Abstract
The immunomodulator FTY720 (FTY) has been shown to be beneficial in experimental models of organ transplantation and autoimmunity. We show that FTY significantly inhibited but did not prevent graft-versus-host disease (GVHD) in lethally irradiated or nonirradiated allogeneic recipients. Although most studies implicate prevention of lymphocyte egress from lymphoid organs as the primary mechanism of action, our data indicate that FTY effects on the host are more likely to be responsible for GVHD inhibition. FTY reduced splenic CD11c+ cells by 50%, and similarly reduced CD4+ and CD8+ T-cell responder frequencies in the spleen early after transplantation. Imaging of GFP+ effectors indicated that FTY modified donor effector T-cell migration to secondary lymphoid organs, but did not uniformly trap T cells in lymph nodes or prevent early effector migration to GVHD parenchymal target organs. Administration of FTY only prior to transplantation inhibited GVHD, indicating that the primary function of FTY may be targeted to host cells. FTY was additive with regulatory T cells for GVHD inhibition. FTY slightly impaired but did not abrogate a graft-versus-leukemia (GVL) effect against C1498, a myeloid leukemia. Our data further define the mechanisms of action and provide insight as to the potential clinical uses of FTY in allogeneic bone marrow transplant recipients.
Introduction
Despite advances in the field, graft-versus-host disease (GVHD) remains a significant cause of morbidity and mortality in patients undergoing bone marrow transplantation (BMT). In this study, we examine the effect of FTY720 (FTY), a potent immunomodulator, in a murine model of GVHD.
FTY, derived from a metabolite of the fungus Isaria sinclairii, is a high-affinity agonist for 4 of the 5 known sphingosine 1-phosphate receptors (S1PRs). S1P1 is expressed on all cell types, while S1P3 is found in endothelial cells, S1P4 is found primarily on lymphoid cells, and S1P5 is expressed on the white matter of the central nervous system.1-4 These receptors are critically involved in cell survival, cytoskeletal rearrangements, cell motility, and cell migration.1-4 FTY induces internalization of the receptor, rendering the cells unresponsive to serum lipid S1P that is produced by platelets.1,5,6 It is generally accepted that FTY exerts its immunomodulatory effects primarily by sequestering lymphocytes within secondary lymphoid organs, thereby denying them the ability to recirculate to peripheral sites of inflammation. FTY is also thought to act on endothelial cells by enhancing the adherens junction assembly, thereby strengthening the endothelial barrier.7 More recent data indicate that in addition to exhibiting profound effects on lymphocyte migration and endothelial barrier integrity, FTY also modulates dendritic cell (DC) trafficking and function.8-11
Accumulating data in animal studies indicate that FTY is a promising immunosuppressive agent for the treatment of various autoimmunities, promotion of engraftment in several models of solid organ transplantation (reviewed in Brinkmann,1 Brinkmann and Lynch,2 Chiba,3 Yopp et al,4 and Brinkmann et al12 ), and inhibition of GVHD.13-15 Although the complete mechanism of FTY is not completely understood, most studies implicate an important role for lymph node (LN) trapping as a mechanism of action. It is generally accepted that sequestration of effector T cells in secondary lymphoid organs is associated with a decrease in T-cell migration and infiltration to sites of inflammation, solid organ grafts, or GVHD target organs, thereby ameliorating autoimmunity, solid organ graft rejection, or GVHD.
Our studies indicate that FTY inhibits but does not prevent GVHD. In contrast to other studies, we did not find that FTY sequestered effector T cells to secondary lymphoid organs, thereby reducing their migration to GVHD target organs. Rather, imaging of donor GFP+ effector T cells revealed that FTY differentially modified effector T-cell migration to lymphoid organs but did not trap effectors in LNs, nor did FTY prevent early effector T-cell migration into GVHD parenchymal target tissues such as liver or lung. A 9-day course of FTY reduced the absolute number of splenic CD11c+ DCs by 50%, and also reduced the responder frequency of both donor CD4+ and CD8+ T cells in the spleen early after transplantation whether FTY was administered before or after transplantation. Moreover, FTY inhibited GVHD if administered only prior to transplantation, indicating that FTY-mediated inhibition of GVHD was at least partly independent of any direct effects on donor effector T cells. Although FTY inhibited GVHD in several different strain combinations, most mice died, and long-term survivors had clinical evidence of GVHD. Therefore, FTY was tested for its compatibility with ex vivo activated and expanded CD4+CD25+ regulatory T (Treg) cells, reported, by us and others, to be potent suppressors of GVHD.16-22 Data indicate that FTY was additive with Treg cells for GVHD inhibition. Lastly, FTY significantly impaired, but did not abrogate, a graft-versus-leukemia (GVL) effect against widely disseminated myeloid leukemia, C1498, given at the time of BMT.
Materials and methods
Mice
BALB/c (H2d) and C57BL/6 (H2b; termed B6) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) or the National Institutes of Health (Bethesda, MD). B6.129S7-Rag1tm1Mom/J (H2b; termed RAG2-deficient) mice were purchased from The Jackson Laboratory. B6 GFP transgenic (Tg) mice were obtained from the laboratory of Dr Jonathan Serody (Chapel Hill, NC) and bred at the University of Minnesota. Mice were housed in a specific pathogen–free facility in microisolator cages and were used at 8 to 12 weeks of age.
GVHD experiments
B6 recipients were lethally irradiated with 8.0 Gy total body irradiation (TBI) by x-ray on day −1 and infused with 10 × 106 T-cell–depleted (TCD) BALB/c bone marrow (BM) cells and whole splenocytes at the indicated number on day 0. FTY at 3.0 mg/kg, a dosage reported to be optimal, or distilled water, was administered daily by oral gavage at the indicated schedule.15 Mice were monitored daily for survival and weighed twice weekly for the first month, then once weekly thereafter as well as examined for the clinical appearance of GVHD. In some experiments, mice were electively killed 7 days after transplantation, and hematoxylin and eosin–stained slides of liver, lung, colon, and spleen were histologically assessed using a semiquantitative GVHD scoring system (0 to 4.0 grades of 0.5 increments) as published.23 Coded sections were graded by 1 investigator (A.P.-M.) without knowledge of the treatment.
GFP in vivo imaging
BALB/c recipients were lethally irradiated with 6.0 Gy TBI on day −1 and infused on day 0 with 10 × 106 B6 BM cells and 2 × 106 CD25-depleted purified T cells obtained from a pool of axillary, inguinal, and mesenteric LNs from B6 GFP mice. T cells were purified by negative selection using Miltenyi LD columns (Miltenyi Biotec, Auburn, CA) and determined to be 98% of the desired phenotype. FTY (3.0 mg/kg) or water was administered daily by oral gavage from day −1 to day 13. To obtain optimal images, mice were killed and dissected for imaging, but no tissue processing was required. A total of 3 mice per group was examined at days 4, 7, and 14. Results were reproduced in a second experiment. Images were taken with a Retiga Exi color camera and QCapture software (Qimaging, Burnaby, BC) mounted onto a Leica MZFLIII stereomicroscope using a GFP2 or a GFP/dsRED-bandpass filter and a 1.0× transfer lens (Leica Microsystems, Bannockburn, IL). Zoom factors from 3.5× to 10× were used for imaging (3.5× for ileum and Peyer patch; 7.0× for femoral bone marrow cavity, liver, spleen, and kidney; and 10.0× for lung). Exposure times were optimized for each organ, and identical times and settings were used for all mice imaged on any given day. Mice within a group yielded very similar results at each time point, so a representative image is illustrated.
Treg cell purification and culture
Ex vivo activated and expanded Treg cells were generated as previously described.21 Briefly, a pool of axillary, inguinal, and mesenteric LNs was harvested from BALB/c donor mice. CD4+ cells were negatively selected by incubation of LN cells with anti-CD19PE, anti-γδPE, and anti-CD8PE (BD Biosciences, San Diego, CA) followed by incubation with anti-PE MicroBeads (Miltenyi Biotec) and passage over a Miltenyi LD column. CD25+ cells were positively selected by incubation with anti-CD25PE followed by incubation with anti-PE MicroBeads and passage over a Miltenyi LS column. Cells were determined to be more than 96% CD4+CD25+. Enriched CD25+ cells were activated with anti-CD3 and anti-CD28 mAb–coated magnetic microspheres (6 beads/1 cell; kindly provided by Drs Bruce Levine and Carl June, University of Pennsylvania, Philadelphia) and cultured for 10 to 14 days with recombinant human interleukin-2 (1000 U/mL; Amgen, Thousand Oaks, CA). Culture medium was Dulbecco complete as previously described.20 Immediately prior to in vivo infusion, Treg cells were washed several times, and magnetic beads were removed with a strong magnet. LSel+ Treg cells, previously determined to be more potent suppressors of GVHD than LSel− Treg cells, were positively selected by incubation with anti-LSel PE followed by incubation with anti-PE MicroBeads and passage over a Miltenyi LS column. Cells were determined to more than 97% of the desired phenotype. The indicated number of cells was infused by separate intravenous injection on day 0 at the time of transplantation. Because Treg cells are very potent suppressors of GVHD under optimal experimental conditions, a single suboptimal dose of Treg cells (3.5 × 106 Treg cells; 0.7 Treg cells to 1 T cell) was used to increase the likelihood of uncovering an additive effect with FTY.
CFSE experiments
BALB/c splenocytes were stained with 2.5 μM CFSE (Molecular Probes, Eugene, OR) at a concentration of 10 × 106/mL in PBS at room temperature for 10 minutes with gentle shaking. Labeling was stopped by the addition of the same volume of FCS followed by washing 2 times with cold PBS. CFSE-stained BALB/c splenocytes (50 × 106) and BM (10 × 106) were infused into lethally irradiated B6 mice. Spleens were harvested 3 or 5 days after transplantation. CFSE divisions were evaluated on gated donor (H2d) CD4+ and CD8+ cells using a FACScalibur (Becton Dickinson, Mountain View, CA) and CellQuest software (Becton Dickinson). A minimum of 5 × 104 to 1 × 105 events was acquired for each evaluation. CFSE-stained splenocytes placed in culture until the day of spleen harvest served as a no-division control. Calculations for responder frequency and proliferative capacity were done as described by Wells et al.24
GVL
B6 recipients were irradiated (8.0 Gy) on day −1 and infused on day 0 with BALB/c BM (10 × 106 cells), splenocytes (15 × 106 cells), and C1498 (3 × 104 cells), a well-characterized myeloid tumor nucleoporated (Amaxa, Gaithersburg, MD) with Sleeping Beauty transposon construct to permit the expression of firefly luciferase (C1498ff). Sleeping Beauty transposon pT2/CAGGS-Luciferase (pT2; kindly provided by Dr Scott McIvor, University of Minnesota) was constructed using standard molecular cloning techniques. Bioluminescent firefly luciferase gene was transcriptionally regulated by a strong composite promoter that is a hybrid of the cytomegalovirus (CMV) intermediate-early enhancer and the chicken β-actin promoter followed by a chicken β-actin/β-globin intron. Transposon was flanked by inverted/direct repeats and the polyadenylation signal from simian virus 40. Plasmid p/CMV-HSB2 (kindly provided by Drs Mark Kay and Steven Yant, Stanford University, Stanford, CA) is a hyperactive transposase mutant expressed from the CMV promoter.25,26 C1498ff allowed for the tracking of tumor persistence in live animals using whole-body imaging (Xenogen, Alameda, CA). Separate cohorts of mice were used for survival and imaging studies to avoid effects on survival data by the repeated anesthesia necessary to image mice. Mice were monitored daily for survival and weighed twice weekly for the first month, then once weekly thereafter as well as examined clinically for presence of GVHD and at death for gross presence of tumor (eg, macroscopic nodules on ovaries, mesentery, liver, spleen, or lung occasionally accompanied by pleural and/or peritoneal fluid). The imaging cohort of mice was imaged 14, 21, and 28 days after transplantation. Mice were anesthetized with pentobarbital (Abbott Laboratories, Abbott Park, IL; 0.1 mL of a 1:10 dilution/10 g body weight), and the abdomen and chest were shaved. Luciferin (30 mg/mL; Xenogen) was injected into the mice at 150 mg/kg intraperitoneally. A grayscale reference image was taken of the position of the mice before luciferase activity was assessed. Bioluminescent signals were assessed at 5 minutes after luciferin injection at an integration time of 2 minutes with an in vivo imaging system that uses a cooled charge-coupled device camera (IVIS100; Xenogen). Pseudocolor images representing the bioluminescent signal intensity (blue is the least intense and red is the most intense) were superimposed over the grayscale reference image. Scales for the pseudocolor intensity plots are displayed with the images.
Statistics
Survival data were analyzed by life-table methods, and actuarial survival rates are shown. Group comparisons were made by log-rank test statistics. Group comparisons of CFSE data were analyzed by Student t test. A P value of .05 or less was considered significant in all tests.
Results
FTY inhibits but does not prevent GVHD
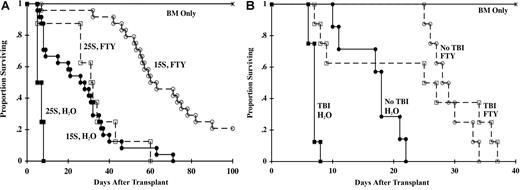
B6 mice were lethally irradiated and infused with BM and either 15 × 106 or 25 × 106 splenocytes from BALB/c donors. FTY (3.0 mg/kg) or water was administered once daily by oral gavage from day −1 to day 28. In recipients of 15 × 106 splenocytes, FTY extended the median survival time (MST) from 28 days to 63 days (Figure 1A; P < .001). In mice receiving 25 × 106 splenocytes, FTY extended the MST from 7 days to 32 days (P = .006). FTY also significantly inhibited, but did not prevent GVHD in 2 additional major histocompatibility complex (MHC)–disparate strain combinations, a MHC class II only–disparate GVHD model, and a T-cell receptor (TCR) Tg GVHD model (data not shown). We hypothesized that irradiation-induced perturbations in homing and chemokine receptors might be preventing FTY from being more effective in inhibiting GVHD. To test this hypothesis, the ability of FTY to inhibit GVHD was compared in nonirradiated versus lethally irradiated B6 RAG2-deficient recipients of BALB/c T cells (Figure 1B). FTY extended the MST of lethally irradiated recipients from 7.2 days to 27 days (P = .006) and of nonirradiated recipients from 18.6 days to 29 days (P = .003). Therefore, FTY was not more effective in mice that received no irradiation versus lethal irradiation (29 days vs 27 days; P = .34), indicating lethal irradiation did not prevent FTY from being a more efficacious therapy for GVHD inhibition.
FTY inhibits GVHD; irradiation does not limit efficacy. (A) Lethally irradiated B6 mice were infused with BALB/c BM. Cohorts received 15 × 106 (15S) or 25 × 106 (25S) BALB/c splenocytes. Mice were administered sterile water or FTY (3.0 mg/kg) by oral gavage daily from days −1 to 28. Survival is indicated (n = 3 experiments pooled for a total of 24 mice per group for 15S; n = 8 per group for 25S; P < .001 for 15S, P = .006 for 25S, FTY vs water). (B) B6 RAG2-deficient mice were lethally irradiated or not irradiated and given 3 × 106 BALB/c purified CD25-depleted T cells. Lethally irradiated mice received BALB/c BM. FTY or water was given as for panel A. Survival is indicated (n = 7-8 per group; P = .003 for no TBI, P = .006 for TBI, FTY vs water).
FTY inhibits GVHD; irradiation does not limit efficacy. (A) Lethally irradiated B6 mice were infused with BALB/c BM. Cohorts received 15 × 106 (15S) or 25 × 106 (25S) BALB/c splenocytes. Mice were administered sterile water or FTY (3.0 mg/kg) by oral gavage daily from days −1 to 28. Survival is indicated (n = 3 experiments pooled for a total of 24 mice per group for 15S; n = 8 per group for 25S; P < .001 for 15S, P = .006 for 25S, FTY vs water). (B) B6 RAG2-deficient mice were lethally irradiated or not irradiated and given 3 × 106 BALB/c purified CD25-depleted T cells. Lethally irradiated mice received BALB/c BM. FTY or water was given as for panel A. Survival is indicated (n = 7-8 per group; P = .003 for no TBI, P = .006 for TBI, FTY vs water).
FTY modulates GVHD effector T-cell migration but does not trap effector T cells in all secondary lymphoid organs
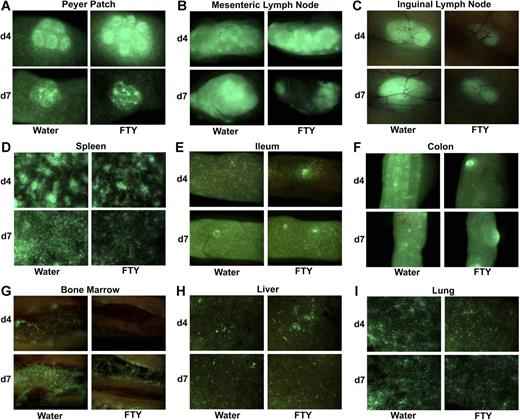
Although the mechanism of action of FTY is multifaceted and not thoroughly understood, it is generally accepted that FTY modulates immune responses primarily by preventing lymphocyte egress from lymphoid organs. Sequestration of GVHD effector T cells in lymphoid organs could theoretically reduce or delay effector T-cell migration into GVHD target parenchymal tissues, thereby ameliorating tissue destruction. To examine the effects of FTY on effector T-cell migration after BMT, lethally irradiated BALB/c recipients were infused with B6 wild-type BM and B6 GFP+ purified T cells and mice were imaged at various times after transplantation (Figure 2). Consistent with the reported effects of FTY on lymphocyte sequestration, day 4 imaging revealed greater donor effector accumulation in Peyer patches and mesenteric LNs in FTY-treated mice (Figure 2A,B). By day 7, there was a reduction in GFP+ T cells relative to day 4 consistent with initial trapping and increased T-cell apoptosis as reported by Hashimoto et al.13 However, FTY-treated mice had fewer donor T-cell effectors present in inguinal and axillary LNs and the spleen on both day 4 and day 7 and in the gut-associated lymphoid tissue in the ileum and colon on day 4 (Figure 2C-F; axillary LNs not shown) as well as in the femoral bone marrow cavity, a primary lymphoid and GVHD target organ (Figure 2G). Significantly and in contrast to data in lymphoid organs, FTY did not reduce effector numbers in either the liver or the lung, 2 major GVHD parenchymal target organs (Figure 2H,I). These data suggested FTY administration differentially modified effector T-cell migration to lymphoid organs but did not uniformly trap effectors in all secondary lymphoid organs, nor did FTY prevent effector T-cell migration and accumulation into GVHD parenchymal target organs.
FTY modulates effector T-cell migration to lymphoid organs but does not uniformly trap effectors in LNs or prevent effector migration to GVHD target parenchymal organs. (A-I) Lethally irradiated BALB/c mice received B6 wild-type BM and 2 × 106 B6 GFP+ purified CD25-depleted T cells. Mice were administered sterile water or FTY by oral gavage daily from day −1 to day of imaging. A total of 3 mice per group per time point was imaged. Data were reproduced in a second experiment in the same strain combination and in a third experiment in a different strain combination; findings were similar. A representative image is shown of the indicated organ on day 4 and day 7. See “Materials and methods” for imaging details.
FTY modulates effector T-cell migration to lymphoid organs but does not uniformly trap effectors in LNs or prevent effector migration to GVHD target parenchymal organs. (A-I) Lethally irradiated BALB/c mice received B6 wild-type BM and 2 × 106 B6 GFP+ purified CD25-depleted T cells. Mice were administered sterile water or FTY by oral gavage daily from day −1 to day of imaging. A total of 3 mice per group per time point was imaged. Data were reproduced in a second experiment in the same strain combination and in a third experiment in a different strain combination; findings were similar. A representative image is shown of the indicated organ on day 4 and day 7. See “Materials and methods” for imaging details.
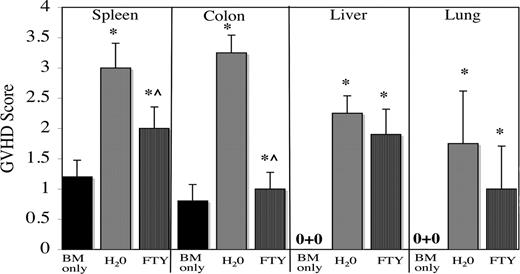
Day 7 GVHD tissue scores were consistent with the imaging data. Compared with GVHD controls, FTY-treated mice had significantly lower GVHD scores in the colon and spleen, but similarly high GVHD scores in the liver and lung (Figure 3). Both groups of mice had significantly higher GVHD scores than mice receiving only TCD BM, indicating that FTY reduced but did not prevent GVHD, also consistent with the survival data.
Consistent with imaging data, FTY decreases GVHD scores in spleen and colon, but not in liver or lung. Lethally irradiated B6 mice were given BALB/c BM and splenocytes (25 × 106) and administered sterile water or FTY by oral gavage from day −1 to time of tissue harvest. Control mice received only BM. On day 7 after transplantation, 5 per group were electively killed, and indicated GVHD target tissues were harvested, sectioned, and stained with hematoxylin and eosin and scored for GVHD histopathology. Shown is average score (± 1 SD). *P ≤ .01 versus BM-only control; ^P ≤ .006, water- versus FTY-treated mice.
Consistent with imaging data, FTY decreases GVHD scores in spleen and colon, but not in liver or lung. Lethally irradiated B6 mice were given BALB/c BM and splenocytes (25 × 106) and administered sterile water or FTY by oral gavage from day −1 to time of tissue harvest. Control mice received only BM. On day 7 after transplantation, 5 per group were electively killed, and indicated GVHD target tissues were harvested, sectioned, and stained with hematoxylin and eosin and scored for GVHD histopathology. Shown is average score (± 1 SD). *P ≤ .01 versus BM-only control; ^P ≤ .006, water- versus FTY-treated mice.
FTY decreases splenic CD11c+ DC numbers and administration of FTY only prior to transplantation delays GVHD mortality
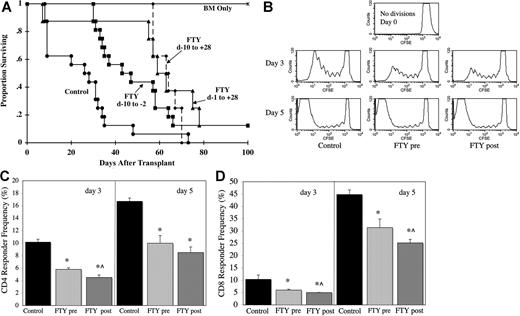
As well as influencing lymphocyte migration, FTY has also been reported to modify the migration and function of DCs. Direct recognition of allogeneic host antigen-presenting cells (APCs) by donor effector T cells is critical for the generation of fulminant GVHD. CD11c+ DCs are the most potent T-cell–stimulatory APCs. Significantly, a 9-day course of FTY administered to normal mice resulted in a 50% reduction in absolute splenic CD11c+ DC numbers (P = .049; n = 3 per group). In contrast to the spleen, FTY did not have a significant impact on absolute CD11c+ DC numbers in mesenteric LNs (P = .32; n = 3 per group). Moreover, FTY given only prior to transplantation (day −10 to −2) extended the MST from 28 days (controls given water) to 47 days (Figure 4A; P = .004). The MST of mice receiving the usual schedule of FTY from day −1 to day 28 was 63 days, which was not statistically better than that of mice receiving FTY only prior to transplantation (P = .157). There was no significant added benefit in administering FTY from day −10 to day 28 (Figure 4; P = .126 vs FTY d −10 to day −2; P = .244 vs FTY d −1 to day 28). These data indicate that FTY effects on the host are sufficient for GVHD inhibition, likely through a reduction in splenic CD11c+ DC numbers.
FTY administered only prior to transplantation inhibits GVHD. (A) Lethally irradiated B6 mice were given BALB/c BM and splenocytes (15 × 106) and administered sterile water or FTY by oral gavage at the indicated schedule. Survival is indicated (Control and FTY: day −10 to −2, pool of 2 experiments, n = 16 per group; FTY day −10 to day 28 and FTY day −1 to day 28 in 1 experiment, n = 8 per group; P < .004, control vs any FTY group; P > .13 for any FTY schedule comparison). (B-D) Lethally irradiated B6 mice were given BALB/c BM and CFSE-stained splenocytes (50 × 106). FTY was administered daily from day −10 to day −3 (FTY pre) or day −1 to day 4 (FTY post) by oral gavage. Controls received sterile water. Spleens were harvested on days 3 and 5, and CFSE divisions were evaluated on gated donor (H2d) CD4+ and CD8+ cells. (B) A representative donor CD4+ CFSE histogram from each group for both days is shown. CFSE-stained splenocytes placed in culture served as control to illustrate no divisions. (C,D) Shown is average CD4 (C) and CD8 (D) responder frequency plus or minus 1 SD calculated from CFSE data according to reference in “Materials and methods.” (n = 3 per group per time point; *P ≤ .014 FTY pre or post vs Control; ^P < .05 FTY pre vs FTY post). Day-3 data were reproduced in a second experiment.
FTY administered only prior to transplantation inhibits GVHD. (A) Lethally irradiated B6 mice were given BALB/c BM and splenocytes (15 × 106) and administered sterile water or FTY by oral gavage at the indicated schedule. Survival is indicated (Control and FTY: day −10 to −2, pool of 2 experiments, n = 16 per group; FTY day −10 to day 28 and FTY day −1 to day 28 in 1 experiment, n = 8 per group; P < .004, control vs any FTY group; P > .13 for any FTY schedule comparison). (B-D) Lethally irradiated B6 mice were given BALB/c BM and CFSE-stained splenocytes (50 × 106). FTY was administered daily from day −10 to day −3 (FTY pre) or day −1 to day 4 (FTY post) by oral gavage. Controls received sterile water. Spleens were harvested on days 3 and 5, and CFSE divisions were evaluated on gated donor (H2d) CD4+ and CD8+ cells. (B) A representative donor CD4+ CFSE histogram from each group for both days is shown. CFSE-stained splenocytes placed in culture served as control to illustrate no divisions. (C,D) Shown is average CD4 (C) and CD8 (D) responder frequency plus or minus 1 SD calculated from CFSE data according to reference in “Materials and methods.” (n = 3 per group per time point; *P ≤ .014 FTY pre or post vs Control; ^P < .05 FTY pre vs FTY post). Day-3 data were reproduced in a second experiment.
FTY reduces the responder frequency of effector T cells
To determine the effect of FTY on early in vivo proliferation of donor T cells, lethally irradiated B6 mice were given BALB/c BM and CFSE-labeled spleen cells. FTY was given either prior to transplantation (day −10 to day −3) or from day −1 to day 4, and donor T-cell proliferation was evaluated in the spleens of mice on days 3 and 5 after transplantation. Division rates can be assessed as CFSE is diluted out as cells divide, and these data can be used to calculate responder frequencies and proliferative capacity (number of daughter cells generated from each precursor cell; Figure 4B-D).24 FTY given only before transplantation reduced the CD4 responder frequency by 43% on day 3 and 40% on day 5 (Figure 4C). FTY, given day −1 to day 4, reduced the CD4 responder frequency by 56% on day 3 and 47% on day 5. The CD8 responder frequency was inhibited by 42% on day 3 and 30% on day 5 by FTY given prior to transplantation (Figure 4D). FTY given from day −1 to day 4 inhibited the CD8 responder frequency by 52% on day 3 and 44% on day 5. FTY did not significantly reduce the proliferative capacity of responding precursor T cells, indicating that FTY did not interfere with the number of divisions a cell underwent once responding cells entered cell cycle. The percentage of apoptotic cells in the spleen was small in all mice (data not shown).
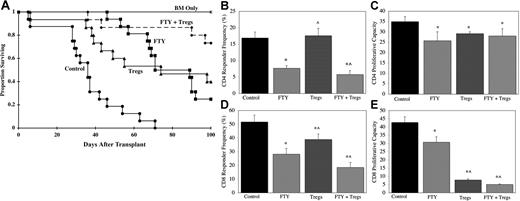
FTY is additive with Treg cells for GVHD inhibition
Although FTY significantly inhibited GVHD in all strain combinations tested, most mice eventually died, and long-term survivors had clinical and histologic evidence of GVHD. As our and others data indicate that Treg cells are potent suppressors of GVHD,16-22 FTY was tested for compatibility with Treg cells. To increase the likelihood of uncovering an additive effect, a single suboptimal dose (approximately 0.7 Treg cells to 1 effector T cell) of ex vivo activated and expanded Treg cells was used in these experiments; therefore, comparisons between FTY and Treg cells as anti-GVHD therapies are not meaningful in these studies. Treg cells and FTY as single therapies extended the MST from 36.5 days to 86 days and 89 days, respectively (Figure 5A; P < .001 for Treg cells or FTY vs control). The combination of Treg cells and FTY resulted in 75% long-term survival that was significantly better than either agent alone (P < .03 vs Treg cells or FTY as single agents). Moreover, the day 100 average body weight of long-term survivors receiving both FTY and Treg cells was not significantly different than that of control mice receiving only TCD BM (19.1 g vs 19.6 g; P = .29). In contrast, long-term survivors receiving FTY or Treg cells had average day 100 weights of 16.6 g or 17.4 g, respectively, that were significantly lower than that of mice receiving only TCD BM, indicative of persistent GVHD (P ≤ .01 vs BM only). In addition, long-term survivors receiving both FTY and Treg cells had no obvious clinical evidence of GVHD, and the day 100 GVHD scores were not significantly different from BM-only controls in the colon, lung, and skin, although they were higher in the liver and spleen (data not shown). The additive benefit of FTY and suboptimal numbers of Treg cells was also demonstrated in a second strain combination (data not shown).
FTY and Treg cells are additive for GVHD inhibition. (A) Lethally irradiated B6 mice were given BALB/c BM and splenocytes (15 × 106). Cohorts received 1 infusion of BALB/c L Sel+ ex vivo activated and expanded Treg cells (3.5 × 106) on day 0. FTY or water was administered daily from day −1 to day 28. Shown is survival (pool of 2 experiments, n = 16 per group; P < .001, control vs any treatment group; P < .03, Treg cells or FTY vs FTY + Treg cells). (B-E) Lethally irradiated B6 mice were given BALB/c BM and CFSE-stained splenocytes (50 × 106). Cohorts received 1 infusion of BALB/c L Sel+ ex vivo activated and expanded Treg cells (10 × 106) on day 0. Sterile water or FTY was administered daily from day −1 to day 4 by oral gavage. Spleens were harvested on day 5, and CFSE divisions were evaluated on gated donor (H2d) CD4+ and CD8+ cells. Shown is average CD4+ and CD8+ responder frequency and proliferative capacity as indicated plus or minus 1 SD calculated from CFSE data according to “Materials and methods” (n = 4 per group per time point; *P ≤ .018 vs control; ^P ≤ .037, FTY vs Treg cells or FTY + Treg cells).
FTY and Treg cells are additive for GVHD inhibition. (A) Lethally irradiated B6 mice were given BALB/c BM and splenocytes (15 × 106). Cohorts received 1 infusion of BALB/c L Sel+ ex vivo activated and expanded Treg cells (3.5 × 106) on day 0. FTY or water was administered daily from day −1 to day 28. Shown is survival (pool of 2 experiments, n = 16 per group; P < .001, control vs any treatment group; P < .03, Treg cells or FTY vs FTY + Treg cells). (B-E) Lethally irradiated B6 mice were given BALB/c BM and CFSE-stained splenocytes (50 × 106). Cohorts received 1 infusion of BALB/c L Sel+ ex vivo activated and expanded Treg cells (10 × 106) on day 0. Sterile water or FTY was administered daily from day −1 to day 4 by oral gavage. Spleens were harvested on day 5, and CFSE divisions were evaluated on gated donor (H2d) CD4+ and CD8+ cells. Shown is average CD4+ and CD8+ responder frequency and proliferative capacity as indicated plus or minus 1 SD calculated from CFSE data according to “Materials and methods” (n = 4 per group per time point; *P ≤ .018 vs control; ^P ≤ .037, FTY vs Treg cells or FTY + Treg cells).
The combination of FTY and Treg cells was assessed for its effect on responder frequency and proliferation capacity (Figure 5B-E). Day 5 was chosen for evaluation, as data indicated that CD8+ T cells took longer to respond than CD4+ T cells (Figure 4C,D). The combination of FTY and Treg cells inhibited the CD4 responder frequency by 66% compared with 54% inhibition by FTY alone (Figure 5B; P = .037). FTY and Treg cells alone and in combination modestly reduced CD4 proliferative capacity (Figure 5C). The CD8 responder frequency was inhibited 64% by FTY plus Treg cells compared with 45% inhibition by FTY alone and by 25% by Treg cells alone (Figure 5D; P = .013 and P < .001, respectively). In contrast to CD4+ cells, the proliferative capacity of CD8+ cells was profoundly reduced by Treg cells alone and in combination with FTY (Figure 5E), with only modest effects by FTY alone. These data indicate that FTY and Treg cells are additive for GVHD inhibition.
FTY impairs but does not abrogate a GVL effect
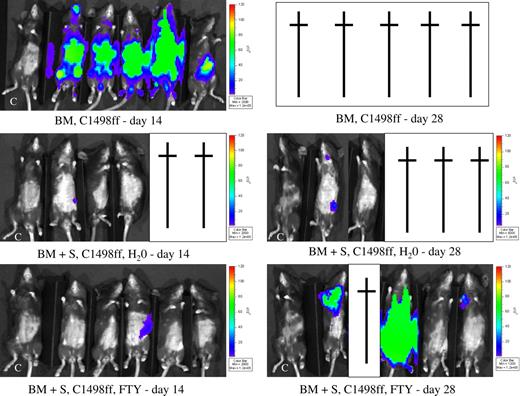
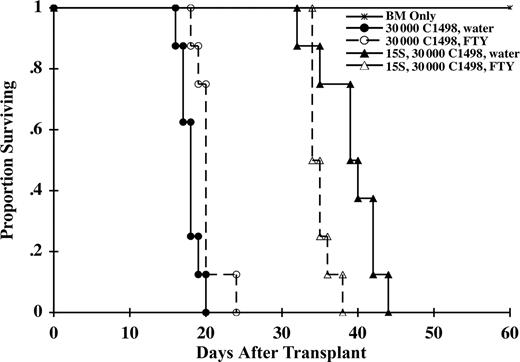
To determine the effect of FTY on GVL early after transplantation, B6 mice were lethally irradiated on day −1 and on day 0, given BALB/c BM and a myeloid tumor, C1498 transduced with firefly luciferase (C1498ff), to allow for the imaging of tumor by bioluminescent imaging. Cohorts of mice received BALB/c splenocytes as a supplemental source of T cells to mediate GVL (and GVHD) and were treated with either water or FTY. Separate cohorts of mice were used for survival versus imaging studies to minimize effects on survival by repeated anesthesia necessary to image mice. By day 14, all mice receiving BM and C1498ff (3 × 104 cells) but no splenocytes had evidence of widely disseminated tumor (Figure 6). In contrast, mice receiving supplemental splenocytes had a profoundly reduced incidence of tumor. Only 1 of 3 water-treated mice (2 had already died of GVHD) and 1 of 5 FTY-treated mice had small tumor foci. By day 28, all mice receiving BM and C1498ff had died of tumor. Of the surviving mice receiving splenocytes, 1 of 2 water-treated mice and 3 of 4 FTY-treated mice had evidence of tumor, with widely metastasized tumor present in 1 FTY-treated mouse. Survival data were consistent with these imaging data (Figure 7). All mice receiving C1498ff (3 × 104 cells) and BM died of tumor by day 24 regardless of whether they received water or FTY. These data indicated that FTY had minimal direct effect on tumor growth. Although splenocytes (15 × 106 cells) significantly extended the MST, most FTY-treated mice died of tumor relapse before water-treated controls that died of GVHD (MST of 35 days vs 40 days; P = .01). These data, confirmed by necropsy, indicated that although FTY reduced GVHD, it also impaired, but did not abrogate, a GVL effect mediated by the T-cell–containing splenocyte inoculum.
FTY does not abrogate a GVL effect. Lethally irradiated B6 mice were given BALB/c BM and C1498ff (3 × 104 cells) on day 0. Cohorts received splenocytes (15 × 106) on day 0 and were administered water or FTY daily by oral gavage from day −1 to day 28. Mice were imaged on days 14 and 28 (n = 5 per group) as indicated in “Materials and methods” after injection with luciferin. Identical exposure times were taken for all pictures. Green indicates a greater tumor load than blue. C indicates a control mouse that did not receive C1498ff. A cross indicates that a mouse died. All mice receiving BM and tumor but no spleen died of tumor by day 28. Although spleen infusions protected most mice from widely metastic tumor growth, several mice died of GVHD. Overall tumor incidence for water-treated mice was 1 of 5 versus 3 of 5 for FTY-treated mice.
FTY does not abrogate a GVL effect. Lethally irradiated B6 mice were given BALB/c BM and C1498ff (3 × 104 cells) on day 0. Cohorts received splenocytes (15 × 106) on day 0 and were administered water or FTY daily by oral gavage from day −1 to day 28. Mice were imaged on days 14 and 28 (n = 5 per group) as indicated in “Materials and methods” after injection with luciferin. Identical exposure times were taken for all pictures. Green indicates a greater tumor load than blue. C indicates a control mouse that did not receive C1498ff. A cross indicates that a mouse died. All mice receiving BM and tumor but no spleen died of tumor by day 28. Although spleen infusions protected most mice from widely metastic tumor growth, several mice died of GVHD. Overall tumor incidence for water-treated mice was 1 of 5 versus 3 of 5 for FTY-treated mice.
FTY impairs but does not abrogate GVL. A separate cohort of mice as described in Figure 6 were evaluated for survival (n = 8 per group). Both FTY-treated and control mice receiving splenocytes survived longer than mice not receiving splenocytes, indicative of a GVL effect (P ≤ .001). However, FTY-treated mice died before controls due to a greater tumor relapse, indicating that FTY impaired GVL effect under these experimental conditions (P = .01).
FTY impairs but does not abrogate GVL. A separate cohort of mice as described in Figure 6 were evaluated for survival (n = 8 per group). Both FTY-treated and control mice receiving splenocytes survived longer than mice not receiving splenocytes, indicative of a GVL effect (P ≤ .001). However, FTY-treated mice died before controls due to a greater tumor relapse, indicating that FTY impaired GVL effect under these experimental conditions (P = .01).
Discussion
FTY significantly inhibited but did not prevent GVHD. The mechanism of action of FTY has been largely, albeit not exclusively, attributed to LN trapping of lymphocytes. We favor the interpretation that our data are most consistent with FTY-mediated GVHD inhibition occurring through multiple mechanisms, including those that directly target the host.
Hashimoto et al reported that GVHD amelioration occurred through initial trapping of effectors within mesenteric LNs and increased apoptosis of donor T cells due to enhanced T cell/APC interactions.13 Our imaging data support their conclusions by demonstrating a transient increase followed by a rapid reduction of effectors in mesenteric LNs, consistent with initial trapping and subsequent apoptosis. Although speculative, imaging data suggest a similar mechanism may be occurring in Peyer patches. At the same time, the imaging data also indicated a reduced number of effectors in other lymphoid organs (eg, inguinal LNs) not consistent with uniform lymphoid trapping. Although it is possible that donor T cells were reduced in other lymphoid organs simply because donor T cells migrated preferentially to mesenteric LNs and Peyer patches in FTY-treated mice, FTY also significantly decreased the total number of splenic CD11c+ DCs, which would be predicted to reduce effector T-cell expansion in the spleen independent of any migratory modulation. Several studies confirm the critical role of the host APC in GVHD generation.27-31 Notably, the 50% reduction of splenic DCs was associated with a reduction in splenic CD4+ and CD8+ responder frequency of an equivalent magnitude that would be predicted to contribute to GVHD inhibition. We did not find evidence of increased apoptosis in the spleen, but given that we did not see evidence of enhanced T cell/APC interactions, that is perhaps not too surprising. And, although FTY decreased the splenic DC number, the DC number was not reduced in mesenteric LN, also consistent with imaging data. Collectively, the data implicate transient LN trapping in mesenteric LNs (and perhaps Peyer patches) and splenic DC reduction accompanied by decreased donor T-cell responder frequency as being important mechanisms of GVHD inhibition. Importantly, FTY did not prevent the early migration of effectors to GVHD target organs.
Since FTY has a short half-life in mice such that there would be no expected direct effects of FTY given from day −10 to day −2 on donor effector cells infused on day 0 (V.B., personal communication, May 2004), the efficacy of FTY administered solely prior to transplantation further indicates that direct effects on donor effector T cells are not essential for GVHD inhibition, and also implicates host DCs as important targets of FTY. Although the effects of FTY on lymphocyte migration have been more widely published, important recent studies indicate that FTY also modulates DC migration and function.8-11 Lan et al found that FTY impaired DC migration from the blood to secondary lymphoid organs, resulting in decreased numbers of DCs in LNs and spleen.10 This DC migratory modulation was associated with the down-regulation of the expression of important intercellular adhesion molecules and CCR7.10 Another study found that FTY blocked FITC-induced migration of skin DCs to the draining LN.8 These authors postulated that as mature DCs depend on proper S1P signaling for migration, and as DCs are required to traffic effectively to mediate their function, the immunomodulatory effect of FTY may be in part due to impaired T-cell priming by DCs in the secondary lymphoid organ.8 This was extended by Muller et al, who showed that in vitro treatment of human DCs with FTY induced changes in DC migration, cytokine production profile, and T-cell stimulation.11 Recent elegant studies by Idzko et al also uncovered an important role for FTY on inhibiting DC migration and function, resulting in suppression of experimental murine asthma.9 They found that local application of FTY via inhalation inhibited migration of lung DCs to the mediastinal LNs.9 Furthermore, FTY-treated DCs had a reduced capacity to form stable DC/T cell interactions and effective immunologic synapses.9 In addition to FTY effects on host DCs, we cannot rule out known effects by FTY on the maintenance of the host endothelial barrier integrity as a potential additional factor contributing to GVHD inhibition.7 It is also important to note that the schedule of administration of FTY is likely to influence the degree to which potential mechanisms are operative.
To our knowledge, this is the first report indicating that FTY is additive with donor Treg cells for GVHD inhibition. Although clearly of additive benefit, it is uncertain whether FTY and Treg cells are functioning by independent but compatible mechanisms to inhibit GVHD, or whether FTY might also be having a direct beneficial effect on the Tregs. It is important to note that FTY was administered on day −1 to day 28; therefore, FTY could have had direct effects on the infused donor Treg cells that could have affected their potency in suppressing GVHD. Consistent with this hypothesis, Sawicka et al found that in comparison to conventional T cells, Treg cells expressed lower levels of S1P1 and S1P4 receptors, resulting in a reduced chemotactic response to S1P, rendering them less susceptible to migration modulation by FTY.32 Consistent with that report, we found that FTY did not discernibly modify the migration of B6 GFP+ activated and expanded Treg cells when coinfused with B6 wild-type BM into a lethally irradiated BALB/c recipient (data not shown), in contrast to Figure 2, which demonstrates that FTY differentially modified the migration of GFP+ effector T cells. In addition to effects on migration, Sawicka et al also found that FTY increased the suppressive activity of Treg cells.32 Regardless of the exact mechanism by which FTY and Treg cells were additive in vivo, the combination is clearly beneficial for GVHD inhibition.
The separation of GVHD from GVL effects is a goal of BMT. Although imaging and survival studies indicate that FTY impaired GVL against a myeloid leukemia, it is important to note that GVL was not abrogated. In addition, the demonstration of GVL and degree of GVL impairment by any given therapeutic modality can be partly dependent on manipulations in experimental design. Although we chose to illustrate a more unfavorable case scenario to most realistically demonstrate that FTY has the potential to reduce GVL effects in the case of high tumor burden, at a 3-fold lower tumor dose than that used for Figures 6 and 7, FTY-treated mice had a longer MST than water-treated control mice (data not shown). These data indicate that increased tumor burden can tip the scale from apparently intact GVL to impaired GVL.
In summary, we provide new insights into the mechanisms by which FTY inhibits GVHD and report the additive effects of FTY and Treg cells on reducing GVHD lethality. FTY may prove to be a useful agent in allogeneic BM transplant recipients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health grants RO1 AI034495, RO1 CA072669, and R37 HL56067 (B.R.B.).
National Institutes of Health
Authorship
Contribution: P.A.T. designed and performed experiments, evaluated data, and wrote the manuscript; M.J.E. conducted murine BMT experiments; C.J.L. performed GVL bioluminescent imaging studies; J.T. made C1498 firefly transductant and advised on bioluminescent imaging studies; B.J.W. assisted with tumor studies; A.P.-M. performed GVHD scoring; J.S.S. provided advice and mice and edited the manuscript; V.B. provided advice and reagents; and B.R.B. designed research, advised on experimental design, and edited manuscript.
Conflict-of-interest disclosure: V.B. is an employee of Novartis and supplied FTY720 for these studies. All other authors declare no competing financial interests.
Correspondence: Bruce R. Blazar, Division of BMT, University of Minnesota Cancer Center and Department of Pediatrics, 420 Delaware St SE, MMC 109, Minneapolis, MN 55455; e-mail: blaza001@umn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal