The canonical Wnt signaling pathway plays key roles in stem-cell maintenance, progenitor cell expansion, and lineage decisions. Transcriptional responses induced by Wnt depend on the association of either β-catenin or γ-catenin with lymphoid enhancer factor/T cell factor transcription factors. Here we show that hematopoiesis, including thymopoiesis, is normal in the combined absence of β- and γ-catenin. Double-deficient hematopoietic stem cells maintain long-term repopulation capacity and multilineage differentiation potential. Unexpectedly, 2 independent ex vivo reporter gene assays show that Wnt signal transmission is maintained in double-deficient hematopoietic stem cells, thymocytes, or peripheral T cells. In contrast, Wnt signaling is strongly reduced in thymocytes lacking TCF-1 or in nonhematopoietic cells devoid of β-catenin. These data provide the first evidence that hematopoietic cells can transduce canonical Wnt signals in the combined absence of β- and γ-catenin.
Introduction
The function and the hierarchical organization of the hematopoietic system depend on the unique ability of rare hematopoietic stem cells (HSCs) to self-renew and to give rise to committed progenitors, which will generate all blood cell lineages. Our understanding of the control mechanisms, which govern the balance of proliferation and differentiation of HSCs, is crucial for the development of therapies in areas such as bone marrow transplantation and the treatment of leukemia
The canonical Wnt signaling pathway plays a key role in stem-cell maintenance, expansion of committed progenitor cells, and control of lineage decisions in a variety of tissues, including the hematopoietic system.1 β-Catenin is the central molecule in the canonical Wnt signaling pathway, which is tightly regulated at the level of protein stability. β-Catenin transmits the Wnt signal into the nucleus, where it acts as transcriptional coactivator by binding to members of the lymphoid enhancer factor/T cell factor (LEF/TCF) family of transcription factors. The related molecule γ-catenin/plakoglobin can fulfill similar functions and activate target genes on Wnt signaling.2 Deregulation of canonical Wnt signaling by aberrant stabilization of β-catenin is linked to a range of diseases including various cancers, whereas ectopic expression of γ-catenin has been linked to acute myeloid leukemias.3,4
Several reports have implicated Wnt signaling in HSC expansion and demonstrated responsiveness of HSCs to Wnt signals.5,6 Conversely, deletion of Wnt genes or the Wnt receptor Fzd9 affects T- and/or B-cell development7,8 and transgenic or retroviral expression of inhibitors of canonical Wnt signaling such as axin or dkk1 further indicated an important function of this pathway in hematopoiesis and thymopoiesis.5,9,10 Individual gene ablations of LEF-1 and TCF-1 transcription factors displayed phenotypes in B-, T-, and NK-cell development.11,–13 TCF-1 deficiency resulted in incomplete blocks within the CD4−CD8− (DN) compartment of early thymocyte development and at the transition from immature single positive (ISP) to the CD4+CD8+ (DP) stage.11,14,–16 LEF-1-deficiency aggravated the phenotype of mice with a hypomorphic TCF-1 allele,17 demonstrating that the 2 factors play to some degree redundant functions during T-cell development. However, a complete block of Wnt signaling in the hematopoietic system has not been analyzed and results obtained by individual gene ablations of the 2 known signal transmitters β- and γ-catenin have thus far failed to support a critical role of this pathway in hematopoiesis.16,18,19
Strikingly, a genetic complementation approach showed that the N-terminal domain of TCF-1 (amino acids 1-117) was essential to rescue thymocyte development in TCF-1 deficient mice.15 This domain in TCF-1 includes the β-catenin binding site (aa 1-50) and a putative γ-catenin interaction site,20 suggesting that catenin binding to TCF-1 is critical for T-cell development. Therefore, there remains a major discrepancy between the phenotypes of LEF-1– and TCF-1–deficient mice and the phenotypes of mice lacking the coactivators β-catenin or γ-catenin. To address these issues, we have analyzed hematopoiesis and thymopoiesis in the combined absence of both β- and γ-catenin. We find that hematopoiesis is not dependent on the 2 known Wnt signal transmitters β- and γ-catenin. Surprisingly, reporter assays show that canonical Wnt signaling is maintained in the combined absence of both genes, indicating a novel mechanism of Wnt signal transduction in the hematopoietic system.
Methods
Mice
CD45.1 congenic C57BL/6 (B6) (Jackson Lab, Bar Harbor, ME), Mx-Cre transgenic,21 β-cateninlox/lox,22 γ-catenin+/−,23 conductinlacZ24 and TCF-1−/−11 mice have been described. Fetal liver cells were obtained at day 12.5 of gestation, genotyped by polymerase chain reaction (PCR) and injected (106) into lethally irradiated (960 rad, 137Cs source) recipient B6 CD45.1+ mice. Primary recipients were allowed to engraft for 6 to 8 weeks. β-Catenin deletion was induced by 3 injections of polyinosine-polycytosine (pI-pC) (Sigma, St Louis, MO; 250 μg each time) on day 0, 3, and 5. BM cells were harvested 10 to 14 days after the last injection. For BM chimeras, 2:1 or 3:1 mixtures of experimental (CD45.2+) and wild-type (CD45.1+) BM cells were used (3-6 × 106 cells total). Chimeras were tail bled at the indicated time points and lymphoid tissues were analyzed 2 to 12 months after reconstitution. TCF-1−/− mice were analyzed at 2 to 3 weeks of age.
Flow cytometry
Thymocytes, BM, or spleen cells were incubated with 2.4G2 (anti-CD16/32) hybridoma supernatant to block Fc receptors before staining for multicolor flow cytometry. The following mAbs were used: CD3ϵ (17A2), CD4 (GK1.5), CD8 (53.6.7), CD11b (Mac1)(M1/70), CD41 (MWReg30), CD45R (B220) (RA3–6B2), CD45.1 (A20.1), CD45.2 (104), CD117 (ACK2), CD127 (A7R34), CD135 (FLT3R)(A2F10.1), CD161 (NK1.1) (PK136), GR1 (RB6–8C5), TCRβ (H57), TCRγδ (GL3), and Ter119. Abs were conjugated to appropriate fluorochromes at the LICR or purchased from BD PharMingen (San Diego, CA) or eBioscience (San Diego, CA).
A cocktail of FITC-conjugated anti-TCRβ, TCRγδ, CD3ϵ, CD4, CD8, Mac-1, B220, NK1.1, Ter119, and GR1 mAbs was used to gate lineage-negative (lin−) cells. Pe-Cy7-conjugated anti-CD4, CD8 CD11b, B220, GR1, and TER119 mAbs were used to gate lin− cells for reporter gene assays. Samples were run on a FACSCanto flow cytometer and analyzed with Cell Quest or FACS Diva software (Becton Dickinson, San Jose, CA). After cell-surface staining, cells were loaded with fluoresceine di-β-galactopyranoside (Molecular Probes, Eugene, OR) by osmotic shock, washed and analyzed by flow cytometry. Dead cells were excluded by 7-AAD uptake (Molecular Probes).
Thymocyte survival
Thymocyte survival was determined by incubating cells overnight at 4°C or 37°C in 96-well plates (at 2-4 × 105 cells/200 μL of complete Dulbecco's modified Eagle medium + 10% fetal calf serum). After collection, the cells were surface stained as described above. A fixed number of microspheres (Bacteria counting kit, Molecular Probes) were added to each sample. Thymocytes and microspheres were discriminated based on FSC and SSC. Dead cells were further excluded based on 7-AAD uptake (Molecular Probes). Thymocyte survival was calculated by comparing the ratio of beads to viable cells at 4°C and 37°C of culture. The ratio obtained for the 4°C culture was assigned to 100%.
Pull down
The NH2-terminal 117amino acids of TCF-1 were cloned into pGEX4T (Pharmacia) for N-terminal GST fusion. TCF-1-GST fusion proteins were reacted with lysates from HEK293T cells, according to the manufacturer's recommendation (Pierce, Rockford, IL). Bound protein was revealed by Western blotting using mAbs to β-catenin (clone 14) and γ-catenin (PG 11E4; Invitrogen, Carlsbad, CA).
Cell sorting
For cell sorting, the indicated cell suspensions were stained with CD45.2-FITC and CD45.1-PE. Appropriate populations were sorted using a FACSAria (Becton Dickinson) cell sorter. The purity was generally more than 99% on reanalysis.
Wnt reporter assays in vitro
HEK293T cells were transiently transfected with expression vectors for TCF-1, β-catenin, stabilized βcatenin (S33A, S37A, T41A, and S45A), truncated β-catenin (starting at M328 and M363), γ-catenin, and lacZ together with a Wnt reporter construct. This artificial Wnt-responsive luciferase reporter was constructed by inserting 20 copies of an optimized LEF/TCF binding site in front of a minimal TATA-box promoter into pGL3 (Promega). After 48 hours, luciferase activity was measured (Promega) and corrected for transfection efficiency as determined by 2-Nitrophenyl-β-d-galacto pyranoside (Sigma) staining of β-galactosidase activity. Lentiviral reporter constructs contained 20 copies of an optimized LEF/TCF binding site in front of a minimal promoter driving Venus (yellow-shifted GFP) expression. This inducible cassette was followed by a constitutive expression cassette consisting of the PGK promoter and cDNAs for Wnt1, a fusion of LEF-1 and the C-terminus of β-catenin or human CD2. Virus production and titration was performed in 293T cells according to standard procedures. For lentiviral reporter assays, splenic CD8+ T cells were purified from chimeric mice using MACS (Miltenyi Biotech). CD8+ T cells (3 × 104) were stimulated for 24 hours using plastic coated anti-CD3 and anti-CD28 mAbs in the presence of huIL-2 (50 ng/mL) followed by infection of T cells with the various reporter viruses in the presence of 5 μg/mL of polybrene. Forty-eight hours after infection, Venus reporter gene expression was determined by flow cytometry. Knockdown of β-catenin was performed using targeting and nontargeting RNAi from Dharmacon according to the manufacturer's recommendations.
Genomic PCR and typing
PCR genotyping of the β- and γ-catenin alleles has been described.22,23 For quantitative PCR, a primer pair (actgcctttgttctcttcccttctg, agcaggtgagggtcagtatgtcg) was used, which detects only the nonrecombined floxed β-catenin allele. The osf2 locus was amplified as a control. For Southern blotting, genomic DNA was digested with Sac1, blotted, and probed with a DNA fragment containing the end of intron 1, exon 2, and the beginning of intron 2 of the β-catenin gene.
Western blot.
Western blots on total cell lysates from total fetal liver or FACS sorted cells (1-2 × 106 cells per lane) were performed as described.15 Blots were incubated overnight at 4°C with primary antibodies: anti-β-catenin (clone 14), anti-γ-catenin (clone 15, Becton Dickinson Transduction Lab or PG 11E4, Invitrogen) or anti-α-tubulin (B-5-1-2, Sigma). Western blots were revealed with HRP-conjugated anti-mouse and anti-rabbit Abs (Sigma) followed by enhanced chemiluminescence detection (Pierce).
Results
Hematopoiesis in the combined absence of β- and γ-catenin
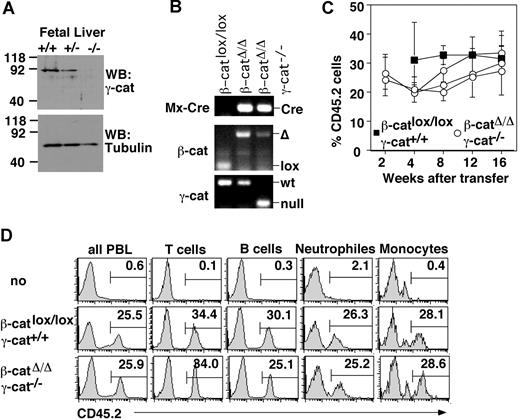
To address the possibility that redundancies between β-catenin and γ-catenin conceal an important role of these canonical Wnt signal transmitters, we analyzed hematopoiesis and lymphopoiesis in mice lacking both β- and γ-catenin. We combined the γ-catenin null allele23 with our inducible β-cateninlox allele22 and a Mx-Cre transgene, which allows pI-pC–mediated, IFN-α,β–induced expression of Cre recombinase and consequently ablation of the β-catenin gene in all cells of the hematopoietic system. Since the null mutation of γ-catenin is embryonic lethal between day 12 and 16 of gestation, fetuses were recovered at day 12.5 of gestation and genotyped by PCR (not shown). Western blot analysis of whole embryos ensured the absence of γ-catenin protein in mice with a targeted γ-catenin locus (Figure 1A). Fetal liver-derived cells were used to reconstitute lethally irradiated, adult recipient mice. Polymorphisms in the CD45 molecule were used to discriminate hematopoietic cells of donor (CD45.2+) and recipient (CD45.1+) origin. After stable engraftment for more than 8 weeks (data not shown), β-catenin deletion was induced by pI-pC injection and monitored by PCR (Figure 1B). The competitive, long-term repopulation potential of β- and γ-catenin double-deficient stem cells was tested in secondary, mixed bone marrow (BM) chimeras using 2:1 or 3:1 mixtures of experimental (CD45.2+) and wild-type (CD45.1+) BM cells. To reduce possible side effects of pI-pC treatment, mutant BM cells were isolated 10 to 14 days after the last injection of primary recipients.
Maintenance of hematopoiesis in the combined absence of β- and γ-catenin. (A) γ-Catenin expression in embryonic day 12.5 fetal liver cells of the indicated genotypes as detected by immunoblot analysis with mAbs specific for the carboxy terminus of γ-catenin (clone 15) and tubulin (to ensure equal protein loading). Numbers indicate the molecular weight in kDa. (B) Genomic DNA from the indicated types of primary chimeras was isolated 10 days after pI-pC injection from sorted CD45.2+ BM cells and subjected to PCR analysis to detect Mx-Cre, γ-catenin, and β-catenin alleles. The mutant (indicated as null) and wild-type (wt) γ-catenin alleles are detected as 150 bp and 300 bp PCR products, respectively. The floxed (indicated as lox) and deleted (Δ) β-catenin alleles yield 183 bp and 481 bp bands, respectively. (C) β-Catenin was deleted in primary recipients by pI-pC. After 10 to 14 days, a mixture of deleted (CD45.2) and wild-type (CD45.1) BM cells were transplanted into lethally irradiated secondary recipients (CD45.1). Recipient mice were bled at the indicated time points. Each point represents the mean percentage of CD45.2+ peripheral blood lymphocyte (± SD) of 4-5 recipient mice per individual fetus. Each curve represents an individual donor fetus. (D) Histograms depict the contribution of CD45.2+ BM precursors to the lymphoid (B and T cells) and myeloid lineages (neutrophiles, CD11b+ GR1+, and monocytes CD11b+ GR1−) at 16 weeks after reconstitution. Numbers on the graphs are percentage of total cells in delineated regions. Data show a representative analysis (of 5-7 performed).
Maintenance of hematopoiesis in the combined absence of β- and γ-catenin. (A) γ-Catenin expression in embryonic day 12.5 fetal liver cells of the indicated genotypes as detected by immunoblot analysis with mAbs specific for the carboxy terminus of γ-catenin (clone 15) and tubulin (to ensure equal protein loading). Numbers indicate the molecular weight in kDa. (B) Genomic DNA from the indicated types of primary chimeras was isolated 10 days after pI-pC injection from sorted CD45.2+ BM cells and subjected to PCR analysis to detect Mx-Cre, γ-catenin, and β-catenin alleles. The mutant (indicated as null) and wild-type (wt) γ-catenin alleles are detected as 150 bp and 300 bp PCR products, respectively. The floxed (indicated as lox) and deleted (Δ) β-catenin alleles yield 183 bp and 481 bp bands, respectively. (C) β-Catenin was deleted in primary recipients by pI-pC. After 10 to 14 days, a mixture of deleted (CD45.2) and wild-type (CD45.1) BM cells were transplanted into lethally irradiated secondary recipients (CD45.1). Recipient mice were bled at the indicated time points. Each point represents the mean percentage of CD45.2+ peripheral blood lymphocyte (± SD) of 4-5 recipient mice per individual fetus. Each curve represents an individual donor fetus. (D) Histograms depict the contribution of CD45.2+ BM precursors to the lymphoid (B and T cells) and myeloid lineages (neutrophiles, CD11b+ GR1+, and monocytes CD11b+ GR1−) at 16 weeks after reconstitution. Numbers on the graphs are percentage of total cells in delineated regions. Data show a representative analysis (of 5-7 performed).
We found β- and γ-catenin double-deficient BM to contribute efficiently and stably to peripheral blood lymphocytes of secondary recipients without apparent differences in lymphoid and myeloid lineages for more than 4 months (Figure 1C,D). After this time, secondary recipients were killed. The analysis of the BM corroborated that β-/γ-catenin double-deficient precursors (CD45.2) mediated sustained multilineage hematopoiesis (Figure 2A,B). PCR and Southern blots of sorted CD45.2 cells confirmed homozygous deletion of γ-catenin and virtually complete cre-mediated recombination of the β-catenin locus (Figure 2C,D). Western blots confirmed that full size β-catenin protein was lacking (Figure 2E). Of note, we also detect smaller β-catenin protein species in hematopoietic cells, which however fail to interact with TCF-1 and do not interfere with TCF/LEF mediated transcription (see “Discussion” and Figure S1). Similar to wild-type controls, recipients reconstituted with β-/γ-catenin double-deficient precursors contained a normal hematopoietic stem-cell compartment (LSK: lineage-negative (lin−) Sca-1+ c-kit+). There was no difference in the abundance of CD135+ LSK cells, which discriminates HSC from lymphoid primed multipotent progenitors,25 although the fraction of common lymphoid precursors (CLP; lineage− CD117int CD127+) was somewhat increased in β-/γ-catenin double-deficient BM (Figure 2A). Importantly, these data show that long-term, multilineage hematopoietic reconstitution of lethally irradiated hosts does occur in the combined absence of β- and γ-catenin.
HSC, progenitor, and lineage reconstitution by β-/γ-catenin negative BM precursors. (A) Density plots show the abundance of CD45.2+ BM cells in the lineage-negative (lin−) hematopoietic progenitor compartment of mice reconstituted with a mixture of experimental (CD45.2+) and wild-type (CD45.1+) BM cells. Shown are the abundances of LSK cells (lin−, Sca-1+ CD117+), multipotent progenitors (lin− Sca-1− CD117+), common lymphoid progenitors (lin− CD127+ CD117low), and lymphoid-primed multipotent progenitor (lin− Sca-1+ CD117+, CD135+) among CD45.2+ cells. (B) Gated CD45.2+ BM cells were analyzed for the presence of myeloid (granulocytes, CD11b+, GR1+, and monocytes, CD11b+ GR1−), erythroid (CD71+ TER119+) and lymphoid (B220+) lineage cells. B-cell development was further dissected by the separation into CD43+ B220+ (fraction A-C′,46 ), CD43− B220+ IgM− (fraction D), and B220+ IgM+ (fraction E, F) subsets. Data show a representative analysis (of a total of 5 to 7 performed using 3 independent donor fetuses) after 7 months of reconstitution of secondary recipients. In panels A and B, numbers on graphs are percentage of total cells in the delineated regions. (C) PCR for β- and γ-catenin deletion on genomic DNA isolated from CD45.2+ BM cells 7 months after competitive reconstitution with catenin deleted BM. The mutant (indicated as null) and wild-type (wt) γcatenin alleles are detected as 150 bp and 300 bp PCR products, respectively. The floxed (indicated as lox) and deleted (Δ-) β-catenin alleles yield 183 bp and 481 bp bands, respectively. (D) Quantification of β-catenin deletion by Southern analysis of Sac1 restricted genomic DNA isolated from CD45.2+ BM cells 7 months after competitive reconstitution with β-catenin–deleted BM. The floxed (indicated as lox), deleted (Δ) and wild-type (wt) β-catenin alleles give rise to 7.4-, 5.2-, and 4.0-kb fragments, respectively. Note the virtually complete deletion of the β-cateninlox allele on Cre-mediated recombination. (E) Total cellular lysates from flow sorted wild-type (CD45.1+) and mutant (CD45.2+) BM cells of the indicated genotypes were subjected to immunoblot analysis with mAbs to the COOH terminus of β-catenin (clone 14) and to tubulin (to ensure equal protein loading). Note the virtually complete absence of the full-size β-catenin protein upon Cre–mediated recombination. The smaller β-catenin protein species fail to interact with TCF-1 and do not interfere with TCF/LEF mediated transcription (see “Discussion” and Figure S1 [available on the Blood website; see the Supplemental Materials link at the top of the online article]).
HSC, progenitor, and lineage reconstitution by β-/γ-catenin negative BM precursors. (A) Density plots show the abundance of CD45.2+ BM cells in the lineage-negative (lin−) hematopoietic progenitor compartment of mice reconstituted with a mixture of experimental (CD45.2+) and wild-type (CD45.1+) BM cells. Shown are the abundances of LSK cells (lin−, Sca-1+ CD117+), multipotent progenitors (lin− Sca-1− CD117+), common lymphoid progenitors (lin− CD127+ CD117low), and lymphoid-primed multipotent progenitor (lin− Sca-1+ CD117+, CD135+) among CD45.2+ cells. (B) Gated CD45.2+ BM cells were analyzed for the presence of myeloid (granulocytes, CD11b+, GR1+, and monocytes, CD11b+ GR1−), erythroid (CD71+ TER119+) and lymphoid (B220+) lineage cells. B-cell development was further dissected by the separation into CD43+ B220+ (fraction A-C′,46 ), CD43− B220+ IgM− (fraction D), and B220+ IgM+ (fraction E, F) subsets. Data show a representative analysis (of a total of 5 to 7 performed using 3 independent donor fetuses) after 7 months of reconstitution of secondary recipients. In panels A and B, numbers on graphs are percentage of total cells in the delineated regions. (C) PCR for β- and γ-catenin deletion on genomic DNA isolated from CD45.2+ BM cells 7 months after competitive reconstitution with catenin deleted BM. The mutant (indicated as null) and wild-type (wt) γcatenin alleles are detected as 150 bp and 300 bp PCR products, respectively. The floxed (indicated as lox) and deleted (Δ-) β-catenin alleles yield 183 bp and 481 bp bands, respectively. (D) Quantification of β-catenin deletion by Southern analysis of Sac1 restricted genomic DNA isolated from CD45.2+ BM cells 7 months after competitive reconstitution with β-catenin–deleted BM. The floxed (indicated as lox), deleted (Δ) and wild-type (wt) β-catenin alleles give rise to 7.4-, 5.2-, and 4.0-kb fragments, respectively. Note the virtually complete deletion of the β-cateninlox allele on Cre-mediated recombination. (E) Total cellular lysates from flow sorted wild-type (CD45.1+) and mutant (CD45.2+) BM cells of the indicated genotypes were subjected to immunoblot analysis with mAbs to the COOH terminus of β-catenin (clone 14) and to tubulin (to ensure equal protein loading). Note the virtually complete absence of the full-size β-catenin protein upon Cre–mediated recombination. The smaller β-catenin protein species fail to interact with TCF-1 and do not interfere with TCF/LEF mediated transcription (see “Discussion” and Figure S1 [available on the Blood website; see the Supplemental Materials link at the top of the online article]).
Normal thymopoiesis in the combined absence of β- and γ-catenin
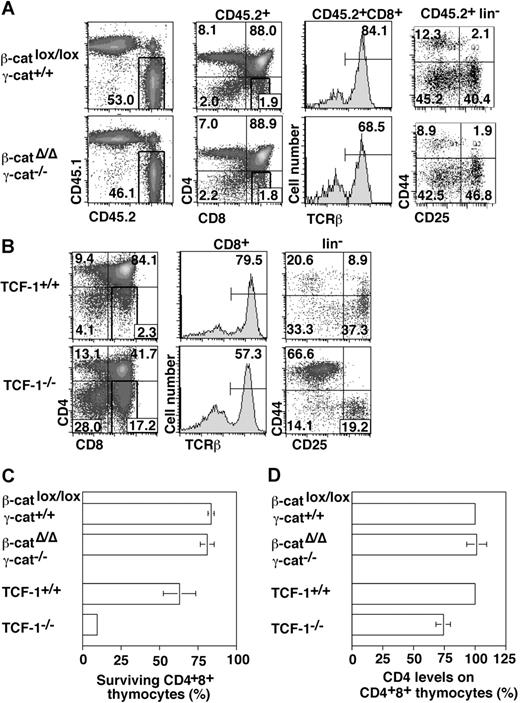
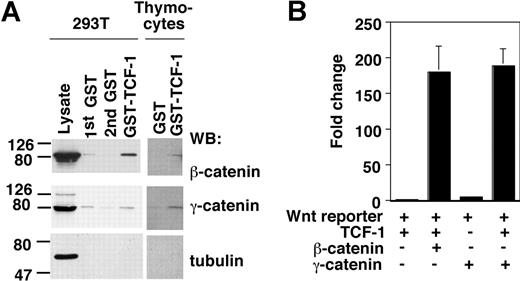
Deletion of TCF-1 impairs thymocyte survival at multiple distinct stages, including the pre-TCR-dependent DN3 to DN4 transition and the DP compartment (Figure 3C, Ioannidis et al,15 and Goux et al16 ). These developmental blocks can be rescued by a TCF-1 transgene, which includes the N-terminal 117 amino acids of TCF-1.15,16 This domain in TCF contains a β-catenin and a putative γ-catenin binding site20 suggesting that catenin binding to TCF-1 might be critical for thymocyte survival. Indeed, conditional stabilization of β-catenin in immature thymocytes bypasses the requirement of pre-TCR signaling for the development past the DN3 stage.26 Pull-down experiments provided direct evidence that both β-catenin and γ-catenin bind to the N-terminal 117 amino acids of TCF-1 (Figure 4A). In addition, in conjunction with TCF-1, both β- and γ-catenin induce the expression of a reporter gene, which is controlled by multimerized TCF binding sites (Figure 4B).
Thymocyte development in the absence of β-/γ-catenin or TCF-1. (A) β-Catenin deletion was induced before transfer as described in the legend to Figure 2. Density plots show gated CD45.2+ thymocytes, which were analyzed for CD4/CD8 expression, TCRβ expression among CD8+ cells, and the distribution of CD44 and CD25 among lin− thymocytes. Numbers indicate the percentage of cells in the respective quadrant or region. Data show a representative analysis of 5-7 performed using 3 independent β-/γ-catenin donor fetuses after 7 months of reconstitution. The absolute number of control and β-/γ-catenin-deficient (CD45.2+) thymocytes was not significantly different. (B) Density plots show a corresponding analysis of thymi from 2- to 3-week-old TCF-1–deficient mice. TCF-1–deficient and control thymi contained 12 (± 7) × 106 and 217 (± 75) × 106 cells, respectively. (C) Survival of β-/γ-catenin–deficient and TCF-1–deficient thymocytes in vitro. Total thymocytes were incubated overnight in complete culture medium without the addition of growth factors. Then cells were surface stained and the presence of viable CD4+8+ (DP) cells was estimated relative to a fixed number of added microspheres. The ratio of beads to DP cells incubated at 4°C was set to 100%. The bar graph shows the mean percentage (± SD) of surviving DP cells at 37°C. (D) Relative CD4 levels on β-/γ-catenin–deficient and TCF-1–deficient DP thymocytes. The mean fluorescence intensity (MFI) of CD4 staining on DP cells of mutant mice was estimated relative to that of corresponding wild-type controls (100%). The bar graph shows the mean percentage (± SD) of CD4 expression on β-/γ-catenin–deficient (n = 4) or TCF-1–deficient (n = 6) DP cells.
Thymocyte development in the absence of β-/γ-catenin or TCF-1. (A) β-Catenin deletion was induced before transfer as described in the legend to Figure 2. Density plots show gated CD45.2+ thymocytes, which were analyzed for CD4/CD8 expression, TCRβ expression among CD8+ cells, and the distribution of CD44 and CD25 among lin− thymocytes. Numbers indicate the percentage of cells in the respective quadrant or region. Data show a representative analysis of 5-7 performed using 3 independent β-/γ-catenin donor fetuses after 7 months of reconstitution. The absolute number of control and β-/γ-catenin-deficient (CD45.2+) thymocytes was not significantly different. (B) Density plots show a corresponding analysis of thymi from 2- to 3-week-old TCF-1–deficient mice. TCF-1–deficient and control thymi contained 12 (± 7) × 106 and 217 (± 75) × 106 cells, respectively. (C) Survival of β-/γ-catenin–deficient and TCF-1–deficient thymocytes in vitro. Total thymocytes were incubated overnight in complete culture medium without the addition of growth factors. Then cells were surface stained and the presence of viable CD4+8+ (DP) cells was estimated relative to a fixed number of added microspheres. The ratio of beads to DP cells incubated at 4°C was set to 100%. The bar graph shows the mean percentage (± SD) of surviving DP cells at 37°C. (D) Relative CD4 levels on β-/γ-catenin–deficient and TCF-1–deficient DP thymocytes. The mean fluorescence intensity (MFI) of CD4 staining on DP cells of mutant mice was estimated relative to that of corresponding wild-type controls (100%). The bar graph shows the mean percentage (± SD) of CD4 expression on β-/γ-catenin–deficient (n = 4) or TCF-1–deficient (n = 6) DP cells.
TCF-1 binding to β- and γ-catenin. (A) The NH2-terminal domain of TCF-1 (amino acids 1-117) was fused to GST and used to test binding to β- and γ-catenin present in HEK 293T or thymocyte cellular lysates. Lysates were pre-cleared with GST alone, and binding proteins were revealed by immunoblot. (B) Wnt reporter gene activity in response to expression of TCF-1 in combination with β-catenin or γ-catenin as analyzed in 293T cells. Error bars are SD.
TCF-1 binding to β- and γ-catenin. (A) The NH2-terminal domain of TCF-1 (amino acids 1-117) was fused to GST and used to test binding to β- and γ-catenin present in HEK 293T or thymocyte cellular lysates. Lysates were pre-cleared with GST alone, and binding proteins were revealed by immunoblot. (B) Wnt reporter gene activity in response to expression of TCF-1 in combination with β-catenin or γ-catenin as analyzed in 293T cells. Error bars are SD.
Despite this evidence, thymopoiesis occurred normally in the combined absence of β- and γ-catenin (Figure 3A), the DP stage was normally represented and pre-TCR-dependent DN3 to DN4 transition was not perturbed (Figure 3A). Unlike TCF-1–deficient thymocytes (Figure 3C, Ioannidis et al,15 and Goux et al16 ), β-/γ-catenin double-deficient DN3, DN4, and CD4+ CD8+ thymocytes did not undergo accelerated cell death on growth factor withdrawal in vitro (Figure 3C and data not shown). Reduced expression of the TCF-1 target gene CD4 on DP thymocytes of TCF-1–deficient mice has recently been reported.27 We did not observe a corresponding reduction of CD4 expression in the combined absence of β- and γ-catenin (Figure 3D). Importantly, the reduction of CD4 expression in TCF-1 deficient mice is unrelated to the reduced survival of CD4+ CD8+ cells and CD4 expression is restored dependent on the presence of the TCF-1 N-terminus.27 These latter data highlight an additional discrepancy between the phenotypes of TCF-1 and β-/γ-catenin–deficient mice, which is clearly independent of DP survival. Collectively, these data imply that the TCF-1 N-terminus mediates T-cell development independent of a functional interaction with β- and γ-catenin.
Canonical Wnt signaling activity in the combined absence of β- and γ-catenin
Several lines of evidence have implicated canonical Wnt signaling in the expansion of hematopoietic stem cells and in B andT lymphocyte development.5,6,12,15,16,26 The double deletion of Wnt1 and Wnt4 resulted in reduced thymic cellularity, and abrogation of Wnt signaling using specific, extracellular signaling inhibitors, such as Dkk1 and secreted Frz receptors, induced a block in T-cell development. Expression of axin, an intracellular inhibitory component of the canonical Wnt signaling pathway, provided similar results.9 In addition, stimulation of thymocytes by Wnt has been shown to result in stabilization of β-catenin28 and to augment T-cell transmigration,29 which clearly demonstrates that all components of the pathway are functional in these cells. Although TCF/LEF mediate functions independent of Wnt signaling, none of these functions requires the TCF/LEF N-terminus. For example, Smad binding to LEF/TCF and activation of target genes require the HMG box,30 while the central domain of LEF/TCF interacts with groucho-related corepressors or ALY to repress or stimulate transcription, respectively.31,32 Wnt is the only pathway known to activate TCF/LEF via the N-terminus.
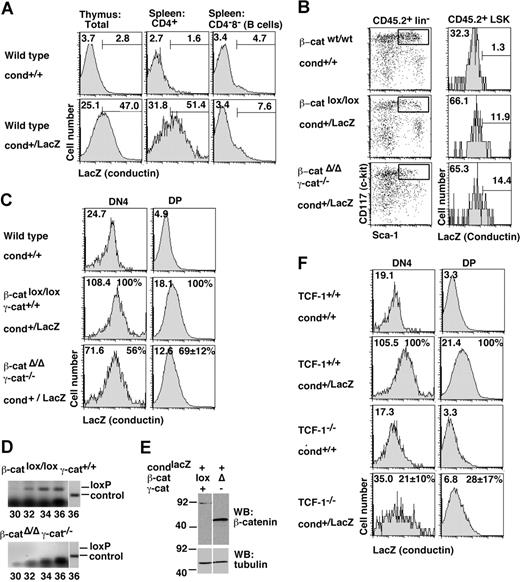
Our results now raised the possibility that canonical Wnt signals were transduced independently from β- and γ-catenin, or alternatively, that canonical Wnt signals might not play any role for normal hematopoiesis. To discriminate between these 2 possibilities, we directly measured endogenous Wnt signaling activity in wild-type and mutant HSCs and thymocytes using 2 independent reporter systems. Conductin/axin2 is a direct target gene ubiquitously activated in response to Wnt signals and encodes a negative regulator of Wnt signal transduction.24,33 We combined our β- and γ-catenin mutant alleles with a “knock-in” conductin allele24 in which the endogenous open reading frame was replaced by a bacterial β-galactosidase reporter gene (termed conductinLacZ). Consequently, β-galactosidase activity can be used to assess Wnt signaling activity in vivo. We detected Wnt signaling in thymocytes and peripheral T cells, but not in peripheral B cells of conductinlacZ mice (Figure 5A). In addition, active Wnt signaling was detected in a subset of LSK cells (Figure 5B). This is similar to results obtained with a distinct Wnt reporter.5 Remarkably, Wnt signaling in LSK cells did not change significantly in the absence of β-catenin and/or γ-catenin (Figure 5B and data not shown). Distinct thymocyte subsets from conductin+/LacZ mice showed variable levels of Wnt signaling with low conductin reporter activity in DP cells and considerably higher activity in DN4 or mature thymocytes (Figure 5C and data not shown). β-/γ-Catenin double-deficient thymocytes retained their ability to transmit Wnt signals, even though the reporter signal was slightly (30%-40%), but reproducibly reduced in both DP and DN4 cells (Figure 5C). We verified by PCR that the respective BM cells and thymocytes were indeed γ-catenin knock-out and that the β-catenin locus was efficiently excised (Figure 5D). Western blots of BM cells confirmed lack of full-size β-catenin protein in these cells (Figure 5E). In contrast to β-/γ-catenin–deficient cells, conductin reporter gene activity was strongly (4-fold) reduced in DN4 and DP thymocytes of TCF-1 deficient mice (Figure 5F). The residual Wnt signaling activity in the absence of TCF-1 is expected to be maintained by LEF-1, which mediates thymopoiesis when TCF-1 levels are reduced.17
Canonical Wnt signaling activity in hematopoietic cells in vivo as measured with the conductinlacZ Wnt reporter. (A) Histograms show β-galactosidase (LacZ) activity (conductin expression) in total thymocytes and gated spleenic CD4+ T cells and CD4−8− cells (B cells) in conductin+/LacZ compared with conductin+/+ controls. (B) β-Catenin was deleted in primary recipients and 10 days later LacZ activity (conductin expression) was determined in gated CD45.2+ LSK cells of the indicated genotypes. Numbers indicate the geometric mean of the green fluorescence intensity of the total population (top left) and the percentage of cells in the indicated gate. The data shown are representative of 2 experiments. (C) Primary recipients were treated and analyzed as described above. In addition, we also used secondary recipients, which were repopulated with β-catenin–deleted BM for more than 8 weeks. LacZ activity was determined in gated CD45.2+ DN4 (lin− CD44− CD25−) and DP (CD4+ CD8+) thymocytes. In panels A-C, numbers on graphs are percentage of total cells in the delineated region. (D) Genomic DNA isolated from chimeras reconstituted with deletion-induced Mx-cre:β-cateninΔ/Δ:γ-catenin−/− BM cells was amplified by PCR specific for the floxed, un-recombined β-catenin allele (loxP) for the indicated number of cycles. The osf2 locus was amplified as a control. (E) Total cellular lysates from BM cells of the indicated types of chimeric mice were subjected to immunoblot analysis with mAbs to the COOH terminus of β-catenin (clone 14) and to tubulin (to ensure equal protein loading). Note that full-size β-catenin protein was not detected after Cre-mediated recombination. (F) LacZ activity was determined in gated DN4 (lin− CD44− CD25−) and DP (CD4+ CD8+) thymocytes of TCF-1-deficient conductin+ LacZ mice. Numbers on graphs are percentage of total cells in the delineated region. (C,E) Numbers indicate the geometric mean of the green fluorescence in the total population. In mutant mice, the mean percentage (± SD) of the geometric mean was calculated relative to the wild-type control (100%) after subtracting the background staining (from conductin+/+ mice). Data are from 3 to 5 independent experiments; a single determination was performed for DN4 cells in β-/γ-catenin–deficient cells.
Canonical Wnt signaling activity in hematopoietic cells in vivo as measured with the conductinlacZ Wnt reporter. (A) Histograms show β-galactosidase (LacZ) activity (conductin expression) in total thymocytes and gated spleenic CD4+ T cells and CD4−8− cells (B cells) in conductin+/LacZ compared with conductin+/+ controls. (B) β-Catenin was deleted in primary recipients and 10 days later LacZ activity (conductin expression) was determined in gated CD45.2+ LSK cells of the indicated genotypes. Numbers indicate the geometric mean of the green fluorescence intensity of the total population (top left) and the percentage of cells in the indicated gate. The data shown are representative of 2 experiments. (C) Primary recipients were treated and analyzed as described above. In addition, we also used secondary recipients, which were repopulated with β-catenin–deleted BM for more than 8 weeks. LacZ activity was determined in gated CD45.2+ DN4 (lin− CD44− CD25−) and DP (CD4+ CD8+) thymocytes. In panels A-C, numbers on graphs are percentage of total cells in the delineated region. (D) Genomic DNA isolated from chimeras reconstituted with deletion-induced Mx-cre:β-cateninΔ/Δ:γ-catenin−/− BM cells was amplified by PCR specific for the floxed, un-recombined β-catenin allele (loxP) for the indicated number of cycles. The osf2 locus was amplified as a control. (E) Total cellular lysates from BM cells of the indicated types of chimeric mice were subjected to immunoblot analysis with mAbs to the COOH terminus of β-catenin (clone 14) and to tubulin (to ensure equal protein loading). Note that full-size β-catenin protein was not detected after Cre-mediated recombination. (F) LacZ activity was determined in gated DN4 (lin− CD44− CD25−) and DP (CD4+ CD8+) thymocytes of TCF-1-deficient conductin+ LacZ mice. Numbers on graphs are percentage of total cells in the delineated region. (C,E) Numbers indicate the geometric mean of the green fluorescence in the total population. In mutant mice, the mean percentage (± SD) of the geometric mean was calculated relative to the wild-type control (100%) after subtracting the background staining (from conductin+/+ mice). Data are from 3 to 5 independent experiments; a single determination was performed for DN4 cells in β-/γ-catenin–deficient cells.
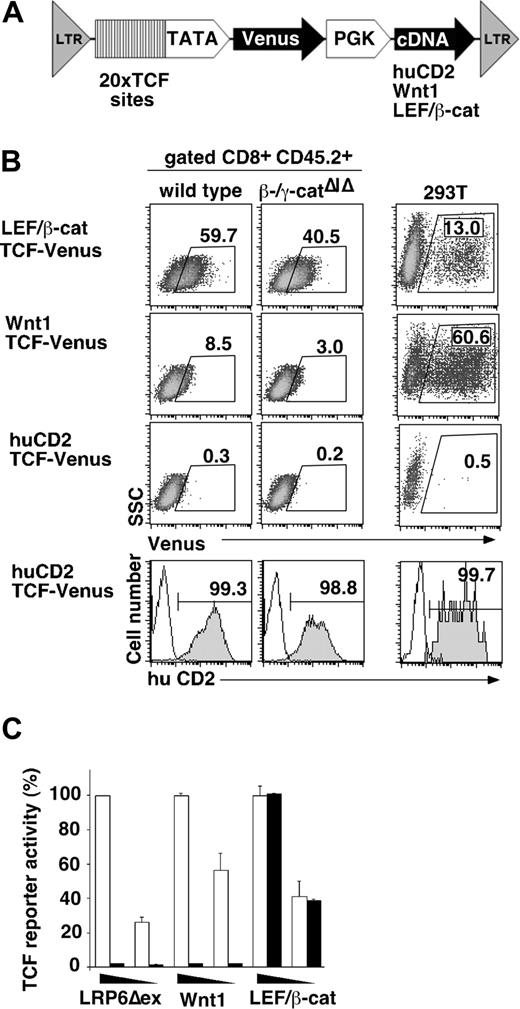
Because the endogenous conductin gene might be regulated by other factors in addition to Wnt, we also analyzed Wnt signaling activity using an artificial, lentiviral reporter system. These viral vectors allow expression of Venus (a yellow-shifted variant of GFP) under the control of multimerized TCF/LEF binding sites. In addition, these vectors constitutively express activating components of the Wnt signaling pathway, such as Wnt1 or a LEF-1/β-catenin fusion or a negative control (huCD2) (Figure 6A). Using this reporter system, we detected substantial, Wnt-induced reporter gene expression in activated, peripheral T cells of control chimeras (Figure 6B), which is in agreement with a recent report.29 Importantly, significant Wnt1 induced reporter gene expression was retained in the combined absence of β-catenin and γ-catenin (Figure 6B). Preliminary experiments similarly indicate Wnt1-mediated signaling in CD48−CD150+ HSC from 5-fluorouracil–treated β-/γ-catenin–deficient mice (data not shown). In contrast, transmission of Wnt signals in epithelial cells is dependent on β-catenin as suppression of β-catenin expression by RNAi completely abolished reporter activation (Figure 6C). We conclude that TCF-1–dependent Wnt signaling in hematopoietic cells is only partly accounted for by β- and γ-catenin. This indicates a thus far unidentified mechanism of Wnt signal transduction toward TCF in hematopoietic cells.
Canonical Wnt signaling activity in hematopoietic cells ex vivo as measured with lentiviral Wnt reporters. (A) Scheme of lentiviral Wnt reporters, which contain multimerized TCF binding sites in front of a minimal promoter driving expression of Venus (a yellow shifted variant of GFP) and a constitutive expression cassette of the PGK promoter driving expression of human CD2 (hCD2), Wnt1 or a fusion of LEF-1 and the C-terminal transactivation domain of β-catenin (LEF/β-cat). (B) Histograms show Venus expression (Wnt signaling) in control (hCD2 expression) or Wnt-stimulated (Wnt1 or LEF/β-cat expression) activated peripheral T cells of control and β-/γ-catenin double-deficient chimeras or 293T cells 48 hours after viral transduction. Numbers indicate the percentage of cells in the indicated gate. (C) The bar graph shows Wnt reporter activity in 293T cells stimulated with different doses of LRP6Δex, Wnt1 or LEF/β-catenin on expression of nontargeting RNAi ( ) or RNAi targeting β-catenin (
) or RNAi targeting β-catenin ( ). The RNAi does not target the C-terminal β-catenin domain contained in the LEF/β-catenin fusion construct. Error bars are SD. All data are from 3 to 5 independent experiments, and representative histograms are shown.
). The RNAi does not target the C-terminal β-catenin domain contained in the LEF/β-catenin fusion construct. Error bars are SD. All data are from 3 to 5 independent experiments, and representative histograms are shown.
Canonical Wnt signaling activity in hematopoietic cells ex vivo as measured with lentiviral Wnt reporters. (A) Scheme of lentiviral Wnt reporters, which contain multimerized TCF binding sites in front of a minimal promoter driving expression of Venus (a yellow shifted variant of GFP) and a constitutive expression cassette of the PGK promoter driving expression of human CD2 (hCD2), Wnt1 or a fusion of LEF-1 and the C-terminal transactivation domain of β-catenin (LEF/β-cat). (B) Histograms show Venus expression (Wnt signaling) in control (hCD2 expression) or Wnt-stimulated (Wnt1 or LEF/β-cat expression) activated peripheral T cells of control and β-/γ-catenin double-deficient chimeras or 293T cells 48 hours after viral transduction. Numbers indicate the percentage of cells in the indicated gate. (C) The bar graph shows Wnt reporter activity in 293T cells stimulated with different doses of LRP6Δex, Wnt1 or LEF/β-catenin on expression of nontargeting RNAi ( ) or RNAi targeting β-catenin (
) or RNAi targeting β-catenin ( ). The RNAi does not target the C-terminal β-catenin domain contained in the LEF/β-catenin fusion construct. Error bars are SD. All data are from 3 to 5 independent experiments, and representative histograms are shown.
). The RNAi does not target the C-terminal β-catenin domain contained in the LEF/β-catenin fusion construct. Error bars are SD. All data are from 3 to 5 independent experiments, and representative histograms are shown.
Discussion
Several lines of evidence have implicated canonical Wnt signaling in hematopoiesis. In BM and thymus, Wnt ligands are produced by the immune cells themselves and might act by autocrine stimulation10,34 as evidenced by Wnt reporter measurements in vivo and ex vivo (our data, Reya et al,5 and Weerkamp et al10 ). Functional studies show that Wnt ligands promote self-renewal of HSCs and allow their long-term expansion in vitro.34,–36 Conversely, genetic ablation of Wnts or the Wnt receptor Fz9 as well as expression of soluble Wnt inhibitors interfere with HSC proliferation and hematopoiesis.5,7,8,10 Importantly, the intracellular signaling cascade responsible for canonical Wnt effects in hematopoietic cells has largely been deduced from gain of function experiments.4,5,9,37 Our results now demonstrate that hematopoiesis and thymopoiesis is sustained on deletion of the 2 known canonical Wnt signal transmitters β- and γ-catenin. In addition, we show that Wnt signaling activity in HSCs and thymocytes is largely preserved in these mutants using reporter gene assays. This is in contrast to TCF-1–deficient thymocytes, where reporter gene activity is strongly reduced. Given that the downstream transcription factors LEF-1 and TCF-1 are known to be essential for thymopoiesis, we postulate a thus far unidentified mechanism of Wnt signaling in thymic development.
This possibility receives strong support by our previous and the present genetic analysis of T-cell development. Thymopoiesis is dependent on the N-terminal domain of TCF-1, which can bind both β-catenin and γ-catenin. However, β- and γ-catenin are not essential for T-cell development. Thus, an additional, currently unidentified factor capable of binding the TCF-1 N-terminus seems to play a key role for T-cell development. Consistent with such a scenario, a novel Wnt signaling transducer has recently been identified in Caenorhabditis elegans,38 which however lacks a vertebrate ortholog. Intriguingly, the situation is different in epithelial cells or in epithelial tissues such as the skin, where LEF-1 deficiency and β-catenin ablation produce consistent phenotypes.22,39 In the skin, Wnt signaling has been shown to depend only on the interaction of LEF-1 and β-catenin, which provides transcriptional activation for this bipartite transcription factor. These data suggest striking tissue-specific differences in the transduction of canonical Wnt signals. Interestingly, recent data suggested that the major role of TCF/LEF in thymocytes might not be gene activation but transcriptional repression.40 However, a TCF-1 transgene construct lacking the N-terminus while preserving the classical TLE/groucho-binding repressor domain has failed to rescue T-cell development,15 suggesting that repression is not the critical function of TCF-1.
Unexpectedly, we detected expression of smaller β-catenin protein species in wild-type and β-catenin mutant BM cells using our or the β-catenin allele used by Cobas et al19 (Figures 2E, S1A-C). Heterozygous animals with either mutant allele (β-cateninΔ/+) exhibit no overt phenotype and display normal hematopoiesis (data not shown). Cloning and expression studies show that the truncated β-catenin protein species fail to interact with TCF-1 and do not interfere with TCF/LEF–mediated transcription (Figure S1D,E and data not shown). Therefore, any functional role of these shorter protein species remains to be demonstrated. In contrast to our loss of function experiments, recent in vivo gain of function experiments clearly demonstrate effects of β-catenin on proliferation and differentiation potential of hematopoietic cells.41,42 In line with this, experimental evidence demonstrates that cells from acute and chronic myelogenous leukemia patients display activated Wnt signaling.43 Proliferation of these cells is dependent on Wnt signals as shown by ectopic expression of axin or inhibition of γ-catenin.3,4 Similarly, overexpression of Wnt ligands has been implicated in lymphoid leukemia and multiple myelomas.44,45 Our results support the feasibility of targeting β- and/or γ-catenin in patients as novel therapeutic strategy. Based on our results in the double-mutant animals, we expect that such a therapy will exhibit no major side effects on normal hematopoiesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to P. Ruiz and B. Jerchow, Berlin, for the γ-catenin and conductinlacZ mice, H. Clevers, Utrecht, for TCF-1−/− mice, and to F. Lévy, Ludwig Institute for Cancer Research Lausanne, for advice.
W.H. was supported in part by the Leenaards Foundation and a grant from the Swiss National Science Foundation. J.H., I.M., F.K., and S.D. were supported in part by the Leenaards Foundation, the Swiss National Center for Competence in Research in Molecular Oncology, and the Swiss National Science Foundation.
Authorship
Contribution: G.J., M.S., L.S., S.D., N.G., J.B., F.K., and I.M. performed research and analyzed data; W.B. contributed vital reagents; A.L. designed the research and analyzed data; J.H. designed the research, analyzed data, and wrote the paper; and W.H. designed the research, analyzed data, and wrote the paper.
J.H. and W.H. contributed equally to this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joerg Huelsken, Ecole Polytechnique Fédérale de Lausanne, ISREC, Chemin des Boveresses 155, 1066 Epalinges, Switzerland; e-mail: joerg.huelsken@epfl.ch; or Werner Held, Ludwig Institute for Cancer, Research, Lausanne Branch, Chemin des Boveresses 155, 1066 Epalinges, Switzerland; e-mail: werner.held@isrec.unil.ch.

![Figure 2. HSC, progenitor, and lineage reconstitution by β-/γ-catenin negative BM precursors. (A) Density plots show the abundance of CD45.2+ BM cells in the lineage-negative (lin−) hematopoietic progenitor compartment of mice reconstituted with a mixture of experimental (CD45.2+) and wild-type (CD45.1+) BM cells. Shown are the abundances of LSK cells (lin−, Sca-1+ CD117+), multipotent progenitors (lin− Sca-1− CD117+), common lymphoid progenitors (lin− CD127+ CD117low), and lymphoid-primed multipotent progenitor (lin− Sca-1+ CD117+, CD135+) among CD45.2+ cells. (B) Gated CD45.2+ BM cells were analyzed for the presence of myeloid (granulocytes, CD11b+, GR1+, and monocytes, CD11b+ GR1−), erythroid (CD71+ TER119+) and lymphoid (B220+) lineage cells. B-cell development was further dissected by the separation into CD43+ B220+ (fraction A-C′,46), CD43− B220+ IgM− (fraction D), and B220+ IgM+ (fraction E, F) subsets. Data show a representative analysis (of a total of 5 to 7 performed using 3 independent donor fetuses) after 7 months of reconstitution of secondary recipients. In panels A and B, numbers on graphs are percentage of total cells in the delineated regions. (C) PCR for β- and γ-catenin deletion on genomic DNA isolated from CD45.2+ BM cells 7 months after competitive reconstitution with catenin deleted BM. The mutant (indicated as null) and wild-type (wt) γcatenin alleles are detected as 150 bp and 300 bp PCR products, respectively. The floxed (indicated as lox) and deleted (Δ-) β-catenin alleles yield 183 bp and 481 bp bands, respectively. (D) Quantification of β-catenin deletion by Southern analysis of Sac1 restricted genomic DNA isolated from CD45.2+ BM cells 7 months after competitive reconstitution with β-catenin–deleted BM. The floxed (indicated as lox), deleted (Δ) and wild-type (wt) β-catenin alleles give rise to 7.4-, 5.2-, and 4.0-kb fragments, respectively. Note the virtually complete deletion of the β-cateninlox allele on Cre-mediated recombination. (E) Total cellular lysates from flow sorted wild-type (CD45.1+) and mutant (CD45.2+) BM cells of the indicated genotypes were subjected to immunoblot analysis with mAbs to the COOH terminus of β-catenin (clone 14) and to tubulin (to ensure equal protein loading). Note the virtually complete absence of the full-size β-catenin protein upon Cre–mediated recombination. The smaller β-catenin protein species fail to interact with TCF-1 and do not interfere with TCF/LEF mediated transcription (see “Discussion” and Figure S1 [available on the Blood website; see the Supplemental Materials link at the top of the online article]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/1/10.1182_blood-2007-07-102558/3/m_zh80030811830002.jpeg?Expires=1769113824&Signature=NoxXwLrwpY4qNCf4SNpc1jEN~O4upvQt0YvI4ijDrOkMo4e-4TAgOVKnmoxVKi5bmTQUOUHVv4D3QZT6QIBaRaS~5maT93cjj4NAta7vN2b5QobfsQHiepIbh92e5fxXtMeJi9ZZtszK7KRLBriW5Gyd0hGuk2hEZu78MVqMN4vcRECYskEqItJn81GCzFEAlHBn21cNpUzFFZnZaGAt0o5ef4TNyJRQ2bhfyYT9DPNVQ8MKJqInUgFAh0cyOf02TKQE2ehU2LGKKV8V-nZ968dszePduCMDn2z0kTFh0ivjIsANqP2mRRDOptsit4nLlhgMCwGOpOPxb5DrM742GQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal