence of FL. (A) Replicate wells of C57BL/6 BM cells depleted of B220+ and CD11c+ cells were incubated for 6 days with M-CSF (20 ng/mL) added at day 0 and again at day 3 or with FL (35 ng/mL) added at day 0 only. On each of days 0 to 6, separate wells were stimulated overnight with CpG-2216 or left unstimulated, and the supernatants were assayed for IFN-α. (B) C57BL/6 BM cells depleted of B220+ and CD11c+ cells were incubated for 6 days with M-CSF (20 ng/mL) added at day 0 and again at day 3. On day 6, the cells were harvested and stained with antibodies to detect CD11c and CD45RA expression. Cells with the phenotype of pDC and cDC populations are shown boxed in the left panel. The number of cells in each of the pDCs and cDCs populations are shown in the right panel and compared with numbers obtained from day 6 FL-generated DC or media only also using BM cells depleted of B220+ and CD11c+ cells. (C) BM cells from mice lacking FL were similarly depleted of B220+ and CD11c+ cells and incubated in replicate wells for 0 to 6 days with FL, of M-CSF (with additional feeding at day 3). On each of days 0 to 6, separate wells were stimulated overnight with CpG-2216 or left unstimulated and the supernatants were assayed for IFN-α (left panel). The pDC and cDC populations present at day 6 in the FLKO cultures are shown in right panel. Data shown are from one experiment representative of 3 similar experiments for the multiple timepoints in panel A: one experiment that was carried out for the multiple timepoints and 3 additional experiments for day 6 and 0 timepoints in panel C left: one experiment representative of 3 experiments of day 6 FL cultures and more than 5 experiments of day 6 M-CSF cultures (B), and one experiment that is representative of 4 experiments (C right panel). In media-only control cultures (B,C right panels), no M-DC were detectable.
The IFN-α producers induced in M-CSF BM cultures displayed characteristics of pDCs but developed without the influence of FL
Depletion of pDCs and cDCs from total BM cells abolished the CpG-induced IFN-α producing capacity of BM cells (data not shown). However, when the DC-depleted BM cells were cultured with M-CSF for 6 days, potent IFN-α producing capacity was again detected, and this activity resided within the nonadherent cells of the M-CSF culture (data not shown). To determine whether any of the nonadherent M-CSF–generated cells displayed the phenotype expected of a pDCs, they were stained with CD11c and CD45RA. Indeed a population of 10% to 20% of cells within the cultures expressed high levels of CD45RA and medium levels of CD11c. Together with the lack of CD3, CD19, CD49b, or NK1.1 expression, low side scatter and forward scatter, this was commensurate with the phenotype of pDCs (Figure 1B and data not shown).
It was clear that M-CSF could drive pDC development, yet the yield of pDCs was substantially less then that obtained with FL. When the DC numbers from the 2 cultures were compared side by side after 6 days of culture, M-CSF was approximately 10-fold less efficient than FL (Figure 1B). Day 6 was chosen for comparison because after this stage, the M-CSF cultures became very acidic and sorted DC populations from the cultures died much more rapidly in culture and failed to produce cytokines (data not shown).
Given the current view that pDCs depend upon FL for development, it was possible that these putative pDCs developed from BM cells in the presence of M-CSF via FL produced endogenously in the cultures. To investigate this possibility, replicate BM cultures were set up using BM cells from mice in which the FL gene was ablated (FLKO mice). IFN-α–producing cells were produced with similar kinetics, but total cell numbers obtained from the BM cultures of FLKO mice were reduced whether cultures were conducted with added M-CSF or FL (Figure 1C). Nevertheless, it was clear that cells with the phenotype and morphology of pDCs were produced by culture of BM first depleted of any DC populations and in the presence of only exogenous M-CSF, even without the potential influence of any FL. These M-CSF–generated pDCs are referred to as M-pDCs.
Detailed surface phenotype of M-pDCs
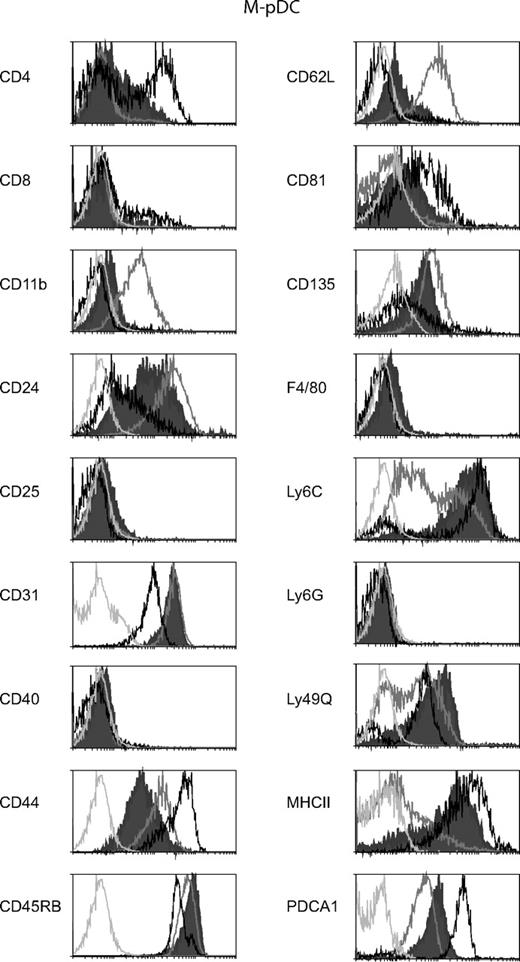
Extensive phenotyping of the M-pDCs from both wild-type and FLKO mice showed that the elicited pDCs displayed an identical phenotype. When M-pDCs were compared with pDCs generated in vitro with FL (FL-pDCs), numerous cell surface markers were different between the pDCs generated with the 2 different cytokines. In fact, as seen in Figure 2, for many surface markers, the M-pDCs displayed an intermediate phenotype between FL-pDCs and ex-vivo spleen pDCs. Certain molecules that are recognised as differentiation or maturation markers of pDCs; Ly49Q,23 CD4,20 and MHCII are higher on M-pDCs than on FL-pDCs and approach or are very similar to the levels on spleen pDCs. The M-pDCs express a high level of Ly6C similar to that of the ex vivo spleen pDCs. Because it has recently been shown that pDCs develop from Ly6C− precursors, this may be an indication that the M-pDCs represent further differentiated pDCs than the FL-pDCs.24 The surface expression of CD81, CD62L, and CD11b on the M-pDCs also more closely resemble the ex-vivo pDCs than the FL-pDCs. However, this trend is not true for all markers examined. Compared with FL-pDCs and ex-vivo pDCs, the M-pDCs express the lowest levels of CD44. The spread of surface CD24 on M-pDCs bridges the high levels expressed by spleen pDCs and the low levels expressed by FL-pDCs. It is noteworthy that M-pDCs express very low levels of CD11b (approximately 10-fold lower than FL-pDCs) and do not express Ly6G.
Surface phenotype of M-pDCs compared with FL-pDCs and ex-vivo isolated spleen pDCs. Stained cells from day 6 M-CSF cultures (filled histograms), FL cultures (gray open histograms), or freshly isolated spleen DC (black histograms) were gated on pDCs by selecting for the expression of CD11c and CD45RA or CD45R among the PI-negative cells. The expression of a range of surface markers on the pDC surface is shown. The light gray histograms represent the background staining of the M-pDCs within each stain. All M-pDCs also lacked expression of CD3, CD19, CD49b, and NK1.1. The surface phenotypes shown are from one experiment representative of 2 to 5 experiments for M-pDCs, 2–3 experiments for FL-pDCs, and 2 experiments for spleen pDCs.
Surface phenotype of M-pDCs compared with FL-pDCs and ex-vivo isolated spleen pDCs. Stained cells from day 6 M-CSF cultures (filled histograms), FL cultures (gray open histograms), or freshly isolated spleen DC (black histograms) were gated on pDCs by selecting for the expression of CD11c and CD45RA or CD45R among the PI-negative cells. The expression of a range of surface markers on the pDC surface is shown. The light gray histograms represent the background staining of the M-pDCs within each stain. All M-pDCs also lacked expression of CD3, CD19, CD49b, and NK1.1. The surface phenotypes shown are from one experiment representative of 2 to 5 experiments for M-pDCs, 2–3 experiments for FL-pDCs, and 2 experiments for spleen pDCs.
Highly purified M-pDCs were activated by ligands for TLR7 and TLR9
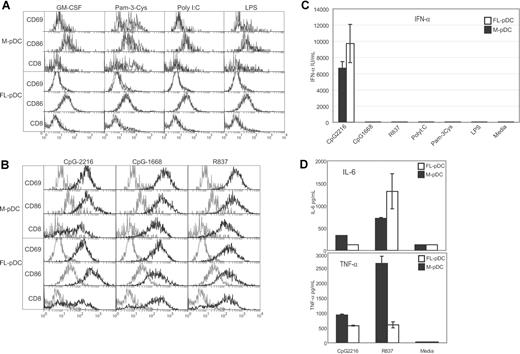
Similar to FL-pDCs and ex-vivo pDCs, the M-pDCs were not activated with ligands to TLR3, TLR4, or GM-CSF but showed a very minor surface activation to the TLR2 ligand Pam-3-Cys (Figure 3A). The survival of the M-pDCs to these stimuli was extremely poor (< 5%; data not shown).
M-pDCs are activated and produce IFN-α in response to TLR9 stimulation and other cytokines in response to TLR7 stimulation. Highly purified, sorted M-pDCs or FL-pDCs were incubated for 18 hours with the TLR ligands shown. The surface phenotype of the pDCs were analyzed (A,B); gray histograms indicate surface expression levels of cells cultured in media only, and black histograms indicate the expression levels of cells cultured in the stimuli indicated. Supernatants were assayed by ELISA for the presence of IFN-α (C) or by cytometric bead assay (CBA) for the production of IL-6 and TNF-α (D). No IFN-γ, IL-10, IL-12p70, or MCP-1 was detected by CBA in the M-pDCs supernatants. The data shown are from one experiment representative of 3 experiments (A,B), 5 experiments (C), and 3 experiments (D). Error bars represent the range of duplicate samples.
M-pDCs are activated and produce IFN-α in response to TLR9 stimulation and other cytokines in response to TLR7 stimulation. Highly purified, sorted M-pDCs or FL-pDCs were incubated for 18 hours with the TLR ligands shown. The surface phenotype of the pDCs were analyzed (A,B); gray histograms indicate surface expression levels of cells cultured in media only, and black histograms indicate the expression levels of cells cultured in the stimuli indicated. Supernatants were assayed by ELISA for the presence of IFN-α (C) or by cytometric bead assay (CBA) for the production of IL-6 and TNF-α (D). No IFN-γ, IL-10, IL-12p70, or MCP-1 was detected by CBA in the M-pDCs supernatants. The data shown are from one experiment representative of 3 experiments (A,B), 5 experiments (C), and 3 experiments (D). Error bars represent the range of duplicate samples.
Similar to FL-pDCs and ex-vivo pDCs, the sorted M-pDCs were activated with ligands for TLR7 and -9, as indicated by elevated expression of CD8α, CD69, CD86, and CD40 (Figure 3B and data not shown). M-pDCs produced detectable cytokines only to TLR7 and 9 ligands (Figure 3C and data not shown). In response to type A CpG-ODN (CpG-2216), the M-pDCs produced high levels of IFN-α (Figure 3C). The absolute amount of IFN-α produced by the M-pDCs was in the range of 2-fold less than that produced by FL-pDCs. Other cytokines induced by TLR7 and -9 ligands included IL-6 and TNF-α (Figure 3D). The M-pDCs produced levels of IL-6 similar to those of the FL-pDCs, but they produced higher levels of TNF-α than the FL-pDCs. The production of IL-10, IL-12p70, MCP-1, and IFN-γ by the M-pDCs was also tested, but none of these cytokines were detected.
cDCs also develop in M-CSF BM cultures
As shown in Figure 1, M-CSF induced the development of CD11c+ cells from BM that did not concomitantly express CD45RA or T-cell, B-cell, or NK-cell markers. Like the M-pDCs, these cells also developed in BM cultures of FLKO mice. Surface phenotypic analyses of these cells revealed that they resembled cDCs, expressing costimulation markers and MHCII (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The M-cDCs generated within the M-CSF BM cultures, like the FL-cDCs, were heterogeneous with respect to activation markers (CD80, CD86, CD40, MHCII) but overall displayed higher levels of these markers than the cDCs induced in FL cultures. In the FL-DC cultures, CD11bloCD24hi cDCs correspond to the CD8+CD11b−CD24hiCD205+ splenic cDCs equivalents. The cDCs generated in the M-CSF cultures contain cells expressing a lower level of CD11b but they lack the very high CD24-expressing cells present among FL-cDCs, suggesting that perhaps they do not produce CD8+ cDCs equivalents.
Upon TLR stimulation, the cDCs were activated to resemble mature, ex-vivo activated cDCs (data not shown).
Of note in the activation of the M-cDCs and M-pDCs is that they responded well to TLR9 ligands. This is quite a different scenario to macrophages within M-CSF cultures that down regulate TLR9 and consequently respond poorly to TLR9 ligands.25
M-pDC and M-cDC generation was dependent upon active c-fms
Two groups have previously presented evidence that Flt3+ cells within the BM CMP and CLP are the precursors of cDCs and pDCs within mouse lymphoid organs.21,26 Consequently, it has been assumed that FL is essential for DC development. Thus the generation of DC, particularly the generation of pDCs with a typical monocytic poietin, in the absence of FL, was unexpected. As shown in Figure 1, the M-pDCs clearly develop in the absence of FL, yet they also show many similarities to FL-pDCs. Given that M-CSF and FL, as well as their respective receptors, Flt3 and c-fms, have structural similarities, we considered the possibility that M-CSF was actually able to signal via Flt3. Thus, whether M-CSF was acting as a FL surrogate that also signaled via Flt3, in effect generating M-CSF-induced “FL-DC,” was examined.
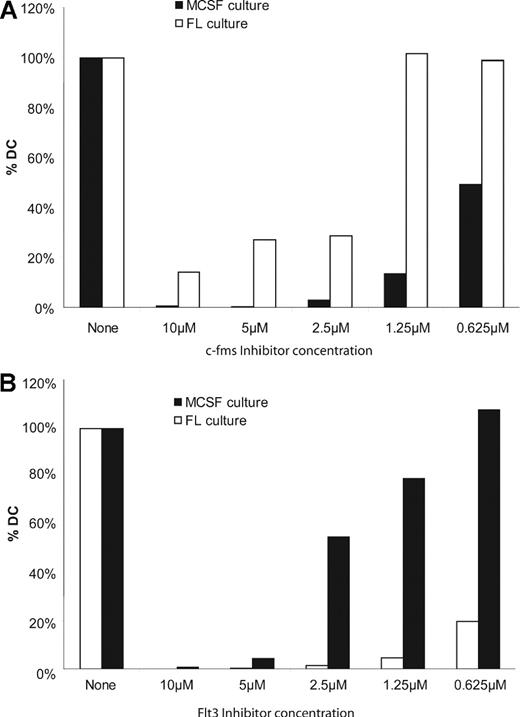
There are numerous inhibitors of receptor tyrosine kinases with various levels of cross-reactivity. We tested the effect of c-fms receptor tyrosine kinase inhibitor (EMD Biosciences), which is reported to be a highly specific c-fms inhibitor, on the generation of cells from total BM. Indeed, c-fms receptor tyrosine kinase inhibitor entirely blocked the hematopoietic effects of M-CSF over a broad concentration range (Figure 4A). The highest concentrations of inhibitor also blocked the generation of DC by FL. However, c-fms inhibitor used in the range of 0.63 to 1.3 μmol/L had only minor effects on FL-mediated FL-DC generation (Figure 4A and Figure S2). Because these same low concentrations still blocked M-DC generation, it is unlikely that M-pDC generation occurs via M-CSF acting through the Flt3 receptor tyrosine kinase. Although the c-fms inhibitor is probably a promiscuous receptor tyrosine kinase inhibitor at high concentrations, or has nonspecific toxic effects, it did not inhibit FL-DC generation, at low concentrations, implying that it was indeed c-fms specific. In stark contrast, a Flt3 inhibitor (Flt3 Inhibitor II; EMD Biosciences) was highly inhibitory for generation of FL-DCs but not M-DCs (Figures 4B, S2). Thus, we propose that M-pDCs and also M-cDCs can be generated by M-CSF via c-fms signaling, independently of Flt3 and FL in vitro.
The generation of M-DC is inhibited by a c-fms inhibitor. Replicate M-CSF and FL cultures were conducted in parallel in the presence or absence of a range of c-fms receptor tyrosine kinase inhibitor (A) or Flt3 inhibitor (B) concentrations. At the end of the culture period, all cells were counted. The number of cells harvested from cultures without inhibitor was set at 100%. Cells from cultures containing inhibitor were expressed as a percentage of cells obtained in the absence of inhibitor. The data shown in each panel are from one experiment each, representative of 2 experiments carried out for each of the inhibitors.
The generation of M-DC is inhibited by a c-fms inhibitor. Replicate M-CSF and FL cultures were conducted in parallel in the presence or absence of a range of c-fms receptor tyrosine kinase inhibitor (A) or Flt3 inhibitor (B) concentrations. At the end of the culture period, all cells were counted. The number of cells harvested from cultures without inhibitor was set at 100%. Cells from cultures containing inhibitor were expressed as a percentage of cells obtained in the absence of inhibitor. The data shown in each panel are from one experiment each, representative of 2 experiments carried out for each of the inhibitors.
M-CSF induces M-pDCs and M-cDCs generation in vivo
It has been reported that op/op mice that carry a mutation in the gene for M-CSF, and thus lack functional M-CSF, exhibit reduced numbers of splenic DC.27 Specifically cDCs were reduced by approximately 2-fold and pDCs by approximately 3-fold. This was substantially less of an effect than that seen in mice lacking FL; nevertheless, lack of M-CSF did result in reduced DC numbers, and this could be in line with our in vitro data that M-CSF is a DC poietin.
To test the other side of this story, initiated by MacDonald et al (2005), we administered M-CSF to mice to analyze whether M-CSF could actually increase DC numbers in vivo. Other reports in the literature have administered a range of exogenous M-CSF concentrations in the range of 10 to 200 μg/day. The source of M-CSF used has varied widely and consequently; so, too, has the specific activity. It is thus impossible to gauge an optimal or saturating level of M-CSF to administer. Moreover, it has been reported that M-CSF has an extremely short half-life of only 10 minutes in the circulation.28 Because of the prohibitive cost of commercial M-CSF with a determined specific activity, we opted for a “proof of principal” trial experiment and administered 10 μg/day of M-CSF to C57BL/6 or FLKO mice for 5 days. We have not titrated this amount or attempted to extend the application period. It was clear, however, that after the 5-day application period, a huge increase in F4/80hi cells was observed in peritoneal lavages of the mice that we examined (data not shown). We interpreted these observations to indicate that indeed M-CSF was inducing an effect in vivo.
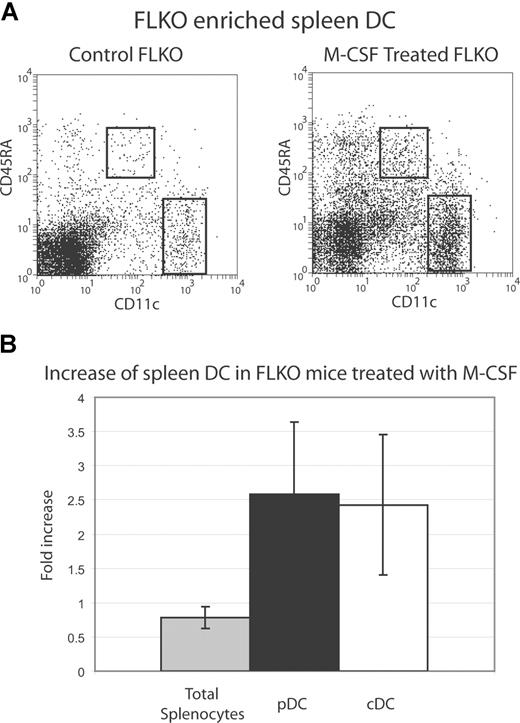
Analysis of DC populations in the spleen revealed that M-CSF was inducing a reproducible increase of approximately 2-fold in both pDC and cDC numbers. This increase was evident in C57BL/6 and FLKO mice (Figure 5). A closer examination of cDC subpopu-lations indicated that CD8+ and CD8− populations were increased fairly uniformly (data not shown). The M-CSF treatment did not induce an increase in total splenocytes (Figure 5B), although there was an increase, as evident in Figure 5A, in light density, non-DCs, purified after M-CSF treatment. The CD11cintCD45RA− and CD45RAhiCD11c−/lo cells could potentially contain immature DC populations but this was not examined further. A more extensive increase (greater than 6-fold) of cells that resembled DC was also evident in the peritoneal lavage of a C57BL/6 mouse examined (data not shown).
M-CSF treatment increases DC numbers in vivo. FLKO mice were treated for 5 consecutive days with 10 μg/day M-CSF i.p. in 0.01% BSA in PBS or with vehicle alone (control). DC were purified from FLKO spleens and stained with CD11c and CD45RA and shown gated in (A). Total splenocytes and pDC and cDC populations were enumerated and are shown as a -fold increase compared with vehicle-treated mice (B). Data are pooled from 3 individual mice within 2 separate experiments compared with control mice analyzed the same day.
M-CSF treatment increases DC numbers in vivo. FLKO mice were treated for 5 consecutive days with 10 μg/day M-CSF i.p. in 0.01% BSA in PBS or with vehicle alone (control). DC were purified from FLKO spleens and stained with CD11c and CD45RA and shown gated in (A). Total splenocytes and pDC and cDC populations were enumerated and are shown as a -fold increase compared with vehicle-treated mice (B). Data are pooled from 3 individual mice within 2 separate experiments compared with control mice analyzed the same day.
Thus, we have the first evidence that as well as inducing pDCs and cDCs in vitro, M-CSF is also capable of increasing DC numbers in vivo, even in the absence of FL.
What progenitor cell populations harbor M-DC precursor potential?
The identification of DC subtypes that could be generated in the absence of FL both in vivo and in vitro immediately raises the question of the nature of their precursor cells. We considered whether this factor acts on an early lineage progenitor or, because M-CSF is traditionally considered a “myeloid” growth factor, on a later precursor within the myeloid lineage to generate M-DC. CMP, GMP, and CLP were purified from mouse BM. Serial dilutions of the purified cell populations were admixed with “feeder” UBC-GFP BM cells, and M-CSF cultures were carried out for 6 days. Analyses of the progeny of each of the 3 precursor populations revealed that committed GMP were not capable of M-DC production in our culture conditions (Figure 6B,C).
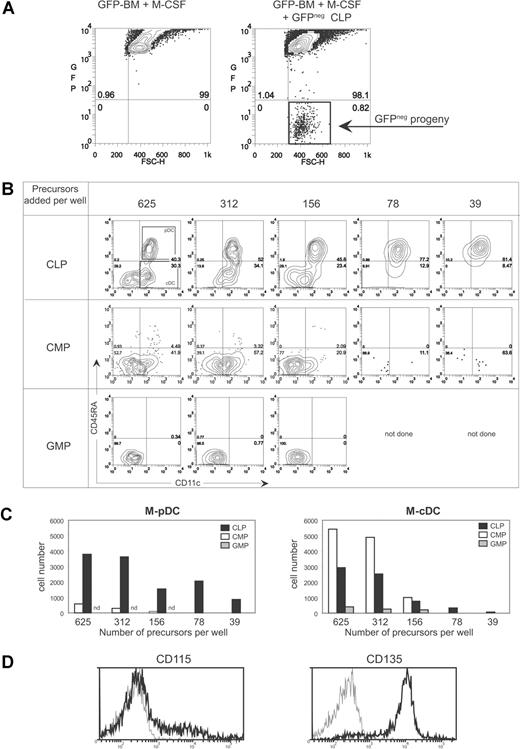
CLP are major producers of M-pDCs and CMP produce mainly M-cDCs. Within control M-CSF cultures containing only UBC-GFP BM cells, all cells expressed high levels of GFP and fluoresced strongly in the FITC (GFP) channel (A left panel). The progeny of C57BL/6 progenitors spiked into UBC-GFP BM cultures were gated as FITC/GFPneg cells (A right panel). Serial dilutions of CLP, CMP, or GMP (625 to 39 cell equivalents are shown) were added to 1.0 × 106 UBC-GFP BM cells in 1 mL, and M-CSF cultures were carried out for 6 days. GFPnegPIneg cells were gated and stained with CD45RA and CD11c. The resulting M-DC plots are shown in panel B, and the gates used to determine M-pDCs and M-cDCs are shown in the top left contour. The absolute number of M-pDCs and M-cDCs obtained in the cultures of panel B are shown in panel C. Data shown are from one experiment. Similar results were obtained in a second experiment. (D) The expression of CD115 (M-CSFR) and CD135 (Flt3) on CLP is shown. Gray histogram represents CLP stained with a PE-conjugated isotype control. Staining was carried out in 2 individual experiments (CD115) with similar results, and one of these experiments also included the CD135 staining.
CLP are major producers of M-pDCs and CMP produce mainly M-cDCs. Within control M-CSF cultures containing only UBC-GFP BM cells, all cells expressed high levels of GFP and fluoresced strongly in the FITC (GFP) channel (A left panel). The progeny of C57BL/6 progenitors spiked into UBC-GFP BM cultures were gated as FITC/GFPneg cells (A right panel). Serial dilutions of CLP, CMP, or GMP (625 to 39 cell equivalents are shown) were added to 1.0 × 106 UBC-GFP BM cells in 1 mL, and M-CSF cultures were carried out for 6 days. GFPnegPIneg cells were gated and stained with CD45RA and CD11c. The resulting M-DC plots are shown in panel B, and the gates used to determine M-pDCs and M-cDCs are shown in the top left contour. The absolute number of M-pDCs and M-cDCs obtained in the cultures of panel B are shown in panel C. Data shown are from one experiment. Similar results were obtained in a second experiment. (D) The expression of CD115 (M-CSFR) and CD135 (Flt3) on CLP is shown. Gray histogram represents CLP stained with a PE-conjugated isotype control. Staining was carried out in 2 individual experiments (CD115) with similar results, and one of these experiments also included the CD135 staining.
CMP were quite efficient precursors of M-cDCs, producing an output of 8 to 10 M-cDCs per input progenitor cell, but this level dramatically dropped off with input cell numbers lower than 156, possibly suggesting that the M-cDCs were arising from a small subpopulation of the CMP. The CLP, on the other hand, were at least 10-fold more efficient than CMP at M-pDCs generation. The CLP produced an output of at least 10 M-pDCs and approximately 5 M-cDCs per input progenitor cell (Figure 6B,C). These data indicate that both CMP and CLP are progenitors that can respond to M-CSF to generate M-DC. Surprisingly the CLP were the most efficient M-pDC progenitors. We stained CLP with antibodies to M-CSFR (CD115; Figure 6D). Approximately 20% of cells within the CLP gate expressed high levels of CD115. Flt3 (CD135) staining done in parallel showed that the majority of the CLP were CD135+ and thus at least some of the CD115+ cells must also be CD135+. Moreover, many of the CLP expressed very low staining of CD115, just above the background of an isotype-matched control, indicating that indeed many CLP are armed with the necessary receptor to respond to M-CSF in the DC cultures that we describe. Thus, the M-DC that we have described most likely arise in vitro and in vivo from precursors within the CLP and CMP progenitor populations.
Discussion
Our data indicate that M-CSF, a growth factor traditionally associated only with monocyte and macrophage development, plays a unique role in the development of pDCs and cDCs. It is noteworthy that we have shown that M-CSF can drive DC development independently of FL, a growth factor seen in recent years as an essential component of steady-state DC development. M-CSF and FL use structurally related receptors that are members of the group III receptor tyrosine kinases. However, with the use of pharmacologic inhibitors, we have shown that the action of M-CSF is via its own receptor, c-fms, and not via potential cross-reactivity with Flt3.
The FLKO mice display a major defect in pDC and cDC numbers. In the spleen, they have only approximately 12% of the DC numbers of C57BL/6 mice. However, because pDCs and cDCs are not completely absent, other factors must be able to drive the generation of these DC subsets in the absence of FL. FLKO mice have reduced myeloid precursors (CFU-GM cells) within BM14 and at least a 10-fold decrease in CLP.29 Because FL acts upon early hematopoietic stem cells as well as later stage DC precursors, it is not clear at which stage in DC development the lack of FL has its most profound effect. There is possibly a lack of DCs that require FL to act upon precursors throughout development and, second, a lack of DCs that require FL for optimal early precursor development but are independent of FL once committed to the DC lineage.
The 12% DC population remaining in the FLKO mice may be driven from FL-independent precursors by M-CSF. Our data indicate that the generation of M-CSF driven DC is reduced in the FLKO, compared with wild type, but at the same time, their generation from wild-type BM cells is not affected by a Flt3-specific pharmacologic inhibitor. This strongly suggests that optimal generation of M-CSF driven DC occurs via a precursor that is optimally regulated by FL and that these precursors are targets of M-CSF, independently of FL. The data of Figure 6 strongly suggest that these precursors consist of both CLP and CMP. Because CLP are reduced at least 10-fold in the FLKO mouse, the potent lack of DC in the FLKO mouse may be due in part to inefficient M-CSF–driven DC development of FL-dependent CLP.
There are numerous reports of infection settings that are DC-enhancing and immune conditions that involve increases in DC numbers that also report increased levels of circulating FL. Some of these same conditions have also been reported to enhance circulating M-CSF levels. For example, the serum of patients with Langerhans cell histiocytosis displays increased FL and M-CSF,30 viral infections and IFN-α, both shown to increase circulating FL, also increase M-CSF,31,,–34 the serum of patients with systemic lupus erythematosus (SLE) has increased FL35 , and animal models of SLE report elevated M-CSF levels.36 Within these settings, the simultaneous elevation of FL and M-CSF, with M-CSF previously not suggested as capable of enhancing DC subsets, potentiates a cooperative role for these growth factors in DC development. Our data presented herein also demonstrate a FL-independent role of M-CSF in the generation of murine pDCs and cDCs.
Of potential clinical interest is the possible connection of our findings with the reports of human pDCs leukemia/lymphoma (pDCL). pDCL are recognised as aggressive neoplastic forms of pDCs that share some surface markers with normal pDCs. At present, these tumors are treated with limited success with aggressive chemotherapy and radiotherapy and occasionally BM transplant. At present, the specific oncogenes responsible for the malignancy of pDCL are not known. Olweus et al37 reported that IL-3Rhi DC resident in T-cell areas of human lymphoid organs (pDCs) arose from M-CSFRhi CD34+ precursors. The murine study by MacDonald et al27 showed that many mouse pDCs develop via an M-CSFR+ stage, although the specific phenotype of murine pDCs precursors has not yet been examined. Our examination of recent reviews and reports of pDCL in the literature38,–40 failed to find any evidence for the examination of CD115 (the M-CSFR) on these tumor cells. However, it has been reported that, along with other chromosomal abnormalities, the long arm of chromosome 5, including the region encoding the M-CSFR (5q33-35), often displays abnormalities, including deletions, translocations, and genetic increases.38,,–41 Whether the expression of the M-CSFR is affected in these cases has not been reported. Dijkman et al41 also reported that chromosome 13 often harbors deletions in pDCL; in one case in which the specific deletion was identified, it was shown to be a monoallelic deletion of Flt3. The pDCL cells of this patient unexpectedly displayed increased mRNA for Flt3.
Based on the available published data and our findings presented herein, it is possible that some of the pDCL tumors develop via aberrant activation of the M-CSFR, with or without Flt3 involvement. We have shown that M-CSF is able to drive pDC and cDC development independently of FL. Both FL and M-CSF can act on CLP and CMP precursor cells, but whether they are actually acting on different or overlapping subsets of these populations has not yet been examined. However, the use of c-fms inhibitors alone or in combination with Flt3 inhibitors might be beneficial for the treatment of pDCL.
The finding that M-CSF apparently acts as a growth factor for the development of pDCs and cDCs broadens the range of leukocytes that it is known to affect. M-CSF and its receptor, c-fms, must also be considered in the basic developmental science of DC subsets and with respect to the treatment of tumors that involve DC or their precursors. Perturbation of M-CSFR using available inhibitors, alone or in combination with Flt3 inhibitors, may also be useful in the short term for disturbing pDC development in cases of unwanted pDC activation, such as SLE.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Li Wu, WEHI, Melbourne, Australia, for advice on the isolation of precursor populations, and Cornelia Wagner and the Animal Facility staff at Bavarian Nordic for excellent technical assistance.
This work was financed by Bavarian Nordic.
Authorship
Contribution: B.F. performed experimental procedures and analyzed data. M.S. provided useful suggestions and experimental mice. H.H. and M.O. designed research, performed some experimental procedures, and analyzed data.
H.H. and M.O. contributed equally to this study.
Conflict-of-interest disclosure: All authors are employees of Bavarian Nordic.
Correspondence: Meredith O'Keeffe or Hubertus Hochrein, Immunology, Research Department, Bavarian Nordic, Fraunhoferstrasse 13, 82152 Martinsried, Germany; e-mail: meredith.okeeffe@bavarian-nordic.com or hubertus.hochrein@bavarian-nordic.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal