Abstract
Coagulation proteases may act as cell signaling molecules via protease-activated receptor (PAR) cleavage, subsequently affecting cellular and inflammatory responses. Activation of PARs in the setting of systemic inflammation and disseminated intravascular coagulation (DIC) might thus exacerbate the inflammatory response contributing to tissue and organ damage. To investigate the role of PAR-4 in these processes, we subjected mice to a model of systemic inflammation and DIC (Shwartzman reaction) in the absence or presence of a cell-penetrating pepducin antagonist of PAR-4 (P4pal-10). P4pal-10 dose-dependently diminished the severity of endotoxemia and preserved liver, kidney, as well as lung function. Moreover, systemic inflammation and local (neutrophilic) inflammatory responses were attenuated. In vitro migration assays and P4pal-10 treatment in neutropenic mice suggest an essential role for neutrophils in PAR-4–mediated pathology. P4pal-10 treatment of thrombocytopenic mice excluded the involvement of platelets in this phenomenon. These results uncover an important role for PAR-4 in the Shwartzman reaction and suggest that inhibition of PAR-4 signaling in neutrophils could be protective in systemic inflammation and DIC.
Introduction
Systemic inflammation due to infection is associated with activation of blood coagulation. The (patho)physiological mechanisms by which inflammatory stimuli induce excessive blood clotting have been firmly established.1,2 However, the role and relative importance of activated coagulation factors as regulators of the inflammatory response are less well understood. The mechanism by which coagulation exacerbates inflammatory disease might be related to the signaling capacity of the different proteases of the coagulation cascade. Thrombin, but also factors VIIa and Xa, are known to act as cell signaling molecules via protease-activated receptor (PAR) cleavage, thereby connecting these (coagulation) proteases to specific cellular/inflammatory responses.
The PAR family currently consists of 4 members (PAR-1, PAR-2, PAR-3, and PAR-4) that have been suggested to play a role in systemic inflammatory disorders (such as sepsis),3 but also in a variety of other complex diseases such as asthma, arthritis, inflammatory bowel disease, and cancer.4-6
Of all PARs, particularly PAR-1 is hypothesized to be a mediator of systemic inflammation; yet, this notion is based mainly on in vitro findings,7-9 whereas an in vivo role for PAR-1 in inflammatory disease has not been confirmed.10,11
A potential role for PAR-4 in the setting of systemic inflammatory disease has been less frequently proposed. PAR-4 is generally recognized as a thrombin receptor, although it can also be activated by other proteases including trypsin,12 tissue kallikrein,4 and the neutrophilic granule protease cathepsin G.13 PAR-4 is expressed on various cell types including endothelial cells,8,14 neurons,15 alveolar macrophages,16 and neutrophils,14 but is most prominently present on platelets.17
Murine platelets express no PAR-1 and therefore depend on PAR-4 for thrombin-induced signaling. PAR-4–deficient mice appear to be normal but hemostasis is impaired due to defective thrombin signaling in platelets.18 Pathogenically, platelets contribute to atherothrombosis, and are recognized to be involved in inflammatory disorders such as sepsis.19 This notion is based on the fact that thrombocytopenia is a common feature of sepsis, that the severity of sepsis correlates with the decrease in platelet count,20 and that endotoxin infusion into mice induces rapid thrombocytopenia.11,21 Platelets accumulate in lung and liver microvasculature,22 where they may secrete their inflammatory content,23 thus contributing to tissue damage.24 Although the precise pathogenic contribution of platelet accumulation in sepsis is not known, platelet activation may exert a proinflammatory response in sepsis. Thrombocytopenia in response to endotoxin is mediated via a neutrophil-dependent sequestration within the pulmonary microvasculature,24 implicating the neutrophil as a key player in sepsis-induced platelet accumulation.
Moreover, PAR-4 may participate in the inflammatory reaction in a neutrophil-dependent manner; local injection of PAR-4–activating peptides in rodents induced neutrophil recruitment and edema formation, which was inhibited by PAR-4 inhibition.25 We consequently hypothesized that PAR-4 activation during systemic inflammation may contribute to platelet- as well as neutrophil-dependent pathologies. In this study, we therefore investigate the role of PAR-4 in a mouse model of systemic inflammation and disseminated intravascular coagulation (DIC), the so-called Shwartzman reaction.
Materials and methods
Animals
Ten-week-old, female C57Bl/6 mice (approximately 25 g; Harlan Sprague-Dawley, Horst, The Netherlands) were maintained at the animal care facility of the Academic Medical Center according to institutional guidelines. Animal procedures were carried out in compliance with Institutional Standards for Humane Care and Use of Laboratory Animals. The Animal Care and Use Committee of the Academic Medical Center approved all experiments.
Pepducins
The palmitoylated peptides pal-RCLSSSAVANRS (PAR1 antagonist, P1pal12; GenScript, Scotch Plains, NJ) and pal-SGRRYGHALR (PAR4 antagonist, P4pal10; Bachem, Weil am Rhein, Germany) were prepared by solid-phase peptide synthesis using the in situ neutralization/HBTU (o-benzotriazole-N,N,N′,N′-tetramethyl-uranium-hexafluoro-phosphate) activation procedure for tBoc chemistry, as described previously.26
Experimental design
The Shwartzman reaction was elicited by 2 consecutive injections of Serratia marcescens LPS (Sigma-Aldrich, St Louis, MO). At t = 0 hours, 5 μg LPS (in 40 μL saline) was injected intradermally in the foot and at t = 24 hours an LPS challenge (375 μg in 100 μL saline) was administered intravenously.27 The mice were pretreated, or not (vehicle control), with P4pal-10 (0.2 or 0.5 mg/kg, intraperitoneally) or P1pal12 (0.5 mg/kg, intraperitoneally) for 30 minutes (t = 23.5 hours) prior to the LPS challenge. Each of the experimental groups included 8 mice, except for baseline values (n = 4). For the survival experiment, 17 mice were pretreated with saline and 17 mice were pretreated with 0.5 mg/kg P4pal-10. Survival of these mice was scored every hour for a maximum of 30 hours.
For neutrophil and platelet depletion experiments, antimouse Ly-6G clone RB6–8C5 (eBioscience, San Diego, CA) and antimouse GP1bα (CD42b; Emfret Analytics, Würzburg, Germany) or IgG control antibodies were used. Ly-6G antibodies (125 μg in 200 μL saline) were injected intraperitoneally 18 hours prior to the LPS challenge (t = 6 hours). This strategy was previously shown to deplete circulating neutrophils from mice by more than 95%.28 GP1bα antibodies were intravenously injected (2 μg/g) 60 minutes prior to the LPS challenge (t = 23 hours) and were described to reduce platelet counts by more than 95%.29,30
Six hours after the LPS challenge, mice were killed (t = 32 hours) by intraperitoneal injection of 0.3 mL Hypnorm (Janssen Pharmaceutical, Beerse, Belgium) and midazolam (Roche, Almere, The Netherlands). Blood was sampled by heart puncture and collected in heparin-coated tubes. Broncho alveolar lavage (BAL) was performed by instilling 3 0.4-mL aliquots of saline by a 22-gauge Abbocath-T catheter (Abbott Laboratories, North Chicago, IL) into the trachea via midline incision. Lung, liver, and kidneys were collected for further analysis.
Enzyme assays
Creatinine, blood urea nitrogen (BUN), lactate dehydrogenase (LDH), and transaminase (ASAT and ALAT) levels in plasma were determined with commercially available kits (Sigma-Aldrich) using a Hitachi analyzer (Boehringer Mannheim, Mannheim, Germany) according to manufacturers' instructions
Total protein concentration
Total protein levels in BAL fluid were determined using a bicinchoninic acid (BCA) protein assay (Pierce, Omnilabo International, Breda, The Netherlands) according to manufacturer's instructions with BSA as standard.
Myeloperoxidase activity assay
Myeloperoxidase (MPO) activity was measured as a marker of the presence of granulocytes within lung tissue as described previously.31 Briefly, lungs were homogenized in potassium phosphate buffer, pH 7.4. After centrifugation, cells were lysed in potassium phosphate buffer, pH 6.0, containing 0.5% hexadecyltrimethylammonium bromide (HETAB) and 10 mM EDTA. MPO activity was determined by measuring the H2O2-dependent oxidation of 3,3′5,5 tetramethylbenzidine (TMB). The reaction was stopped with glacial acetic acid followed by reading the absorbance at 655 nm. MPO activity was expressed as units of MPO activity per gram lung tissue per minute ([OD655 × dilution factor minute−1] tissue weight−1). All reagents were purchased from Sigma-Aldrich.
Wet-dry weight ratio
Lungs were weighed and subsequently dried overnight (o/n) in a 60°C oven. The ratio of wet weight to dry weight represents tissue edema.32
Cytokine/chemokine assays
IL-6, MCP-1, IFN-γ, and TNF-α were measured using the BD Cytometric Bead Array (CBA) Mouse Inflammation Kit (Becton Dickinson, Lincoln Park, NJ) following the manufacturer's instructions. Detection limits were 10 pg/mL.
Granulocyte staining
Fresh liver, kidney, and lung sections were fixed in 10% buffered formalin and embedded in paraffin. Slides (4-μm thick sections) were deparaffinized and rehydrated. Endogenous peroxidase activity was quenched with 0.03% H2O2 in methanol. Sections were then digested with 0.25% pepsin (Sigma) in 0.01 M HCl. After rinsing, the sections were incubated in 10% goat serum in PBS (Dako, Glostrup, Denmark) and exposed to FITC-labeled antimouse Ly6G monoclonal antibody (1:1000 in PBS; Pharmingen, San Diego, CA). Slides were incubated with a rabbit anti-FITC antibody (Dako) (1:1000 in PBS + 5% mouse serum) followed by incubation with Power-Vision poly HRP-antirabbit IgG (Dako), rinsed, and developed using DAB (Sigma-Aldrich). The sections were counterstained and mounted in pertex and analyzed. Granulocytes were scored in 10 microscopic fields at a magnification of 40 ×.
Neutrophil migration assay
Neutrophil migration was assayed as described previously.33 Isolated human neutrophils were washed and labeled for 1 hour with 10 μM Cell Tracker Green in serum-free DMEM medium. The dye was fixed by 1-hour incubation in medium with 10% serum, and subsequently cells were washed in PBS. Next, cells (105) were preincubated with P4pal-10 (5 μM) for 30 minutes, and transferred to 8-μM pore size HTS FluoroBlok Cell Culture Inserts (BD Bioscience, San Jose, CA) that were inserted in fitting 24-well plates containing DMEM supplemented with different chemoattractants. Subsequently, fluorescence values representing the number of cells on the bottom side of the insert were read during 30 cycles (each cycle comprising 4 readings spanning 1 minute) at 37°C on a Series 4000 CytoFluor Multi-Well Plate Reader (Perseptive Biosystems, Framingham, MA). The raw fluorescence data were corrected for background fluorescence and no-attractant controls were subtracted from each condition to correct for any effects not due to active migration to the chosen attractant. Data shown are the mean fluorescence of all measured cycles from baseline to plateau migration. These are expressed as values relative to non–P4pal-10–treated conditions, which were arbitrarily set to 100% (mean ± SEM of at least 2 independent experiments).
Statistical analysis
Statistical analyses were conducted using GraphPad Prism version 3.00 (GraphPad Software, San Diego, CA). Data are expressed as mean (± SEM). Comparison between 2 groups was analyzed using (one-tailed) Mann-Whitney U tests.
Results
Effect of PAR-4 inhibition using P4pal-10 on organ damage
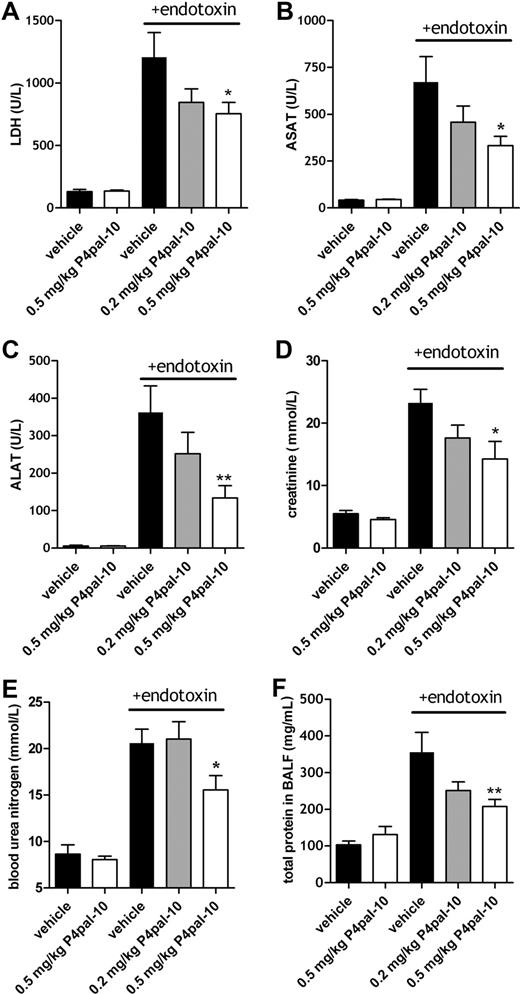
Severe systemic inflammation complicated by coagulation is characterized by (multi)organ failure. Figure 1 shows that various (systemic) markers of organ damage were increased 6 hours after the induction of the Shwartzman reaction (in the figures referred to as + endotoxin) (Figure 1A-F). To determine the effect of PAR-4 inhibition on organ failure, we analyzed the effect of P4pal-10 (0.2 or 0.5 mg/kg) on markers of tissue injury in plasma of mice subjected to the Shwartzman reaction. These doses of P4pal-10 were chosen because these were previously shown to be able to prevent thrombin-mediated aggregation of mouse platelets in vivo.34 No significant differences in baseline levels were observed for all measured markers among P4pal-10–treated and control animals.
Systemic markers of organ damage after P4pal-10 treatment in the Shwartzman reaction. General indication of tissue injury (A; LDH) and organ damage (B-E) in the Shwartzman reaction (t = 6 hours after endotoxin challenge) and the effect of P4pal-10–dependent PAR-4 inhibition. ASAT (B) and ALAT (C) represent liver injury. Creatinine (D) and BUN (E) are markers of renal failure. Total BALF protein (F) is an indicator of permeability of the pulmonary microvasculature. Values are depicted as mean (± SEM). *P < .05, **P < .01.
Systemic markers of organ damage after P4pal-10 treatment in the Shwartzman reaction. General indication of tissue injury (A; LDH) and organ damage (B-E) in the Shwartzman reaction (t = 6 hours after endotoxin challenge) and the effect of P4pal-10–dependent PAR-4 inhibition. ASAT (B) and ALAT (C) represent liver injury. Creatinine (D) and BUN (E) are markers of renal failure. Total BALF protein (F) is an indicator of permeability of the pulmonary microvasculature. Values are depicted as mean (± SEM). *P < .05, **P < .01.
Lactate dehydrogenase (LDH) is a surrogate marker for the severity of sepsislike syndromes,35 and Figure 1A demonstrates that the cumulative release of LDH in response to endotoxin-induced injury was significantly reduced in P4pal-10–treated mice. Moreover, hepatic injury (reflected by leakage of transaminases into plasma) was dose-dependently reduced by P4pal-10 treatment (Figure 1B,C). In addition, mice subjected to the Shwartzman reaction developed renal failure as represented by increased levels of creatinine and BUN. As shown in Figure 1D,E, renal failure was significantly reduced by P4pal-10 pretreatment. Finally, systemic inflammation may provoke lung injury characterized by pulmonary endothelial-epithelial leakage represented by increased protein levels in BALF. Figure 1F demonstrates that P4pal-10 significantly reduced protein leakage into the airspace.
In addition to PAR-4 inhibition, P4pal-10 may partially block PAR-1 signaling.34 To exclude partial PAR-1 inhibition as being responsible for the observed effects on organ damage, we analyzed the effect of cell-penetrating peptide P1pal-12 pepducin (which is specific for PAR-134 ) in a separate set of experiments. Table 1 demonstrates that P1pal-12 has no significant effect on transaminases, creatinine, BUN, and LDH and therefore suggests that the observed effects of P4pal-10 are independent of PAR-1 inhibition. Altogether these results suggest that P4pal-10–dependent inhibition of PAR-4 dose-dependently controls specific organ functions in the Shwartzman model.
Systemic markers of tissue injury
| . | Vehicle . | P1pal-12 . | P . |
|---|---|---|---|
| LDH, U/L | 864 ± 161 | 940 ± 139 | .96 |
| ASAT, U/L | 498 ± 47 | 488 ± 81 | .79 |
| ALAT, U/L | 138 ± 31 | 135 ± 22 | .79 |
| Creatinine, μM | 22.5 ± 6.5 | 21.7 ± 7.8 | .78 |
| BUN, mM | 20.7 ± 2.9 | 18.0 ± 3.1 | .39 |
| . | Vehicle . | P1pal-12 . | P . |
|---|---|---|---|
| LDH, U/L | 864 ± 161 | 940 ± 139 | .96 |
| ASAT, U/L | 498 ± 47 | 488 ± 81 | .79 |
| ALAT, U/L | 138 ± 31 | 135 ± 22 | .79 |
| Creatinine, μM | 22.5 ± 6.5 | 21.7 ± 7.8 | .78 |
| BUN, mM | 20.7 ± 2.9 | 18.0 ± 3.1 | .39 |
LDH, transaminases, creatinine, and BUN were determined in plasma of mice 6 hours after the endotoxin challenge (Shwartzman reaction) in mice subjected to P1pal-12 (0.5 mg/kg) or vehicle control. Data are means (± SEM).
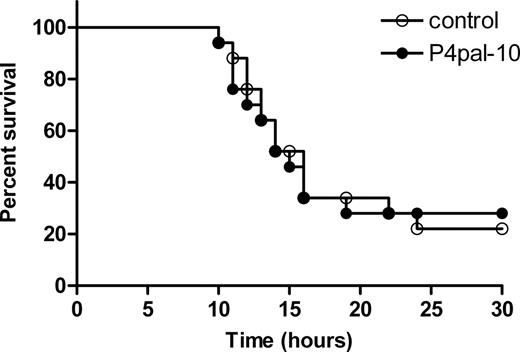
Effect of PAR-4 inhibition on DIC-induced mortality
To evaluate the consequence of the improvement in organ function by P4pal-10, we performed a survival experiment. Induction of the Shwartzman reaction in mice resulted in approximately 70% lethality within 30 hours after the challenge injection with LPS in both the control group and the P4pal-10–treated group (Figure 2). Thus, the improvement in organ function seems not to confer a survival advantage in this particular model.
Effect of PAR-4 inhibition on endotoxin-induced mortality. Survival was monitored in P4pal-10–treated (0.5 mg/kg; n = 17, ●) and saline-treated (n = 17, ○) mice subjected to the Shwartzman reaction. Indicated on the x-axis is the time after the challenging LPS reaction.
Effect of PAR-4 inhibition on endotoxin-induced mortality. Survival was monitored in P4pal-10–treated (0.5 mg/kg; n = 17, ●) and saline-treated (n = 17, ○) mice subjected to the Shwartzman reaction. Indicated on the x-axis is the time after the challenging LPS reaction.
Effect of PAR-4 inhibition on inflammation
To determine the effect of PAR-4 inhibition on inflammatory processes, we studied the effect of P4pal-10 on systemic inflammatory mediator production, neutrophil influx, and pulmonary edema formation in the Shwartzman reaction.
As shown in Table 2, the production of interleukin-6 (IL-6), monocyte chemotactic protein-1 (MCP-1), interferon γ (IFNγ), and tumor necrosis factor-α (TNF-α) was prominently induced by the Shwartzman reaction. Plasma levels of these proinflammatory mediators were decreased by approximately 40% to 70% as a result of P4pal-10 (Table 2). Thus, the production of proinflammatory mediators in plasma is attenuated as a result of PAR-4 inhibition.
Levels of systemic inflammatory mediators
| . | Baseline . | +DIC . | P . | ||
|---|---|---|---|---|---|
| Vehicle . | P4pal-10 . | Vehicle . | P4pal-10 . | ||
| IL6, ng/mL | nd | nd | 22.6 ± 6 | 7.8 ± 2.8* | <.05 |
| MCP-1, ng/mL | 48.5 ± 9 | 34.6 ± 15 | 57.1 ± 9 | 24.6 ± 6.8* | <.05 |
| IFNγ, pg/mL | nd | nd | 698 ± 145 | 327 ± 65* | <.05 |
| TNFα, pg/mL | nd | nd | 435 ± 83 | 255 ± 67 | .05 |
| . | Baseline . | +DIC . | P . | ||
|---|---|---|---|---|---|
| Vehicle . | P4pal-10 . | Vehicle . | P4pal-10 . | ||
| IL6, ng/mL | nd | nd | 22.6 ± 6 | 7.8 ± 2.8* | <.05 |
| MCP-1, ng/mL | 48.5 ± 9 | 34.6 ± 15 | 57.1 ± 9 | 24.6 ± 6.8* | <.05 |
| IFNγ, pg/mL | nd | nd | 698 ± 145 | 327 ± 65* | <.05 |
| TNFα, pg/mL | nd | nd | 435 ± 83 | 255 ± 67 | .05 |
IL-6, MCP-1, IFNγ, and TNFα levels were determined in mouse plasma before (baseline) and after the induction of the Shwartzman reaction (+DIC) in mice subjected to P1pal-10 (0.5 mg/kg) or vehicle control. Data are means (± SEM).
nd indicates nondetectable, below detection limit.
P < .05.
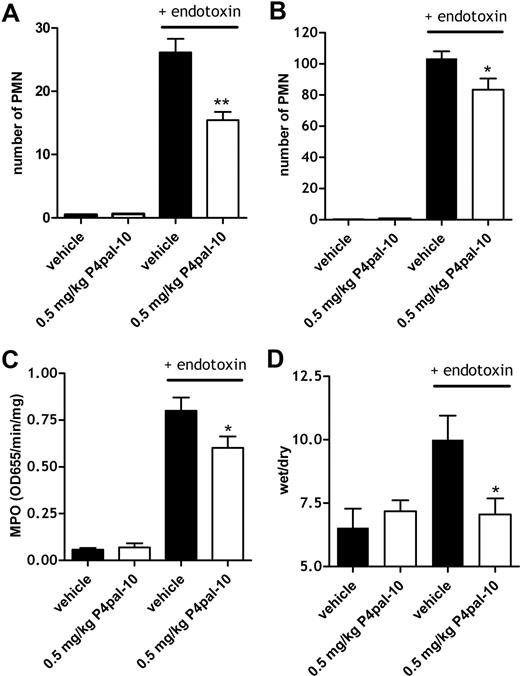
The influx of inflammatory cells such as neutrophils into susceptible organs (including lung, liver, and kidney) is an important feature of endotoxin-induced tissue injury. As shown in Figure 3, the induction of the Shwartzman reaction increased the number of neutrophils in kidney, liver, and lung. The influx of neutrophils in lung tissue was so abundant that it was difficult to score with precision. Therefore, to quantify the effect of PAR-4 inhibition on pulmonary neutrophil influx, we determined MPO activity in lung tissue (without performing the BAL procedure) as a marker for neutrophil inflammatory activity. Lung MPO activity was concomitantly increased by the Shwartzman reaction, and this increase was partly abolished by P4pal-10 pretreatment (Figure 3C), suggesting that total neutrophil activity within lung tissue was reduced by PAR-4 inhibition.
PAR-4 inhibition, neutrophil influx, and edema formation. Number of neutrophils (Ly6G-positive cells) for 10 microscopic fields (40 ×) in kidney (A) and liver (B) after the induction of the Shwartzman reaction with or without P4pal-10 (0.5 mg/kg) pretreatment. (C) Myeloperoxidase (MPO) activity as a marker of the presence of granulocytes in lung tissue after the induction of the Shwartzman reaction with or without (vehicle) 0.5 mg/kg P4pal-10 pretreatment. (D) Effect of P4pal-10 pretreatment (0.5 mg/kg) on endotoxin-induced pulmonary edema formation as represented by wet-dry ratios. Values are depicted as mean (± SEM). *P < .05
PAR-4 inhibition, neutrophil influx, and edema formation. Number of neutrophils (Ly6G-positive cells) for 10 microscopic fields (40 ×) in kidney (A) and liver (B) after the induction of the Shwartzman reaction with or without P4pal-10 (0.5 mg/kg) pretreatment. (C) Myeloperoxidase (MPO) activity as a marker of the presence of granulocytes in lung tissue after the induction of the Shwartzman reaction with or without (vehicle) 0.5 mg/kg P4pal-10 pretreatment. (D) Effect of P4pal-10 pretreatment (0.5 mg/kg) on endotoxin-induced pulmonary edema formation as represented by wet-dry ratios. Values are depicted as mean (± SEM). *P < .05
As an alternative marker for pulmonary injury, we determined edema formation, which is formed secondary to an increase in the permeability of the microvasculature in response to inflammatory stimuli. Figure 3D demonstrates that baseline wet-dry ratios (which are representative of edema formation) were not affected by P4pal-10 pretreatment, while induction of the Shwartzman reaction increased this wet-dry ratio. Pulmonary edema formation was significantly decreased after P4pal-10 treatment as can be seen in Figure 3D.
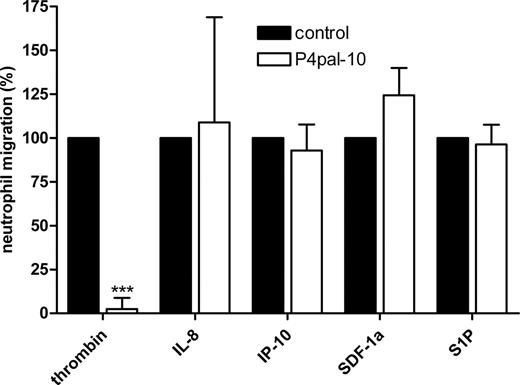
Effect of P4pal-10 on neutrophil migration
The above-mentioned results suggest that PAR-4 inhibition diminishes inflammation in a neutrophil-dependent manner. To substantiate that PAR-4 influences neutrophil function during acute inflammation, we preincubated freshly isolated neutrophils with 5 μM P4pal-10 and allowed these cells to migrate toward different G-protein–coupled receptor (GPCR) ligands that are known to function in migration of immune cells. As demonstrated in Figure 4, neutrophils efficiently migrate toward thrombin, and pretreatment with P4pal-10 completely abolishes this migration. In addition, neutrophils also migrate to other GPCR ligands, but P4pal-10 pretreatment did not affect migration toward these ligands. Thus, P4pal-10 specifically inhibits neutrophil migration toward thrombin.
P4pal-10 and neutrophil migration. Effect of P4pal-10 (5 μM) on migration of peripheral blood neutrophils toward thrombin (1 U/mL), interleukin-8 (IL-8; 100 ng/mL), interferon gamma–inducible protein-10 (IP-10; 10 ng/mL), stromal-derived factor 1 α (SDF-1α; 100 ng/mL), and sphingosine-1-phosphate (S1P; 100 nM). Data are expressed as values relative to non–P4pal-10–treated controls, which were set to 100% (mean ± SEM). ***P < .005.
P4pal-10 and neutrophil migration. Effect of P4pal-10 (5 μM) on migration of peripheral blood neutrophils toward thrombin (1 U/mL), interleukin-8 (IL-8; 100 ng/mL), interferon gamma–inducible protein-10 (IP-10; 10 ng/mL), stromal-derived factor 1 α (SDF-1α; 100 ng/mL), and sphingosine-1-phosphate (S1P; 100 nM). Data are expressed as values relative to non–P4pal-10–treated controls, which were set to 100% (mean ± SEM). ***P < .005.
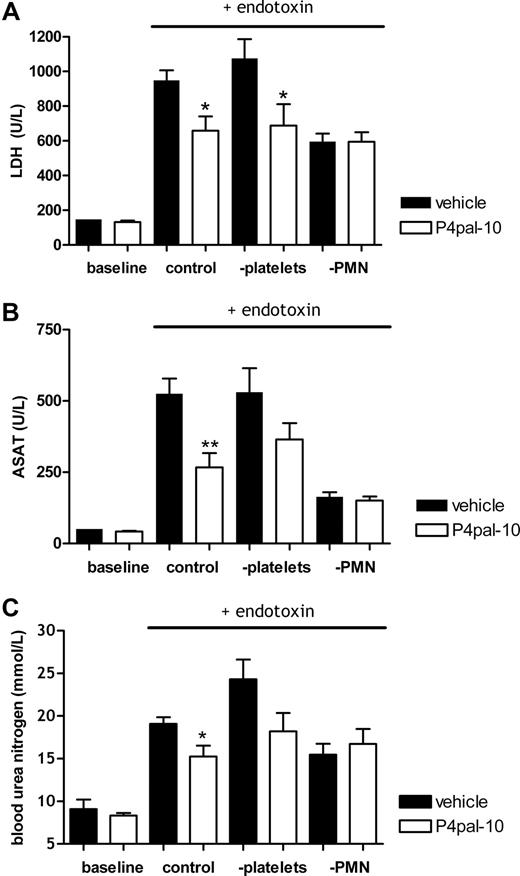
The role of neutrophils and platelets and the inhibition of PAR-4
Based on the above, we hypothesized that PAR-4 expressed on neutrophils plays a detrimental role in systemic inflammation and consecutive organ damage. Alternatively, one could speculate (“Introduction”) that PAR-4–dependent platelet activation plays an important role in this process. To discriminate between these 2 hypotheses, we subjected neutrophil-depleted or platelet-depleted mice to the Shwartzman reaction with or without P4pal-10 pretreatment.
Figure 5 again shows that subjection to the Shwartzman reaction induced tissue injury (as indicated by LDH increase), and PAR-4 inhibition significantly decreased LDH levels in control mice (Figure 5A) and, concurrently, inhibited liver and kidney failure (Figure 5B,C). With respect to LDH levels, platelet depletion did not influence P4pal-10–induced protection (Figure 5A). In accordance, liver and kidney damage (as represented by ASAT and BUN levels, respectively) revealed a trend toward improvement induced by P4pal-10 treatment in platelet-depleted mice (Figure 5B,C), which suggests that platelets are not crucial for P4pal-10–induced protection.
PAR-4 inhibition in neutrophil- and platelet-depleted mice. Effect of P4pal-10 (0.5 mg/kg) pretreatment in mice on injury provoked by the Shwartzman reaction. (A) LDH levels as a general marker for tissue injury. Liver damage as represented by (B) ASAT levels in plasma and (C) BUN as an indicator of renal failure. PMN indicates polymorphonuclear cell. Values are depicted as mean (± SEM). *P < .05
PAR-4 inhibition in neutrophil- and platelet-depleted mice. Effect of P4pal-10 (0.5 mg/kg) pretreatment in mice on injury provoked by the Shwartzman reaction. (A) LDH levels as a general marker for tissue injury. Liver damage as represented by (B) ASAT levels in plasma and (C) BUN as an indicator of renal failure. PMN indicates polymorphonuclear cell. Values are depicted as mean (± SEM). *P < .05
In contrast, the protective effect of PAR-4 inhibition disappeared after neutrophil depletion (Figure 5A-C right side), signifying that P4pal-10–inhibited neutrophil function contributes to the protection against the Shwartzman reaction.
Discussion
Ongoing systemic inflammation and coagulation may result in the obstruction of blood supply to various organs and the secretion of cytotoxic mediators, which contributes to the development of organ failure. In this setting, PAR-mediated cellular responses may add to inflammation,36,37 suggesting that coagulation-dependent PAR activation in systemic inflammation may promote injury and tissue damage. PAR-4 has been shown to (at least partly) mediate thrombin-induced pulmonary inflammation,16 thereby promoting a proinflammatory reaction via the release of inflammatory mediators such as cytokines or prostaglandins.38 Furthermore, in addition to a role in thrombin-induced platelet aggregation, PAR-4 activation has been suggested to contribute to motility disturbances during intestinal inflammation.39
To elucidate potential functional roles of PAR-4 in a setting of systemic inflammation accompanied by coagulation, we treated mice with a cell-penetrating pepducin of PAR-4 (P4pal-10) in the Shwartzman reaction. The most important finding of our study was that PAR-4 inhibition diminished the severity of the reaction as indicated by partial protection of individual organ function. Although the pretreatment of P4pal-10 diminished endotoxin-induced failure of liver, lung, and kidney function, it did not prevent mortality, indicating that the residual organ damage (and/or ongoing inflammatory response) after PAR-4 inhibition is still considerable. Overall, these data not only suggest that PAR-4 is involved in inflammatory disease but also indicate that mortality might not always be a suitable endpoint for investigating the involvement of specific host factors in extreme inflammatory models such as the Shwartzman reaction.
Interestingly, PAR-1 or -2 deficiencies in endotoxemia studies fail to support an important role for these PARs in systemic inflammation and survival.10,11 In agreement with our study, PAR-4 deficiency has been shown not to affect survival in the endotoxemia model. However, the effect of PAR-4 deficiency on inflammatory mediators and/or organ failure was not addressed in this previous study.11 Future experiments should clarify whether PAR-4 deficiency also protects against organ damage in the single-hit endotoxemia model or whether the protective effect observed in our study is dependent on the chosen model and/or the method of inhibition (pharmacological PAR-4 inhibition in our study versus genetic PAR-4 deficiency in the other study).
Previous studies in mice using P4pal-10 pepducin for PAR-4 inhibition demonstrated an extended bleeding time and protection against systemic platelet activation.34 In addition to PAR-4 inhibition, P4pal-10 also partially blocked PAR-1 signaling (5 μM P4pal-10 inhibited 36% of the PAR-1–dependent Ca2+ response in human platelets while inhibiting 97% of the PAR-4 response34 ). Although PAR-1 is often proposed as a mediator of coagulation-dependent inflammatory responses, PAR-1 deficiency had no effect on the outcome of murine endotoxemia.10,11 In line with these results, a specific intracellular PAR-1 antagonist pal-RCLSSSAVANRS (P1pal-12; 0.5 mg/kg) had no effect on sepsis severity or organ function in the Shwartzman reaction (Table 1), which supports PAR-4 rather than PAR-1 dependency in P4pal-10–mediated protection.
PAR-4–mediated signaling has been suggested to contribute to a proinflammatory reaction.33 Indeed, PAR-4 activation plays a critical proinflammatory role in neutrophil rolling and adherence,14 and the endogenous activation of PAR-4 via neutrophil granule protease cathepsin G13 may link neutrophil presence and PAR-4 activation to systemic inflammation.39 In addition to controlling granulocyte influx, PAR-4 activation may regulate edema formation,25 a phenomenon also observed in this study.
In the setting of the Shwartzman reaction, we have demonstrated that systemic inflammation is partially reduced as a result of PAR-4 inhibition (P4pal-10). Furthermore, local inflammation and in particular neutrophilic responses are diminished as a result of PAR-4 inhibition. Our results suggest an important role for PAR-4 in the control of systemic inflammation, possibly via reduced neutrophil influx into different organs, which was corroborated in an in vitro migration assay.
PAR-4 is also expressed on platelets, which upon endotoxin infusion (neutrophil dependently) accumulate in lung and liver,22 where they theoretically contribute to the development of tissue damage. Based on these observations, we expected that protection conferred by PAR-4 inhibition in the Shwartzman reaction model would be dependent on platelet as well as neutrophil function.
However, platelet depletion in mice did not protect against tissue injury (Figure 5), minimizing the contribution of platelets to the development of tissue damage. Thrombocytopenic mice were, however, (to some extent) protected by PAR-4 inhibition (Figure 5), implying that platelets do not play a major role in the protection mediated by P4pal-10.
Neutrophil depletion, on the contrary, abolished the protective effect of P4pal-10 in the Shwartzman reaction, implying that protection via PAR-4 inhibition was actually neutrophil dependent. Then again, in the absence of neutrophils, mice were protected from organ damage compared with the control mice (Figure 5), which we expect to depend on the absence of neutrophil-secreted inflammatory mediators and cytotoxic enzymes contributing to the development of tissue damage. The distinct protection of neutrophil deficiency against tissue injury may, however, conceal protection induced by PAR-4 inhibition. In addition, various other cell types besides neutrophils and platelets express PAR-4 (including endothelial cells and macrophages), and it is conceivable that these other cell types are also involved in PAR-4–mediated pathologies.
As already indicated, the Schwartzman model causes a more severe coagulopathy compared with single-dose endotoxin models. Whether this coagulopathy contributes to the PAR-4–dependent induction of multiple organ damage as observed in this study remains speculative. However, we show that thrombin is a strong (PAR-4 dependent) chemoattractant for neutrophils and that reduced neutrophil influx into affected organs likely contributes to the protective effect of PAR-4 deficiency. It is therefore tempting to speculate that the induction of extravascular thrombin formation (which is more profound in the Shwartzman reaction than in a single-dose endotoxin model27 ) would lead to increased neutrophil influx (in a PAR-4–dependent manner) and consequently cytokine release, thereby augmenting the inflammatory response and inducing subsequent organ damage. However, as thrombin is certainly not the only protease capable of PAR4 activation, future experiments are needed to prove or refute the involvement of thrombin in neutrophil influx and organ damage in this particular model.
We conclude that inhibition of PAR-4 using P4pal-10 in the Shwartzman reaction controls systemic inflammation and (multi)organ failure. Reduced neutrophil influx is likely to contribute to this protection.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a grant from the Netherlands Organization of Scientific Research (C.A.S) and the Dutch Thrombosis Foundation (T.L.). H.C. was a Clinical Established Investigator of the Netherlands Heart Foundation.
We would like to thank Joost Daalhuisen, Marieke ten Brink, and Maartje Obdeijn for expert technical assistance.
Authorship
Contribution: S.H.S designed and performed research, analyzed data, and wrote the paper; A.P.G. and M.F.B. performed research and analyzed data; P.H.R. designed research and wrote the paper; T.L. contributed vital reagents; H.C. designed research and wrote the paper; C.A.S. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: C. Arnold Spek, Center for Experimental and Molecular Medicine, H2–257, Academic Medical Center, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: c.a.spek@amc.uva.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal