The first leukocytes that arise in the development of vertebrate embryos are the primitive macrophages, which differentiate in the yolk sac and then quickly invade embryonic tissues. These macrophages have been considered to constitute a separate lineage, giving rise to no other cell type. Using an in vivo photoactivatable cell tracer in the transparent zebrafish (Danio rerio) embryo, we demonstrated that this lineage also gave rise to an equal or higher number of neutrophilic granulocytes. We were surprised to find that the differentiation of these primitive neutrophils occurs only after primitive myeloid progenitors have dispersed in the tissues. By 2 days after fertilization, these neutrophils have become the major leukocyte type found wandering in the epidermis and mesenchyme. Like the primitive macrophages, all primitive and larval neutrophils express PU.1 and L-plastin and they are highly attracted to local infections, yet only a small fraction of them phagocytose microbes, and to a much lesser extent per cell than the macrophages. They are also attracted to variously stressed or malformed tissues, suggesting a wider role than antimicrobial defense.
Introduction
In all vertebrate embryos examined so far, the first leukocytes to appear are macrophages.1 They belong to a specific, so-called “primitive macrophage” lineage that arises from mesoderm and differentiates in the yolk sac in parallel with the primitive wave of erythrocytes. In zebrafish (Danio rerio) as well as in Xenopus laevis embryos, these primitive macrophages were found to originate from the rostral-most lateral mesoderm, adjacent to the cardiac field.2,3 They differentiate in the neighboring yolk sac between the 20- and 30-somite stages (24 hours after fertilization [hpf]), just before the onset of blood circulation, and then quickly spread throughout the mesenchyme of the embryo.4
The next type of leukocyte that appears in zebrafish development is the neutrophilic granulocyte (neutrophils). These cells have been documented by electron microscopy in the trunk and tail by 48 hpf.5,6 Searching for a molecular marker of neutrophils, 2 groups cloned the same zebrafish gene through similarity with the mammalian myeloperoxidase (mpo) gene.5,7 Sequence comparisons revealed that this gene was equally close to 4 related mammalian peroxidases—myelo-, eosinophil, lacto-, and salivary peroxidase (3 of which lie on the same human chromosome within 100 kb)—strongly suggesting that the diversification of these peroxidases through gene duplications occurred in tetrapods after their divergence from fishes. Therefore, Lieschke et al5 appropriately named the zebrafish gene “myeloid peroxidase” (mpx), rather than myeloperoxidase. In adult zebrafish, mpx was found to be expressed in neutrophils of the kidney5,7 (the definitive hematopoietic organ in fish) and spleen.5 In embryos, mpx was found expressed in dispersed leukocytes, which were assumed to be neutrophils.5,7 However, in mammals, even though myeloperoxidase is expressed most strongly in neutrophils, it is also expressed during monocytic differentiation and has been used as a marker of immature cells of the monocyte/macrophage, as well as of the primitive macrophage lineages, that becomes down-regulated upon terminal differentiation.8 In zebrafish embryos, many cells of the primitive macrophage lineage are likely to not yet be terminally differentiated, so they may express the mpx gene but not necessarily become granulocytes. Consistent with this, in X laevis embryos, all cells of the primitive macrophage lineage were found to express xpox2, the likely frog homolog of mpx.3
In the present study, we have investigated the embryologic origins of neutrophilic granulocytes, their subsequent deployment in tissues throughout zebrafish development, and their behavior upon experimental infections. We found that the primitive macrophage lineage also gives rise to neutrophils, most of which acquired their granules only once in the tissues. These primitive neutrophils, as well as larval neutrophils later born in the tail, were distributed mostly in subepidermal mesenchyme, rather than concentrated in the blood as in mammals. They steadily circulate within the tissues and were quickly attracted to any local infection, as well as to stressed tissues. Yet they were barely phagocytic relative to the other myeloid tissue leukocytes, the primitive macrophages, suggesting that their main function may be something other than phagocytosis.
Methods
Zebrafish stocks and embryo treatments
Wild-type AB and spadetailb104 and mindbombta52b mutant zebrafish were raised and maintained as described previously.9 The anti-runx1 morpholino10 dose (1 ng) injected in 1- to 4-cell stage embryos suppressed larval hematopoiesis11 but preserved blood circulation.
Escherichia coli bacteria expressing Discosoma sp. red fluorescent protein (DsRed)12 or green fluorescent protein (GFP)13 were microinjected either in the caudal vein at 52 hpf or in the otic cavity at 3 to 3.5 dpf [days after fertilization]. Overnight stationary-phase cultures (109 bacteria/mL) of E coli expressing GFP13 or DsRed12 were concentrated 4× for intravenous injection or 2× for otic vesicle injection. Zebrafish larvae were injected using a Picospritzer III microinjector (Parker Hannifin, Fairfield, NJ) and a mechanical micromanipulator (M-152; Narishige, Tokyo, Japan); bacteria were loaded in a pulled borosilicate glass capillary (GC100F-15; Harvard Apparatus, Holliston, MA). For intravenous injection, 3 to 5 nL were injected under a pressure of 40 psi and injection time of 40 milliseconds. For the ear injections shown in Figure 7, 0.5 to 1 nL were injected under a pressure of 20 psi and injection time of 20 milliseconds. For the ear injections shown in Figure S5, an injection pressure of 40 psi and injection time of 40 milliseconds were used.
Sudan Black staining
Embryos were fixed with 4% methanol-free formaldehyde (Polysciences, Warrington, PA) in phosphate-buffered saline (PBS) for 2 hours at room temperature, rinsed in PBS, incubated in Sudan Black (SB; Sigma-Aldrich, Saint-Quentin Fallavier, France) for 20 minutes, washed extensively in 70% ethanol in water, then progressively rehydrated to PBS and 0.1% Tween 20 (PBT).
Tyramide-based detection of endogenous peroxidase activity
Fluorescein isothiocyanate (FITC)- and Cyanine 3 (Cy3)- conjugated tyramides were synthesized as described previously.14,15 Embryos fixed as above were washed in PBS, incubated in 1/100 tyramide in PBS, 0.1 mol/L imidazole, and 0.001% H2O2 in the dark for 10 to 30 minutes, then washed 3 times for 10 minutes each in PBT. The reaction was stopped by incubation in 2% H2O2 in PBT for 30 minutes.
Immunohistochemistry
The generation of rabbit anti-zebrafish and anti-mouse L-plastin antibodies have been described previously.16,17 Both antibodies gave the same results and were used interchangeably. Anti-zebrafish PU.1 antibody was generated by injecting rabbits with a fusion protein composed of the amino-terminal 190 amino acids of zebrafish PU.1 fused to glutathione transferase. The resulting antisera were purified over an affinity column bearing the same 190 amino acids of zebrafish PU.1.
Whole-mount immunohistochemistry was performed as described previously,18 omitting the acetone treatment, which destroys the SB staining. In brief, after fixation as indicated above, larvae were treated with collagenase,18 washed in PBDT (PBT and 1% dimethyl sulfoxide), incubated for 3 hours in PBDT containing 10% sheep serum (PBDTS) and then with the primary antibody in PBDTS overnight at 4°C, and washed in PBDT twice for 15 minutes each. Then, when relevant, endogenous peroxidase was inactivated by a 50-minute incubation in PBDT containing 2% H2O2. Embryos were rinsed for 3 hours in PBDT, incubated in PBDTS for 2 to 3 hours, incubated with the secondary antibody overnight at 4°C, and washed in PBDT and then PBT for 2 hours. The secondary rabbit antibody used was coupled either to Alexa Fluor 488 (1:200; Invitrogen, Cergy-Pontoise, France), or to horseradish peroxidase (HRP; 1:800; GE Healthcare, Les-Ulis, France), in which case revelation was performed using aminoethylcarbazol (AEC) as a substrate.18 Rabbit antibodies against mouse or zebrafish L-plastin, zebrafish PU.1, and GFP (MBL International, Woburn, MA) were used at 1:500, 1:800, and 1:300 dilutions, respectively.
Cell tracing
Embryos were injected at the 1- to 4-cell stage with 1 nL of a 1:3 mix of fixable and nonfixable caged fluorescein-dextran 10 000 (Invitrogen) together with 1/10 (vol/vol) rhodamine-dextran 10.000 (all from 50 mg/mL stock solutions in 120 mmol/L KCl), and then left to develop in the dark, except for their manipulation, during which illumination was kept minimal. Only those embryos that displayed homogeneous rhodamine fluorescence were used in uncaging experiments. Fluorescein uncaging was performed through the epifluorescence port of a Nikon 90i microscope, using a Micropoint pulsed laser generating light at 365 nm (Photonic Instruments, Saint Charles, IL), and a 40× water immersion objective.
The targeted primitive myeloid progenitors in the 20 hpf yolk sac are visualized by differential interference contrast (DIC) microscopy as large, nonadherent, round cells just beneath the yolk sac epidermis, next to the lateral and anterior borders of the pericardium.2 These characteristics, together with the very simple histology of the 20 hpf yolk sac (one giant yolk cell covered by an epidermal monolayer, with the myeloid progenitors in between2 ), make the labeling of myeloid progenitors easily achievable at single-cell resolution.
To detect simultaneously granulocytes and uncaged fluorescein at 72 hpf, we used either of 2 methods: (1) Sudan Black staining followed by anti-fluorescein immunohistochemistry revealed in AEC,18 as indicated above except that an HRP-conjugated anti-fluorescein antibody (1:300; Roche, Neuilly-sur-Seine, France) was used; (2) Cy3-tyramide based detection of endogenous peroxidase, followed by incubation with HRP-conjugated anti-fluorescein antibody as above, then detection of HRP activity with FITC-tyramide as above.
Microscopy
All fixed embryos, larvae, and juveniles were transferred gradually from PBT to 100% glycerol for conservation and microscopic observation. Live specimens were anesthesized with tricaine in embryo medium,9 and observed in depression slides. Video-enhanced (VE)-DIC/fluorescence microscopy was performed either on a Reichert Polyvar2 (under a 40×/1.00 NA or 100×/1.32 NA oil objective) or a Nikon 90i (under a 60×/1.00 NA water-immersion objective) microscope (Nikon-France, Champigny-sur-Marne, France); VE-DIC images were generated using a 3-CCD video camera (HVD-25, Hitachi, Leeds, United Kingdom) tuned as described,2 and captured with the BTVPro software; fluorescence images were captured with a DS5c digital camera driven by the NIS software (Nikon-France). Electron microscopy was performed as described previously.6 Confocal fluorescence microscopy was performed on a Leica SPE (Leica-France, Rueil-Malmaison, France), with a 40×/1.15 NA oil-immersion objective. All pictures were taken at room temperature, and images were processed using Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA).
Results
Deployment and dynamics of granulocytes from embryo to juvenile fish
Sudan black B is a classic lipid stain that stains the granules of granulocytes much more avidly than any other cell structures. Thus, staining followed by extensive 70% ethanol washes results in a highly specific staining of the granules of granulocytes.20 We found this staining method to be strikingly efficient when applied to whole fixed zebrafish embryos, larvae, and even juvenile (more than 1 month old) fish (Figure 1). At medium magnification, the staining nicely delineates the often amoeboid granulocytes throughout the embryo, leaving their nucleus unstained (Figure 1Aii, iii,Biv,Cii,iii). At high magnification, the individually stained granules can be discerned wherever the cell is flat enough (Figures 1Aiv and S1J [available on the Blood website; see the Supplemental Materials link at the top of the online article]). Combining the SB staining with VE-DIC microscopy reveals the precise histologic location of these cells in whole-mount embryos (Figure 1Aii-iv,Biii-vi,Cii,iii).
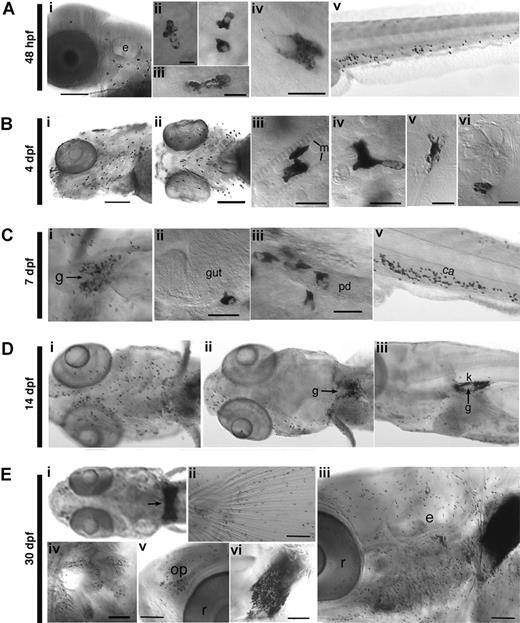
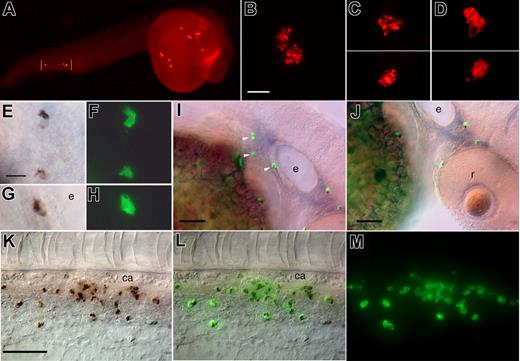
Deployment of Sudan Black-stained granulocytes from embryo to juvenile zebrafish. (A) 48 hpf embryo. (i) Lateral view of the head; the granulocytes are mainly dispersed in the mesenchyme; granulocytes in the yolk sac (ii), head epidermis (iii) and mesenchyme (iv); (v) Lateral view of the tail, where larval hematopoiesis is beginning. (B) 4 dpf larvae, head region. Ventrolateral (i) and ventral (ii) views, showing the increasing number of granulocytes. Examples of granulocytes between 2 cephalic muscle fibers (iii), in the mesenchyme (iv), in the epidermis (note the characteristic actin ridges of the overlying periderm in the lower half of the image) (v), and at the border of a neuromast (vi). (C) 7 dpf larvae. (i) Granulocytes gathered around the pronephric glomerulus (arrow), revealing the beginning of definitive granulopoiesis in the kidney. Granulocytes along the basal lamina of the gut (ii) and the left pronephric duct (iii); (iv) lateral view of the tail showing the growing granulocyte population in the CHT, the site of larval hematopoiesis. (D) 14 dpf larvae. Ventral (i) and dorsal (ii) views of the anterior region; note the paucity of granulocytes in the dorsal head, except most anteriorly around the olfactory pits, and the increasing number of granulocytes in the kidney around the glomerulus (arrow), also visible in lateral view in (iii). (E) 30 dpf juvenile fish. (i) The head kidney (remnant of the pronephros) is now full of granulocytes (arrow). Granulocyte populations in the caudal fin (ii), all over the head (iii), in the gills (iv), in the left olfactory pit (v), and in the head kidney (vi) (left lateral side). e indicates inner ear; ca, caudal artery; g, pronephric glomerulus; k, kidney; m, muscle fiber; op, olfactory pit; pd, pronephric duct; and r, retina. (Scale bars, 10 μm in Ab-d, Bc-f; 20 μm in Cb,c; 100 μm in Aa, Ba,b, Ca, Eb-f.)
Deployment of Sudan Black-stained granulocytes from embryo to juvenile zebrafish. (A) 48 hpf embryo. (i) Lateral view of the head; the granulocytes are mainly dispersed in the mesenchyme; granulocytes in the yolk sac (ii), head epidermis (iii) and mesenchyme (iv); (v) Lateral view of the tail, where larval hematopoiesis is beginning. (B) 4 dpf larvae, head region. Ventrolateral (i) and ventral (ii) views, showing the increasing number of granulocytes. Examples of granulocytes between 2 cephalic muscle fibers (iii), in the mesenchyme (iv), in the epidermis (note the characteristic actin ridges of the overlying periderm in the lower half of the image) (v), and at the border of a neuromast (vi). (C) 7 dpf larvae. (i) Granulocytes gathered around the pronephric glomerulus (arrow), revealing the beginning of definitive granulopoiesis in the kidney. Granulocytes along the basal lamina of the gut (ii) and the left pronephric duct (iii); (iv) lateral view of the tail showing the growing granulocyte population in the CHT, the site of larval hematopoiesis. (D) 14 dpf larvae. Ventral (i) and dorsal (ii) views of the anterior region; note the paucity of granulocytes in the dorsal head, except most anteriorly around the olfactory pits, and the increasing number of granulocytes in the kidney around the glomerulus (arrow), also visible in lateral view in (iii). (E) 30 dpf juvenile fish. (i) The head kidney (remnant of the pronephros) is now full of granulocytes (arrow). Granulocyte populations in the caudal fin (ii), all over the head (iii), in the gills (iv), in the left olfactory pit (v), and in the head kidney (vi) (left lateral side). e indicates inner ear; ca, caudal artery; g, pronephric glomerulus; k, kidney; m, muscle fiber; op, olfactory pit; pd, pronephric duct; and r, retina. (Scale bars, 10 μm in Ab-d, Bc-f; 20 μm in Cb,c; 100 μm in Aa, Ba,b, Ca, Eb-f.)
The very first detectable SB stained granules appear by 33 to 35 hpf, already in diverse locations (Figure S1A-J): in amoeboid cells in the yolk sac, between pericardium and epidermis, in the cephalic mesenchyme, and the ventral tail. The small number of granules per cell and their frequent clustering close to the nucleus (Figure S1H,I) suggests that most of these cells are still immature neutrophils (ie, myelocytes).6,21
By 2 to 4 dpf, heavily stained amoeboid cells are present in all embryos (Figure 1A,B), mostly in the ventral moiety (Figure 1Bi,ii): in the cephalic mesenchyme, notably in contact with muscles (Figure 1Biii), in the epidermis and around the base of olfactory pits and neuromasts of the lateral line (Figure 1Aiii,Bv,vi); medially in the ventral tail (Figure 1Av), and sub-epidermally in the trunk and tail. In the next days of larval development (Figure 1C,D), their number increases, especially in the ventral head (jaw and gills), along the gut and pronephric ducts (Figure 1Cii,iii), and prominently in the ventral tail (ie, the caudal hematopoietic tissue (CHT) that we recently characterized18 ) (Figure 1Civ). By 7 dpf, most larvae display a local concentration of SB+ granulocytes in the pronephros around the glomerulus (Figure 1Ci) (ie, where definitive hematopoiesis has just been initiated), and all of them do so by 10 dpf. The number of stained cells in the pronephros increases steadily over the ensuing days and weeks (Figure 1Dii-v,Ei,iii,vi). Unlike primitive macrophages, these SB stained granulocytes were never seen in the brain and retina (Figure Dii).
In juvenile (1-month-old) fish, whole-mount SB staining reveals the entire population of granulocytes throughout the fish (Figure 1E). Access to deep tissues is demonstrated by the heavy staining of the entire head kidney, resolved into individual leukocytes at higher magnification (Figure 1Ei,iii,vi, and data not shown). We noted numerous, mostly amoeboid granulocytes in the gills (Figure 1Eiv), below or within the epidermis, in the fins (Figure 1Eii), at the midline between the somitic muscles (not shown), around the nasal pits (Figure 1Ev), and dorsally in the head, between the skull and epidermis (Figure 1Eiii).
In embryos and larvae, electron microscopy revealed bona fide neutrophilic granulocytes in the same locations as SB staining (ie, not only in the ventral tail as previously documented,5,6 but also in peripheral tissues, including the epidermis [Figure S2]). Figure S2B shows a subepidermal neutrophil in extended contact with a macrophage.
We found that in live larvae, the granules of these neutrophils are readily noticeable through VE-DIC microscopy, for they are refractile and constantly moving in Brownian motion in the cytoplasm (Videos S1Video 2. Interaction of a neutrophil with two macrophages in the yolk sac circulation valley (MOV, 5.39 MB)–3). Just like with SB staining, the first granules were discerned in vivo by 35 hpf, clustered in the indentation of a kidney-shaped nucleus (Figure S1K); such cells could be followed undergoing mitosis (Figure S1L-P, arrow), indicative of myelocytes.21 From 48 hpf, leukocytes with numerous mobile granules were found at all the same locations as the SB stained cells (Figure 2A-H). When the cell is moving, its granules are moved collectively by the cytoplasmic flow that accompanies the cell's protrusive activity and anticipates its motility (Video S1). In the mesenchyme, these cells can migrate quite rapidly (15-20 μm/min). Even within the epidermis, at these stages still a monolayer, we found them wandering by slipping between epidermal cells at a velocity of 10 μm/min (Figure 2A and Video S1). In fact, by 48 hpf, all leukocytes moving within the epidermis display these granules. In contrast, all leukocytes resting and dendritically spread between epidermal cells,4 lack granules, and are likely to be primitive macrophages, that by 48 hpf have stopped wandering to settle down between epidermal cells.
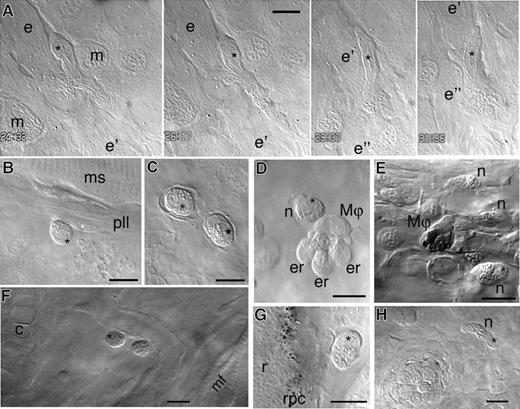
In vivo imaging of neutrophilic granulocytes by video-enhanced DIC microscopy. The nucleus of each neutrophil, whenever visible, is indicated by a black asterisk. (A) 48 hpf: a neutrophil migrating within the epidermis (at this stage still a monolayer). Time is indicated in minutes and seconds. In this 24-minute sequence, the neutrophil moves successively along 3 long epidermal cells (e, e′ and e″), at a mean velocity of 10 μm/min; the worm-like elements visible in these cells are mitochondria. See also Video S1. (B) 50 hpf: a neutrophil with visibly bilobed nucleus in addition to its granules, in contact with a Schwann cell (with white asterisk on its nucleus) wrapped around the posterior lateral line nerve. (C) 48 hpf: 2 neutrophils on the anterior aspect of the pericardium. (D) 50 hpf: still image from Video S2. A neutrophil interacting with a macrophage, itself interacting with 3 dying erythrocytes in the yolk sac circulation valley. (E) 56 hpf: a macrophage that accumulated methylene blue from the outer medium, and 3 granulocytes, in the CHT. See also Video S3. (F) 5 dpf: 2 neutrophils along a blood vessel in the ventral head. (G) 56 hpf: a neutrophil in the mesenchyme along the retinal pigment cell layer. (H) 7 dpf: a neutrophil near a neuromast of the ventral head (white asterisk). n indicates neutrophilic granulocyte; Mφ: macrophage; e-e′-e″, bulk epidermal cells; m, epidermal mucous cell; er, degenerating primitive erythrocytes; pll, posterior lateral line nerve; c, cartilage; mf, cephalic muscle fibers; s, somitic muscle; r, retina; and rpc, retinal pigment cell layer. Scale bars, 10 μm.
In vivo imaging of neutrophilic granulocytes by video-enhanced DIC microscopy. The nucleus of each neutrophil, whenever visible, is indicated by a black asterisk. (A) 48 hpf: a neutrophil migrating within the epidermis (at this stage still a monolayer). Time is indicated in minutes and seconds. In this 24-minute sequence, the neutrophil moves successively along 3 long epidermal cells (e, e′ and e″), at a mean velocity of 10 μm/min; the worm-like elements visible in these cells are mitochondria. See also Video S1. (B) 50 hpf: a neutrophil with visibly bilobed nucleus in addition to its granules, in contact with a Schwann cell (with white asterisk on its nucleus) wrapped around the posterior lateral line nerve. (C) 48 hpf: 2 neutrophils on the anterior aspect of the pericardium. (D) 50 hpf: still image from Video S2. A neutrophil interacting with a macrophage, itself interacting with 3 dying erythrocytes in the yolk sac circulation valley. (E) 56 hpf: a macrophage that accumulated methylene blue from the outer medium, and 3 granulocytes, in the CHT. See also Video S3. (F) 5 dpf: 2 neutrophils along a blood vessel in the ventral head. (G) 56 hpf: a neutrophil in the mesenchyme along the retinal pigment cell layer. (H) 7 dpf: a neutrophil near a neuromast of the ventral head (white asterisk). n indicates neutrophilic granulocyte; Mφ: macrophage; e-e′-e″, bulk epidermal cells; m, epidermal mucous cell; er, degenerating primitive erythrocytes; pll, posterior lateral line nerve; c, cartilage; mf, cephalic muscle fibers; s, somitic muscle; r, retina; and rpc, retinal pigment cell layer. Scale bars, 10 μm.
Some neutrophils were found in the circulation; Figure 2D and Video S2 show one neutrophil interacting with one, then 2 macrophages in the duct of Cuvier. VE-DIC microscopy also readily reveals that, unlike the primitive macrophages, these neutrophils do not phagocytose cell debris. Nor are they particularly endocytic: in some embryo batches, the primitive macrophages take up large amounts of methylene blue (present in the standard growth medium of the embryos), whereas neutrophils never do so (Figure 2E, Video S3, and Figure 7).
Thus SB staining, electron microscopy, and DIC video-microscopy of live specimens altogether show that neutrophils are abundant and rapidly moving in the mesenchyme and epidermis of healthy embryos and larvae.
Are these cells the same as the peroxidase positive leukocytes detected previously?5 To test this, we combined SB staining with the detection of peroxidase activity, using fluorescent tyramide as a substrate. This method revealed a 100% coincidence of SB staining with intense tyramide-based labeling (Figure 3E-M) in all embryos examined (n = 15). The only difference was that with the fluorescent tyramides, we could easily detect peroxidase-positive leukocytes in the yolk sac, head mesenchyme, and ventral tail already by 24 hpf (Figure 3A-D) (ie, 10 hours before the first SB-stained granules appeared).
Sudan Black (SB)–stained granulocytes coincide with peroxidase-positive leukocytes in zebrafish embryos. (A-D) At 24 hpf, 10 hours before the first detection of SB-stained granules, Cy3-tyramide based detection of endogenous peroxidase activity already reveals numerous peroxidase-positive leukocytes in the yolk sac (A,C), cephalic mesenchyme (B), and tail (A,D); note the unstained nuclei in panel D. Unlike at later stages, the staining appears concentrated in foci or granules (B,C). In panel A, vertical lines indicate a picture element captured at a slightly different focus. (E-M) 48 hpf embryos; detection of endogenous peroxidase activity with FITC-tyramide followed by the detection of granules with SB demonstrates a perfect coincidence of the 2 stainings. (E-H) High magnification of cells stained with both SB (E,G) and FITC-tyramide (F,H) near the ear. (I,J) Lateral right views of the eye and ear regions. Arrowheads point at the 3 cells shown at higher magnification in (E-H). (K-M) ventral tail (rostral to the left): DIC optics (K), fluorescence (M), and overlay (L). ca indicates caudal artery; e, ear; and r, retina. Scale bars, 15 μm in panels B and E, 40 μm in panel K, and 50 μm in panels I and J.
Sudan Black (SB)–stained granulocytes coincide with peroxidase-positive leukocytes in zebrafish embryos. (A-D) At 24 hpf, 10 hours before the first detection of SB-stained granules, Cy3-tyramide based detection of endogenous peroxidase activity already reveals numerous peroxidase-positive leukocytes in the yolk sac (A,C), cephalic mesenchyme (B), and tail (A,D); note the unstained nuclei in panel D. Unlike at later stages, the staining appears concentrated in foci or granules (B,C). In panel A, vertical lines indicate a picture element captured at a slightly different focus. (E-M) 48 hpf embryos; detection of endogenous peroxidase activity with FITC-tyramide followed by the detection of granules with SB demonstrates a perfect coincidence of the 2 stainings. (E-H) High magnification of cells stained with both SB (E,G) and FITC-tyramide (F,H) near the ear. (I,J) Lateral right views of the eye and ear regions. Arrowheads point at the 3 cells shown at higher magnification in (E-H). (K-M) ventral tail (rostral to the left): DIC optics (K), fluorescence (M), and overlay (L). ca indicates caudal artery; e, ear; and r, retina. Scale bars, 15 μm in panels B and E, 40 μm in panel K, and 50 μm in panels I and J.
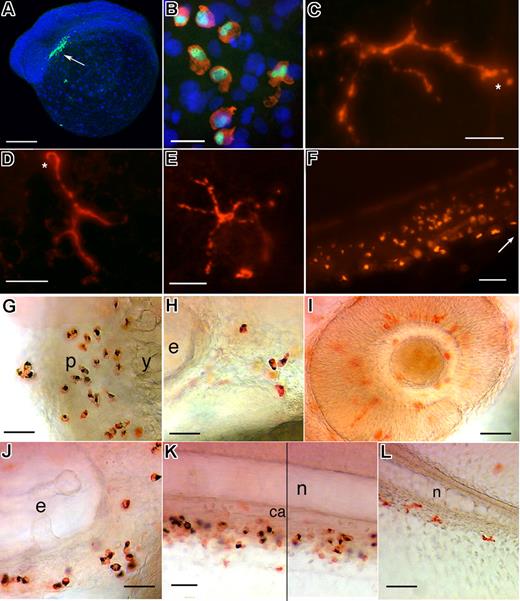
We then tested whether SB staining could be combined with whole-mount immunohistochemistry for leukocyte proteins, using polyclonal antibodies that we developed against the zebrafish homologs of the transcription factor PU.1 and the actin-bundling protein Leukocyte-specific plastin (L-plastin). Immunostaining revealed the PU.1 protein throughout the differentiation sequence of the primitive macrophage lineage from the rostral lateral mesoderm (Figure 4A,B), as previously found by in situ hybridization,22,23 and still at 2, 3, and 5 dpf in numerous tissue leukocytes (Figure 4C-L). Intriguingly, the PU.1 protein localized to the nucleus until 18-somites, then to the whole cell, and later on predominantly to the cytoplasm (Figure 4A-F). Immunostaining after SB staining was successful and revealed that all granulocytes express PU.1 at 2 and 3 dpf (Figure 4G,H,J,K) (n = 15 embryos/stage). The PU.1-positive, SB-negative leukocytes included macrophages of the retina and brain (Figure 4I), and ramified leukocytes in the epidermis (Figure 4C,D) clearly corresponding to the ramified sessile leukocytes observed in vivo by VE-DIC microscopy.4
All primitive and larval granulocytes express PU.1. (A) Whole-mount immunodetection of PU.1 with a polyclonal antibody directed against zebrafish PU.1. Left lateral view of a 14-somite embryo counterstained with DAPI, showing the immunostained cells in the rostral-most, “myelopoietic” lateral mesoderm ( ). At this early stage, the staining is only nuclear. (B) Coimmunodetection of PU.1 (green) and L-plastin (red) in the yolk sac of a 24 hpf embryo; nuclei stained with DAPI. (C-F) Immunostaining for PU.1 at 5 dpf; (C,D) Ramified nongranulocytic myeloid leukocytes in the trunk epidermis; an asterisk points at the nucleus, positioned at one end of the cell; (E) caudal fin; (F) CHT (
). At this early stage, the staining is only nuclear. (B) Coimmunodetection of PU.1 (green) and L-plastin (red) in the yolk sac of a 24 hpf embryo; nuclei stained with DAPI. (C-F) Immunostaining for PU.1 at 5 dpf; (C,D) Ramified nongranulocytic myeloid leukocytes in the trunk epidermis; an asterisk points at the nucleus, positioned at one end of the cell; (E) caudal fin; (F) CHT ( , rostral end). (G-L) Immunostaining for PU.1 after Sudan Black staining at 48 hpf (G-I, L) and 72 hpf (J, K). All SB+ cells also express PU.1. For example, in the pericardial region (G), posterior (H) and ventral to the ear (J), and in the CHT (K); in panel K, a vertical line separates 2 parts captured at a slightly different focus. The PU.1+, SB cells are probably macrophages, notably in the retina and brain (I). y indicates yolk sac; p, pericardial area; e, inner ear; n, notochord; and ca, caudal artery. Scale bars, 200 μm in panel A, 15 μm in panel B, 20 μm in panels C,G,L, 10 μm in panel D, 5 μm in panel E, and 50 μm in panel F.
, rostral end). (G-L) Immunostaining for PU.1 after Sudan Black staining at 48 hpf (G-I, L) and 72 hpf (J, K). All SB+ cells also express PU.1. For example, in the pericardial region (G), posterior (H) and ventral to the ear (J), and in the CHT (K); in panel K, a vertical line separates 2 parts captured at a slightly different focus. The PU.1+, SB cells are probably macrophages, notably in the retina and brain (I). y indicates yolk sac; p, pericardial area; e, inner ear; n, notochord; and ca, caudal artery. Scale bars, 200 μm in panel A, 15 μm in panel B, 20 μm in panels C,G,L, 10 μm in panel D, 5 μm in panel E, and 50 μm in panel F.
All primitive and larval granulocytes express PU.1. (A) Whole-mount immunodetection of PU.1 with a polyclonal antibody directed against zebrafish PU.1. Left lateral view of a 14-somite embryo counterstained with DAPI, showing the immunostained cells in the rostral-most, “myelopoietic” lateral mesoderm ( ). At this early stage, the staining is only nuclear. (B) Coimmunodetection of PU.1 (green) and L-plastin (red) in the yolk sac of a 24 hpf embryo; nuclei stained with DAPI. (C-F) Immunostaining for PU.1 at 5 dpf; (C,D) Ramified nongranulocytic myeloid leukocytes in the trunk epidermis; an asterisk points at the nucleus, positioned at one end of the cell; (E) caudal fin; (F) CHT (
). At this early stage, the staining is only nuclear. (B) Coimmunodetection of PU.1 (green) and L-plastin (red) in the yolk sac of a 24 hpf embryo; nuclei stained with DAPI. (C-F) Immunostaining for PU.1 at 5 dpf; (C,D) Ramified nongranulocytic myeloid leukocytes in the trunk epidermis; an asterisk points at the nucleus, positioned at one end of the cell; (E) caudal fin; (F) CHT ( , rostral end). (G-L) Immunostaining for PU.1 after Sudan Black staining at 48 hpf (G-I, L) and 72 hpf (J, K). All SB+ cells also express PU.1. For example, in the pericardial region (G), posterior (H) and ventral to the ear (J), and in the CHT (K); in panel K, a vertical line separates 2 parts captured at a slightly different focus. The PU.1+, SB cells are probably macrophages, notably in the retina and brain (I). y indicates yolk sac; p, pericardial area; e, inner ear; n, notochord; and ca, caudal artery. Scale bars, 200 μm in panel A, 15 μm in panel B, 20 μm in panels C,G,L, 10 μm in panel D, 5 μm in panel E, and 50 μm in panel F.
, rostral end). (G-L) Immunostaining for PU.1 after Sudan Black staining at 48 hpf (G-I, L) and 72 hpf (J, K). All SB+ cells also express PU.1. For example, in the pericardial region (G), posterior (H) and ventral to the ear (J), and in the CHT (K); in panel K, a vertical line separates 2 parts captured at a slightly different focus. The PU.1+, SB cells are probably macrophages, notably in the retina and brain (I). y indicates yolk sac; p, pericardial area; e, inner ear; n, notochord; and ca, caudal artery. Scale bars, 200 μm in panel A, 15 μm in panel B, 20 μm in panels C,G,L, 10 μm in panel D, 5 μm in panel E, and 50 μm in panel F.
Granulocytes arise from 2 origins in the zebrafish embryo
What is the embryologic origin of all these tissue neutrophils? We recently showed that the CHT is a site of granulopoiesis between at least 3 and 14 dpf, seeded by the definitive hematopoietic precursors arisen in the trunk beneath the dorsal aorta (the fish homolog of the aorta-gonad-mesonephros (AGM) area of amniote embryos).19 This caudal granulopoiesis, which was documented by electron microscopy, obviously accounts for many of the numerous SB+ cells seen in the CHT and its surroundings from at least 3 dpf onward and possibly earlier. Yet the first appearance of SB + leukocytes by 32 to 35 hpf simultaneously in the CHT, yolk sac, and cephalic mesenchyme suggested that the rostral “primitive macrophage lineage” might also be a source of granulocytes.
Therefore, we undertook a cell tracing analysis of the clearly identifiable hematopoietic precursors of the primitive macrophages present in the yolk sac before 24 hpf, which we previously named the “premacrophages.”2 Embryos were injected at the 1-cell stage with the photoactivatable cell tracer caged fluorescein-dextran. At the 22-somite stage (20 hpf), when mesodermal precursors had emigrated from anterocardiac mesoderm to the neighboring yolk sac, and evolved into the characteristic, round (12-μm) blastlike premacrophages,2 we uncaged the fluorescein with an ultraviolet laser in single premacrophages: 10 per embryo (Figure 5A). At 72 hpf, the larvae were fixed, and we combined immunodetection of the uncaged fluorescein (revealing the progeny of the initially photoactivated cells) with a detection of granulocytes, either through their SB+ granules or through their peroxidase activity. Both approaches led to the same results. Figure 5B shows a typical outcome obtained with the first detection scheme. From the 10 cells initially photoactivated in the yolk sac at 20 hpf, a progeny of 17 cells was detected at 72 hpf, dispersed around the eye, in the CHT, along the pronephric duct, and dorsal to the somites. Eleven of 17 of these cells were also stained by SB and hence were granulocytes. Figure 5C shows an example after the second detection scheme. From the 10 initially photoactivated cells, a progeny of 34 cells was detected, mostly around the left eye, in the CHT, and dorsal to the somites. Seventeen of 34 also displayed bright red fluorescence, indicative of peroxidase activity, hence of granulocytic identity; the non granulocytic progeny included 5 microglial cells in the retina (eg, Figure 5Cvi). Over 14 different embryos, the mean detected pro-geny was 20.1 cells, of which 57% (± 9%) were granulocytes (Figure S4).
In vivo cell labeling with a photoactivatable cell tracer demonstrates the double potential of the primitive (rostral) myeloid progenitors. (A) At 20 hpf, after injection of caged fluorescein-dextran in the embryo at the 1-cell stage, the fluorescein was uncaged (hence its natural fluorescence was restored with a pulsed ultraviolet [365 nm] laser beam) in 10 primitive myeloid progenitors, located along the left lateral border of the pericardium. Fifteen hours later (35 hpf), some of the green-fluorescent labeled cells are observed in the yolk sac circulation valley ( ). (B) 72 hpf; immunodetection of uncaged fluorescein (AEC staining, red) after SB staining reveals the granulocyte vs. macrophage nature of the progeny of the primitive myeloid progenitors photolabeled in panel A. The drawing recapitulates the locations of all red (uncaged fluorescein-positive) cells identified in this embryo; red dots represent macrophages, stained only by AEC (6/17 cells); these are located along the retina, along the base of the dorsal fin (ii,iii, red arrowheads), in the CHT. Black dots represent cells stained by both AEC and SB (11/17 cells), hence granulocytes; those are located around the eye, ventral to the trunk somites, in the CHT (i,iv,v, black arrowheads). Note that the dextran-coupled uncaged fluorescein revealed by AEC is in the whole cell, whereas SB is confined to the cytoplasm, hence the red label is most apparent in the nucleus. Scale bars, 25 μm. (C) 72 hpf; fluorescent combined immunodetection of uncaged fluorescein (FITC-tyramide, green) with a detection of the peroxidase activity of granulocytes (Cy3-tyramide, red), after uncaging of fluorescein in 10 primitive myeloid progenitors in the yolk sac at 20 hpf. Green dots on the drawing represent macrophages (17/34 cells) that are located in the head mesenchyme, around the eye, in the ganglion cell layer (rgc) of the retina (vi), dorsal to the somites (ii,iii), along the yolk tube (i), and in the CHT (v, vii, green arrowheads). Yellow dots and arrowheads stand for granulocytes, stained in both green and red (17/34 cells). Those cells are located in the head mesenchyme, dorsal to the somites (ii,iii), in the CHT (vii). Scale bars, 50 μm.
). (B) 72 hpf; immunodetection of uncaged fluorescein (AEC staining, red) after SB staining reveals the granulocyte vs. macrophage nature of the progeny of the primitive myeloid progenitors photolabeled in panel A. The drawing recapitulates the locations of all red (uncaged fluorescein-positive) cells identified in this embryo; red dots represent macrophages, stained only by AEC (6/17 cells); these are located along the retina, along the base of the dorsal fin (ii,iii, red arrowheads), in the CHT. Black dots represent cells stained by both AEC and SB (11/17 cells), hence granulocytes; those are located around the eye, ventral to the trunk somites, in the CHT (i,iv,v, black arrowheads). Note that the dextran-coupled uncaged fluorescein revealed by AEC is in the whole cell, whereas SB is confined to the cytoplasm, hence the red label is most apparent in the nucleus. Scale bars, 25 μm. (C) 72 hpf; fluorescent combined immunodetection of uncaged fluorescein (FITC-tyramide, green) with a detection of the peroxidase activity of granulocytes (Cy3-tyramide, red), after uncaging of fluorescein in 10 primitive myeloid progenitors in the yolk sac at 20 hpf. Green dots on the drawing represent macrophages (17/34 cells) that are located in the head mesenchyme, around the eye, in the ganglion cell layer (rgc) of the retina (vi), dorsal to the somites (ii,iii), along the yolk tube (i), and in the CHT (v, vii, green arrowheads). Yellow dots and arrowheads stand for granulocytes, stained in both green and red (17/34 cells). Those cells are located in the head mesenchyme, dorsal to the somites (ii,iii), in the CHT (vii). Scale bars, 50 μm.
In vivo cell labeling with a photoactivatable cell tracer demonstrates the double potential of the primitive (rostral) myeloid progenitors. (A) At 20 hpf, after injection of caged fluorescein-dextran in the embryo at the 1-cell stage, the fluorescein was uncaged (hence its natural fluorescence was restored with a pulsed ultraviolet [365 nm] laser beam) in 10 primitive myeloid progenitors, located along the left lateral border of the pericardium. Fifteen hours later (35 hpf), some of the green-fluorescent labeled cells are observed in the yolk sac circulation valley ( ). (B) 72 hpf; immunodetection of uncaged fluorescein (AEC staining, red) after SB staining reveals the granulocyte vs. macrophage nature of the progeny of the primitive myeloid progenitors photolabeled in panel A. The drawing recapitulates the locations of all red (uncaged fluorescein-positive) cells identified in this embryo; red dots represent macrophages, stained only by AEC (6/17 cells); these are located along the retina, along the base of the dorsal fin (ii,iii, red arrowheads), in the CHT. Black dots represent cells stained by both AEC and SB (11/17 cells), hence granulocytes; those are located around the eye, ventral to the trunk somites, in the CHT (i,iv,v, black arrowheads). Note that the dextran-coupled uncaged fluorescein revealed by AEC is in the whole cell, whereas SB is confined to the cytoplasm, hence the red label is most apparent in the nucleus. Scale bars, 25 μm. (C) 72 hpf; fluorescent combined immunodetection of uncaged fluorescein (FITC-tyramide, green) with a detection of the peroxidase activity of granulocytes (Cy3-tyramide, red), after uncaging of fluorescein in 10 primitive myeloid progenitors in the yolk sac at 20 hpf. Green dots on the drawing represent macrophages (17/34 cells) that are located in the head mesenchyme, around the eye, in the ganglion cell layer (rgc) of the retina (vi), dorsal to the somites (ii,iii), along the yolk tube (i), and in the CHT (v, vii, green arrowheads). Yellow dots and arrowheads stand for granulocytes, stained in both green and red (17/34 cells). Those cells are located in the head mesenchyme, dorsal to the somites (ii,iii), in the CHT (vii). Scale bars, 50 μm.
). (B) 72 hpf; immunodetection of uncaged fluorescein (AEC staining, red) after SB staining reveals the granulocyte vs. macrophage nature of the progeny of the primitive myeloid progenitors photolabeled in panel A. The drawing recapitulates the locations of all red (uncaged fluorescein-positive) cells identified in this embryo; red dots represent macrophages, stained only by AEC (6/17 cells); these are located along the retina, along the base of the dorsal fin (ii,iii, red arrowheads), in the CHT. Black dots represent cells stained by both AEC and SB (11/17 cells), hence granulocytes; those are located around the eye, ventral to the trunk somites, in the CHT (i,iv,v, black arrowheads). Note that the dextran-coupled uncaged fluorescein revealed by AEC is in the whole cell, whereas SB is confined to the cytoplasm, hence the red label is most apparent in the nucleus. Scale bars, 25 μm. (C) 72 hpf; fluorescent combined immunodetection of uncaged fluorescein (FITC-tyramide, green) with a detection of the peroxidase activity of granulocytes (Cy3-tyramide, red), after uncaging of fluorescein in 10 primitive myeloid progenitors in the yolk sac at 20 hpf. Green dots on the drawing represent macrophages (17/34 cells) that are located in the head mesenchyme, around the eye, in the ganglion cell layer (rgc) of the retina (vi), dorsal to the somites (ii,iii), along the yolk tube (i), and in the CHT (v, vii, green arrowheads). Yellow dots and arrowheads stand for granulocytes, stained in both green and red (17/34 cells). Those cells are located in the head mesenchyme, dorsal to the somites (ii,iii), in the CHT (vii). Scale bars, 50 μm.
These cell tracing data demonstrate that the hematopoietic precursors of the primitive macrophages also gave rise to granulocytes, in even slightly higher number. To distinguish the granulocytes from the 2 lineages, those arisen from the same rostral lineage as the primitive macrophages we called “primitive granulocytes,” and those arisen from AGM-derived precursors that homed to the CHT we called “larval granulocytes,” as this is essentially a site of larval hematopoiesis.
We then turned to mutants and morphants that are defective in definitive, AGM-derived hematopoiesis but not in the primitive, rostral myeloid lineage: the spadetail23,26 and mindbomb26 mutants and runx1 knockdown morphants,12,27 obtained by injection of an anti-runx1 morpholino in the fertilized eggs. In both spadetail and mindbomb mutants and in runx1 morphants, SB staining revealed numerous granulocytes throughout the head and in the yolk sac. They were present also in the trunk and tail but in a pattern different from that of wild-type siblings (Figure 6 and data not shown): all confined to superficial (epidermal and subepidermal) locations; none (in spadetail mutants) or very few (in mindbomb mutants and runx1 morphants) were seen in the CHT (which lies deep at the midline, ventrally). This pattern confirms the absence of granulopoiesis in the CHT and the functionality of the rostral primitive lineage in spadetail and mindbomb mutants and runx1 morphants. Because spadetail has no blood circulation in the trunk and tail (but normal circulation in the head), its SB staining pattern further showed that the neutrophils of rostral origin can easily disperse in the whole body up to the tail tip in the absence of blood circulation (Figure 6B).
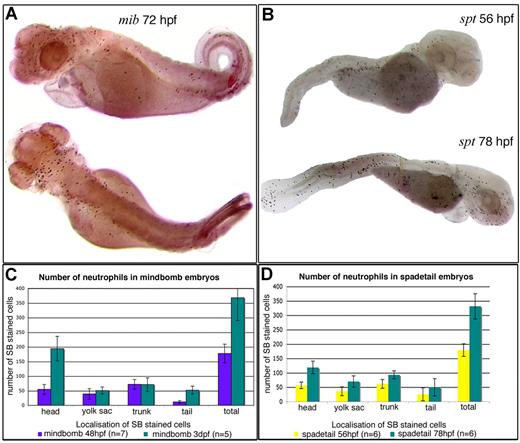
Sudan Black (SB)–stained granulocytes in mindbomb and spadetail mutants. (A) Lateral and dorsal views of a 3 dpf mindbomb mutant. Note the accumulation of SB+ cells dorsally in the head. (B) Lateral views of 56-hpf and 78-hpf spadetail mutants. Note the increase in SB+ cells all over the body between the 2 stages, even though these mutants have no circulation in the trunk and tail (but normal circulation in the head, data not shown). At both stages, all SB+ cells in the tail and trunk are superficial (mostly subepidermal); none is in the CHT. (C,D) Histograms showing the number of SB+ granulocytes sorted by their localization throughout the embryo, in mindbomb at 48 and 72 hpf (C) and spadetail at 56 and 78 hpf (D) mutants. In both mutants, the granulocyte population increases 2-fold between the 2 stages examined. Cell numbers for the wild-type siblings are not shown because they are irrelevant to the point examined here.
Sudan Black (SB)–stained granulocytes in mindbomb and spadetail mutants. (A) Lateral and dorsal views of a 3 dpf mindbomb mutant. Note the accumulation of SB+ cells dorsally in the head. (B) Lateral views of 56-hpf and 78-hpf spadetail mutants. Note the increase in SB+ cells all over the body between the 2 stages, even though these mutants have no circulation in the trunk and tail (but normal circulation in the head, data not shown). At both stages, all SB+ cells in the tail and trunk are superficial (mostly subepidermal); none is in the CHT. (C,D) Histograms showing the number of SB+ granulocytes sorted by their localization throughout the embryo, in mindbomb at 48 and 72 hpf (C) and spadetail at 56 and 78 hpf (D) mutants. In both mutants, the granulocyte population increases 2-fold between the 2 stages examined. Cell numbers for the wild-type siblings are not shown because they are irrelevant to the point examined here.
It is noteworthy that in both mindbomb and spadetail, the granulocyte population still increased 2-fold between 2 and 3 dpf (Figure 6C,D), indicating that once in the peripheral tissues, the cells of the primitive, rostral lineage are still capable of proliferation and granulocytic differentiation. It is intriguing that mindbomb embryos displayed a striking accumulation of SB+ granulocytes by 3 dpf on the dorsal side of the head, subepidermally (Figure 6A), possibly related to the brain malformation in this mutant, a malformation, however, that does not correlate with increased apoptosis, as revealed by acridine orange vital staining (data not shown).
Primitive and larval granulocytes are barely phagocytic yet highly attracted to infected or stressed tissues during zebrafish development
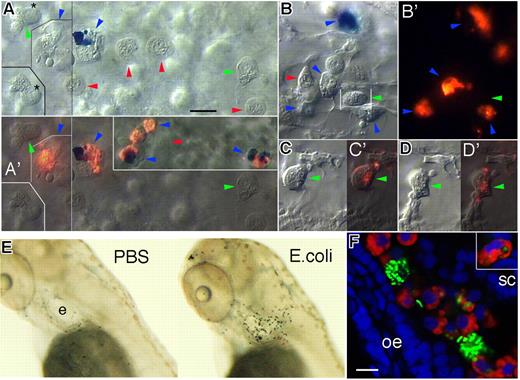
The mpx+ or peroxidase-positive leukocytes of zebrafish larvae—presumed to be neutrophils—have been shown to be attracted to a caudal fin wound,5 but their behavior toward infections has not been investigated. We were surpised to find that on injection of E coli in the blood by 52 hpf, the bacteria immediately stuck to the macrophages but not to the neutrophils present in the blood flow or in the CHT. Thirty minutes to 2 hours later, the macrophages were loaded with engulfed bacteria, but the neutrophils contained none (Figure 7A,B). Only a few neutrophils in the CHT, close to the injection site, had phagocytosed bacteria, although in much smaller amounts than the macrophages (Figure 7C,D).
Neutrophils are attracted to infection foci, yet much less phagocytic than macrophages, in zebrafish larvae. (A-D) In vivo observations in a swimming larva injected with red fluorescent E coli in the caudal vein at 52 hpf. Blue, green, and red arrowheads, respectively point at macrophages, neutrophils, and erythrocytes. (A-D) are video-enhanced DIC images, (A′-D′) show the corresponding overlay of DIC and red fluorescence of the bacteria; all images are at the same magnification. (A,A′) 1.5 hours after injection, yolk sac circulation valley: 2 macrophages, one of which loaded with methylene blue, have already phagocytosed many bacteria, whereas 2 neutrophils nearby are free of them; the neutrophil marked by an asterisk at the border of its nucleus is also displayed 45 seconds later in the lower left inset in A, to show its fast motility. The inset in A′ shows 4 more macrophages farther in the valley, 2 of which also loaded with methylene blue. (B,B′): 2.25 hours after injection, CHT (close to the site of bacteria injection): 4 macrophages, one loaded with methylene blue, have phagocytosed many bacteria, whereas a neutrophil among them has none. (C,C′) 2.5 hours after injection, CHT/ventral fin junction: a neutrophil that phagocytosed bacteria; (D,D′) images of the same neutrophil 8 minutes later. In (A,A′,B) thin straight lines indicate picture elements captured at a slightly different focus. (E) SB staining of neutrophils at 3 dpf, 5 hours after microinjection of either PBS or E coli bacteria in the left inner ear. (F) Confocal fluorescence image of a portion of the left ear at 3 dpf, 5 hours after injection of green fluorescent (GFP-expressing) E coli in the ear cavity. After fixation, the peroxidase activity of neutrophils was revealed with Cy3-tyramide (red), then GFP by anti-GFP immunohistochemistry with Alexa Fluor 488 as fluorophore (green), and the nuclei were stained with DAPI (blue). Two macrophages are highlighted by their large amount of phagocytosed bacteria, whereas only some of the recruited neutrophils show a few small bacteria-containing phagosomes. The upper right inset shows a neutrophil in an other focal plane, with 4 obvious such phagosomes. e indicates ear; oe, otic epithelium; and sc, semicircular canal. Scale bars, 10 μm.
Neutrophils are attracted to infection foci, yet much less phagocytic than macrophages, in zebrafish larvae. (A-D) In vivo observations in a swimming larva injected with red fluorescent E coli in the caudal vein at 52 hpf. Blue, green, and red arrowheads, respectively point at macrophages, neutrophils, and erythrocytes. (A-D) are video-enhanced DIC images, (A′-D′) show the corresponding overlay of DIC and red fluorescence of the bacteria; all images are at the same magnification. (A,A′) 1.5 hours after injection, yolk sac circulation valley: 2 macrophages, one of which loaded with methylene blue, have already phagocytosed many bacteria, whereas 2 neutrophils nearby are free of them; the neutrophil marked by an asterisk at the border of its nucleus is also displayed 45 seconds later in the lower left inset in A, to show its fast motility. The inset in A′ shows 4 more macrophages farther in the valley, 2 of which also loaded with methylene blue. (B,B′): 2.25 hours after injection, CHT (close to the site of bacteria injection): 4 macrophages, one loaded with methylene blue, have phagocytosed many bacteria, whereas a neutrophil among them has none. (C,C′) 2.5 hours after injection, CHT/ventral fin junction: a neutrophil that phagocytosed bacteria; (D,D′) images of the same neutrophil 8 minutes later. In (A,A′,B) thin straight lines indicate picture elements captured at a slightly different focus. (E) SB staining of neutrophils at 3 dpf, 5 hours after microinjection of either PBS or E coli bacteria in the left inner ear. (F) Confocal fluorescence image of a portion of the left ear at 3 dpf, 5 hours after injection of green fluorescent (GFP-expressing) E coli in the ear cavity. After fixation, the peroxidase activity of neutrophils was revealed with Cy3-tyramide (red), then GFP by anti-GFP immunohistochemistry with Alexa Fluor 488 as fluorophore (green), and the nuclei were stained with DAPI (blue). Two macrophages are highlighted by their large amount of phagocytosed bacteria, whereas only some of the recruited neutrophils show a few small bacteria-containing phagosomes. The upper right inset shows a neutrophil in an other focal plane, with 4 obvious such phagosomes. e indicates ear; oe, otic epithelium; and sc, semicircular canal. Scale bars, 10 μm.
Because the primitive macrophages are able to sense and migrate to an infected body cavity to clear the microbes,2 we devised a similar test for neutrophils by injecting E coli into the left ear, another closed space naturally devoid of leukocytes, at 3 dpf. SB staining at various time points after injection revealed that the neutrophils were massively attracted to the infected ear, where their number peaked by 4 to 5 hours after injection (Figure 7E). Initially those of the head were recruited, then the large caudal population of the CHT also became visibly depleted by the recruitment (data not shown). Red fluorescent tyramide-based staining of the peroxidase activity of the recruited neutrophils allowed us to analyze in detail their relation with the green fluorescent bacteria, by confocal microscopy (Figure 7F). Again, many bacteria were phagocytosed by peroxidase-negative macrophages, whereas only a minority of the numerous recruited neutrophils phagocytosed bacteria—2 to 10 per cell, instead of up to 100 per macrophage. Similar results were obtained upon injection of Gram-positive bacteria or zymosan (data not shown). Because the production of extracellular thread-like “nets” by neutrophils from adult zebrafish has been described,28 we stained the infected embryos with 4,6-diamidino-2-phenylindole (DAPI)27 but failed to detect such structures.
The number of neutrophils in the ear gradually decreased past 5 hours after injection. In vivo VE-DIC microscopy, which readily detects any type of cell death or damage,4 revealed no trace of it among the neutrophils (data not shown). They gradually left the site, and most were gone by 24 hours after injection, whereas some macrophages still remained in the cavity.
It is noteworthy that if the microinjection in the otic cavity was performed slightly less smoothly (see “Zebrafish stocks and embryo treatments”), injected sterile PBS attracted neutrophils to the same extent as injected bacteria (Figure S5), further indicating that these neutrophils are exquisitely sensitive and attracted to local perturbations of homeostasis. Figure S6 gives another example, which occurred naturally in a 3-week-old wild-type larva that suffered some malformation and displayed a striking accumulation of neutrophils all over the outer and vitreal surface of both lenses, as well as around the intestinal bulb. This massive mobilization had entirely depleted the kidney and CHT from their neutrophils.
Discussion
The results presented here clarify the identity, distribution, origins, and functional traits of neutrophils through zebrafish development. SB staining of the granules that define these cells, along with their visualization in vivo by VE-DIC microscopy, and by EM, in the tissues of the whole animal provides a firm, unequivocal basis on which molecular and behavioral traits can be safely superimposed. Thus, we find that from 48 hpf onward, the granulocytes coincide completely with the peroxidase-positive leukocytes, as previously proposed. On the other hand, unlike previous claims,7,23 all these granulocytes express L-plastin, as well as PU.1, even once fully differentiated and wandering in peripheral tissues.
The simultaneous appearance of granulocytes over the whole embryo by 48 hpf was intriguing. Our cell tracing results demonstrate that they have 2 origins: not only the CHT seeded by the definitive hematopoietic stem cells born in the trunk19 but also the rostral lineage that produced the primitive macrophages a day earlier, from anterocardiac mesoderm. The morphologically homogeneous hematopoietic progenitors of the primitive macrophages, in the yolk sac,2 actually give rise to both macrophages and granulocytes in similar numbers. So far, in vertebrates, especially in mammals, where they have been most studied, the primitive macrophages have always been considered to constitute a separate lineage giving rise to no other cell type.1 Either the primitive granulocytes found in the fish do not exist in mammals or they have been overlooked because of the peculiar features of their differentiation, which occurs almost a day after that of the primitive macrophages, and mostly after emigration from the yolk sac and dispersal in embryonic tissues. In fact, to our knowledge, the very notion of myeloid progenitors that emigrate to the peripheral tissues before differentiating into neutrophilic granulocytes in situ is unprecedented. The peroxidase-positive myeloid cells found in the yolk sac and embryonic tissues in the 24 to 32 hpf interval (ie, before the appearance of the first SB-stained granules), are likely to represent such migrating progenitors, because we no longer detect perox + SB- cells in the tissues (nor morphologically identifiable primitive myeloid progenitors in the yolk sac) by 48 hpf. Still, it is not possible to know whether these migrating mpx + progenitors are already committed to a granulocytic fate, for mpx may be expressed by the progenitors both of granulocytes and of macrophages, as myeloperoxidase is in mammals.8 It is remarkable that despite the absence of such progenitors in the tissues by 48 hpf, we still observed a doubling of the primitive granulocyte population in the tissues between 48 and 72 hpf, in spadetail and mindbomb mutants. In wild-type embryos, more than half of the progeny at 72 hpf of primitive myeloid progenitors labeled in the yolk sac at 20 hpf consisted of neutrophils, scattered all over the swimming larva. All together, these data suggest that such mature tissue neutrophils have a much longer lifespan than previously assumed for neutrophils in mammals and might even be capable of some proliferation.
Our cell tracing data regarding both the rostral/primitive (this work) and caudal/larval leukopoiesis18 lead to a coherent picture of how the cells from these 2 origins contribute to the total myeloid population. When blood circulation starts, by 26 hpf, some of the primitive myeloid leukocytes born in the yolk sac are taken by the blood that flows freely over the yolk surface.2 Many of them stop in the caudal vein and settle there and in the surrounding mesenchyme. As in the other tissues that these cells colonize, some become macrophages—here the stromal macrophages of the CHT18 —and others start differentiating into neutrophils by 32 to 35 hpf. From 33 hpf onward, the definitive hematopoietic stem cells born in the trunk between aorta and axial vein start seeding the CHT (K.K. et al, manuscript submitted), where they expand and differentiate, leading to a steady larval granulopoiesis for at least several days.18
We found that, overall, the embryonic granulocytes and macrophages share the same tissue localizations, with the clearcut exception of the brain and retina, in which granulocytes are never seen. In this respect, our previous finding that the colonization of brain and retina by macrophages requires the M-CSF receptor4 takes a further meaning. It provides a straightforward explanation for the absence of neutrophils in the CNS, because the M-CSF receptor is the very marker of the monocyte/macrophage lineage among all leukocytes.
We found that these neutrophils are potently attracted to bacteria and able to cross epithelia to reach them; surprisingly, however, only a minority of them phagocytose microbes, and many times less per cell than the macrophages of the same embryologic origin.
A macrophage-restricted expression of microbial sugar binding lectin receptors may be responsible for the fast sticking of microbes to the macrophage surface but not to the neutrophil, observed in the embryo's blood. Phagocytosis by the neutrophils could require an opsonization of the microbes by antibodies or complement, which would be lacking in embryos/larvae. Alternatively, these cells may display some intrinsic immaturity, as documented for perinatal neutrophils in mammals.29 However, our preliminary data indicate that in juvenile zebrafish, neutrophils behave as in early larvae, with strong attraction to but poor phagocytosis of injected bacteria (E.C. and P.H., unpublished observations, August 2006). If they are barely phagocytic, what could be the function of these neutrophils patrolling in the tissues? Because they are attracted to microbes together with the macrophages, they might assist the latter in killing the microbes, through some controlled degranulation, of a kind that would not compromise their viability, because once the bacteria have been cleared by the macrophages, these neutrophils appear to just leave and resume their wandering.
But their action may be broader than the release of microbicidal compounds upon infection,30 for we found these cells to be also strongly attracted to an aseptic local tissue damage (in the otic cavity after a larger PBS injection) or to some developmental abnormalities (the abnormal brain of mindbomb mutants or the abnormal lenses of a naturally occurred case). Overall, these long-lived neutrophils steadily patrolling in the dermis and epidermis display a behavior that we so far attributed to tissue-resident macrophages.4 Along this line, a nonphagocytic, interleukin-1 producing resident population of neutrophils was recently identified in the testis of the gilthead seabream, a seasonal breeding fish, suggesting a role of these cells in seasonal testicular involution.31 Thus the possible roles in tissue remodelling and homeostasis beyond microbe elimination that have been advocated for tissue macrophages,4 notably on the basis of their extremely diverse secretory activity,32,33 should be investigated as well for fish tissue neutrophils. Their study might ultimately lead to uncovering neutrophil subsets of similar functions in mammals.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Isabelle Godin, Véronique Witko-Sarsat, and Jean-Pierre Levraud for their critical reading of the manuscript, and J. M. Ghigo and W. Bitter for the GFP and DsRed expressing E coli bacteria, respectively.
This work was initially supported by an Avenir grant from Inserm (P.H.). D.L.G. was supported by a fellowship from Ministère de l'Education Nationale de la Recherche et de la Technologie.
Authorship
Contribution: D.L.G., M.J.R., E.C., E. Murayama, K.M., V.B., E. Mordelet, A.Z., and P.H. performed experiments, analyzed data, and checked or improved the manuscript; H.S. contributed an important reagent (anti-mouse L-plastin antibody). P.H. drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Philippe Herbomel, Unité Macrophages et Développement de l'Immunité, Institut Pasteur, 25 rue du Dr Roux, 75724 Paris cedex 15, France; e-mail: herbomel@pasteur.fr.

![Figure 5. In vivo cell labeling with a photoactivatable cell tracer demonstrates the double potential of the primitive (rostral) myeloid progenitors. (A) At 20 hpf, after injection of caged fluorescein-dextran in the embryo at the 1-cell stage, the fluorescein was uncaged (hence its natural fluorescence was restored with a pulsed ultraviolet [365 nm] laser beam) in 10 primitive myeloid progenitors, located along the left lateral border of the pericardium. Fifteen hours later (35 hpf), some of the green-fluorescent labeled cells are observed in the yolk sac circulation valley (). (B) 72 hpf; immunodetection of uncaged fluorescein (AEC staining, red) after SB staining reveals the granulocyte vs. macrophage nature of the progeny of the primitive myeloid progenitors photolabeled in panel A. The drawing recapitulates the locations of all red (uncaged fluorescein-positive) cells identified in this embryo; red dots represent macrophages, stained only by AEC (6/17 cells); these are located along the retina, along the base of the dorsal fin (ii,iii, red arrowheads), in the CHT. Black dots represent cells stained by both AEC and SB (11/17 cells), hence granulocytes; those are located around the eye, ventral to the trunk somites, in the CHT (i,iv,v, black arrowheads). Note that the dextran-coupled uncaged fluorescein revealed by AEC is in the whole cell, whereas SB is confined to the cytoplasm, hence the red label is most apparent in the nucleus. Scale bars, 25 μm. (C) 72 hpf; fluorescent combined immunodetection of uncaged fluorescein (FITC-tyramide, green) with a detection of the peroxidase activity of granulocytes (Cy3-tyramide, red), after uncaging of fluorescein in 10 primitive myeloid progenitors in the yolk sac at 20 hpf. Green dots on the drawing represent macrophages (17/34 cells) that are located in the head mesenchyme, around the eye, in the ganglion cell layer (rgc) of the retina (vi), dorsal to the somites (ii,iii), along the yolk tube (i), and in the CHT (v, vii, green arrowheads). Yellow dots and arrowheads stand for granulocytes, stained in both green and red (17/34 cells). Those cells are located in the head mesenchyme, dorsal to the somites (ii,iii), in the CHT (vii). Scale bars, 50 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/1/10.1182_blood-2007-06-095398/3/m_zh80020811270005.jpeg?Expires=1770406755&Signature=W7egLbDDaCoG28GO2F~m9CF7j182SxKqnRxvUqTrqWZ64I~2JSj1hpePTbftpVUiU-SV0LwlbN-hZzoFB3TLy1C9sUTQ9pdr0DnGHA~Ir0y9r2eogNDbwB~cxQXP9ZUm1I8aPJktiIP24Yr6DDeS5pBaToaHdPt2yOAczSKVBc0OV2dOmWxqF0m1sMw3Og6DI9coSZwEfhopkmCRKcQUsEYmLAFYMX12LaqIuPXPaKX01JGUHjOspDmj~7UfYNifYKXeiqgp2yTPyLEpX-~hJXXu7NzT-75F88~5~22q1T~QDtQV8XEf2R-~IccsAhVtsvqAx9gj~LoVHxNdAttM2w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)