Abstract
Neonates have an increased rate of infection with encapsulated bacteria compared with older children and adults because of diminished antibody responses to T-independent (TI) antigens such as bacterial polysaccharides. Because the interactions of tumor necrosis factor (TNF) family ligands BAFF and APRIL with the TNF family receptors (TNFRs) TACI, BCMA, and BAFF-R are crucial to TI antibody responses, we measured the expression of these receptors on adult and cord blood–derived term and preterm neonatal B cells. Preterm neonatal B cells expressed less TACI, BCMA, and BAFF-R compared with adult B cells and had significantly less proliferation compared with adult B cells after stimulation with human recombinant BAFF and anti-IgM in an assay in which TACI-Fc fusion protein inhibits B-cell proliferation. In addition, neonatal dendritic cells had diminished expression of B7–1, B7–2, and CD40 compared with adult cells. Finally, neonatal B cells, particularly preterm B cells, exhibited markedly decreased production of IgG and IgA in response to CD40L and IL-10. Overall, this study shows that maturational delay in TNFR expression particularly by preterm neonatal B cells may interfere with effective antibody responses to TI antigens, cognate T- and B-cell interactions and normal isotype switching.
Introduction
Term and preterm neonates are considered to be relatively “immunocompromised” and have diminished capacity to prevent the spread of bacterial and viral infections and to mount antibody responses particularly against T-independent (TI) antigens such as bacterial polysaccharides (PS).1-3
The conjugation of PS to protein renders these antigens immunogenic in term neonates because of the induction of T-cell help by the carrier protein.4,5 Preterm infants, however, make significantly less anti-PS antibodies than do term infants after immunization with conjugate vaccines at the same chronologic age as term infants.6-12 In addition, preterm infants are more prone to Haemophilus influenzae type b (Hib) infection after vaccine failure,5,9-11 and preterm infants that become infected after vaccine failure make 10-fold less convalescent anti-Hib Ab titers than do term infants, suggesting inferior memory responses after the initial immunization with the Hib conjugate vaccine.11 Similar low antibody responses have also been observed in preterm infants receiving the pneumococcal conjugate vaccine.12 These data suggest an important maturational delay in antibody responses to TI antigens (PS) and in the induction of T-cell help by a carrier protein in preterm neonates.
Components of the immune system that may be developmentally relevant to the observed delay in maturation of the adaptive immune system in term and preterm neonates include several members of the tumor necrosis factor receptor (TNFR) superfamily that are known to be important to B-cell survival, antibody production, isotype switching, and cognate interaction with T cells. The CD40-CD40 ligand and BAFF-BAFF receptor (BAFF-R) systems, both members of the TNF superfamily, play critical roles in the regulation of B-cell functions.13-17 The CD40-mediated pathway is crucial to T-cell–mediated B-cell activation. Ligation of CD40 expressed on B cells by CD40 ligand expressed on activated T cells stimulates B-cell survival, proliferation, differentiation, isotype switching, antigen presentation, germinal center (GC) development, and the memory responses by B cells.18 This costimulatory mechanism is required for the enhanced immune response to glycoconjugate vaccines.19 There is disagreement in the literature, however, whether CD40L is expressed and functions normally in human neonatal T cells and whether CD40 is present in normal quantity and functionality on neonatal B cells.20-23
Although antibody responses to TI antigens such as bacterial PS do not require CD40L-CD40 interactions, data show that antibody responses to bacterial proteins and PS that are associated with intact bacteria, such as encapsulated Escherichia coli, group B streptococcus (GBS), and polysaccharide-protein conjugate vaccines, are dependent on the interaction between CD28 on T cells and B7 on B cells.24,25 Thus, the antibody responses to bacteria likely to colonize or infect term and preterm neonates and the vaccines required to prevent infection require a coordinated interaction between B cells and CD4+ T cells.
BAFF (B-cell activating factor or B-lymphocyte stimulator also called BLyS) and APRIL (a proliferation-inducing ligand), 2 recently defined members of the TNF superfamily, are important regulators of B- and T-cell responses. BAFF is expressed primarily by monocytes, macrophages, and dendritic cells16,26-32 and is necessary for normal B-cell development and antibody production. BAFF binds to 3 receptors within the TNFR family: BAFF-R; B-cell maturation antigen (BCMA); and transmembrane activator, calcium-modulator, and cyclophilin ligand interactor, called TACI. APRIL appears to bind only to TACI and BCMA, and it is also important to TI antibody responses and isotype switching particularly to IgA.28,31 Deficiency of BAFF, TACI, or the expression of transgenic TACI-Ig, which blocks receptor ligand engagement, reduces B-cell numbers and causes deficient humoral immunity to TI antigens, including encapsulated bacterial pathogens such as Streptococcus pneumoniae.32 Finally, humans with common variable immunoglobulin and IgA deficiency have TACI mutations, further showing the functional importance of these TNFRs to human adaptive immunity.32 The ontogeny of these TNFRs, however, remains unknown as is their role in the maturational delay in the human antibody response to TI antigens.
We now report that the cell-surface expression, gene expression, and function of the B- and T-cell costimulatory molecules and the TNF family ligands in neonates and particularly preterm neonates are markedly diminished compared with healthy adults. The delayed ontogeny of expression of these molecules may significantly decrease the adaptive immune response of preterm neonates, particularly in terms of antibody responses to various polysaccharide components of colonizing or invading bacteria and to bacterial glycoconjugate vaccines.
Materials and methods
This study contains human subjects, and the study has been done in accordance to the Institutional Review Board (IRB) approval from the University of Minnesota. A verbal informed consent in accordance with the Declaration of Helsinki was obtained for this study as required by our IRB approval.
Materials
Purified human recombinant human BAFF, soluble CD40, and CD40L were purchased from R&D Systems (Minneapolis, MN). Purified recombinant human TACI-Ig was purchased from Axxora (San Diego, CA). All the antibodies for staining cells analyzed by flow cytometry were purchased from R&D Systems or from BD Pharmingen (San Diego, CA). Goat anti–human IgG, IgM, and IgA were obtained from Southern Biotechnology (Birmingham, AL). Human recombinant granulocyte-macrophage colony-stimulating factor (hGM-CSF), IL-4, IL-2, IL-10, and IL-7 were obtained from BD Biosciences (San Diego, CA).
Isolation of PBMCs
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood of healthy adult volunteers (AB) or cord blood (CB) by centrifugation over Ficoll-Paque gradients (Amersham Pharmacia, Piscataway, NJ) as previously described.33 CB of healthy term and preterm newborns was collected at the time of delivery at the University of Minnesota Medical Center and the University of Minnesota Children's Hospital, Fairview, in accordance with IRB approval. Mononuclear cells were prepared as just described. Red blood cell contamination was removed by lysis with lysing buffer (0.15 M NH4Cl, 10 mM NaHCO3, and 0.1 mM Na2EDTA.2H2O). PBMCs were incubated with lysing buffer for 10 minutes at room temperature and washed with complete medium containing RPMI 1640 supplemented with 10% fetal bovine serum, penicillin and streptomycin (100 U/mL), and l-glutamine (2 mM).
Purification and activation of T cells
Purified T cells were isolated from blood using CD3 magnetic cell sorting (MACS) magnetic beads (Miltenyi Biotech, Auburn, CA) by positive selection according to the manufacturer's instructions. These cells were stained with FITC-conjugated anti-CD3 and analyzed by flow cytometry. Freshly isolated T cells were cultured in complete medium with 10 ng/mL of PMA and 1 μg/mL of ionomycin (Sigma-Aldrich, St Louis, MO) for 6 hours, and CD40L gene expression was measured by quantitative polymerase chain reaction (PCR) using gene-specific primers. For kinetic experiments, T cells were incubated for 1 to 18 hours, and PCR was performed at the different time intervals.
T-cell priming experiments used T cells that were cultured in complete medium with PHA and PMA (Sigma-Aldrich), with recombinant human IL-2 (BD Pharmingen) for 3 days. Cells were then washed, resuspended in complete medium with recombinant human IL-7 (BD Pharmingen), and incubated for 2 days to allow quiescence of the cells, as defined by the CD69 surface expression to the baseline levels.15 These primed T cells were then again activated by PMA and ionomycin for 6 hours and mixed after fixation with B cells to measure Ig production as described in “Induction of immunoglobulin production by B cells.”
Purification and activation of B cells
B cells were purified from AB or CB PBMCs, using CD19+ MACS magnetic beads (Miltenyi Biotech) by positive selection according to the manufacturer's instructions, and purity of B cells was measured by staining with FITC-conjugated anti-CD19 followed by FACS analysis (>90% purity was obtained). B cells were activated with anti-IgM for 24 hours and stained for analysis with antibodies against BAFF-R, BCMA, and TACI as described in “Flow cytometry.”
Isolation of naive B cells from adult PBMCs
Naive B cells were obtained from AB PBMCs using a naive B-cell isolation kit II (MACS; Miltenyi Biotech) according to the manufacturer's specifications by depletion of nonnaive magnetically labeled cells (negative selection). Non-B cells (ie, T cells, natural killer cells, dendritic cells, monocytes, granulocytes, and erythroid cells) as well as nonnaive B cells (ie, memory B cells, activated B cells, and plasma B cells) were indirectly magnetically labeled with a cocktail of biotin-conjugated monoclonal antibodies against CD2, CD14, CD16, CD27, CD36, CD43, and CD235a (glycophorin A), and anti-biotin microbeads. Highly purified naive B cells were obtained by depletion of magnetically labeled cells by passing thorough a MACS column. The purity of enriched naive adult B cells was confirmed by flow cytometry in which B cells were stained with anti-CD19 FITC, anti-IgD FITC, and anti-CD27 PE.
Generation of monocyte-derived dendritic cells
Human monocytes were purified from PBMCs with the use of a Monocyte Isolation Kit II according to the instructions provided by the manufacturer (MACS; Miltenyi Biotech). To generate immature dendritic cells (iDCs), monocytes were cultured in RPMI 1640 medium containing 10% FCS with GM-CSF (800 U/mL) and IL-4 (500 U/mL; BD Pharmingen) at a concentration of 2 × 106 cells/mL, and half of the medium was replaced every other day with medium containing GM-CSF and IL-4. Mature dendritic cells were generated by stimulating iDCs that had been cultured with LPS (026:B6; Sigma-Aldrich; 1μg/mL) for an additional 24 hours as previously described.23
Flow cytometry
Surface staining of cells was accomplished by washing 1 × 105 cells in phosphate-buffered saline (PBS) containing bovine serum albumin (BSA) and incubated with blocking buffer (10 μg of each human IgG, IgM, and IgA). Monoclonal antibodies directly conjugated to FITC or PE against CD19, CD20, CD40, CD14, CD3, CD4, CD40L, CD69, CD45RA, CD11c (BD Pharmingen), CD80, and CD86 (R&D Biosystems) and against TACI, BAFF-R, and BCMA (Apotech, San Diego, CA) were subsequently used for cell staining. A volume of 100 μL of each antibody or isotype-matched control was added to appropriate tubes and further incubated for 30 minutes. Cells were washed with PBS containing 0.5% BSA and resuspended in the same buffer containing 1% paraformaldehyde before analysis. Flow cytometric acquisition was performed on a FACSCalibur and associated CellQuest software (BD Biosciences). These data were analyzed using FlowJo software (Tree Star, Ashland, OR).
B-cell proliferation assay
The proliferation of B cells in response to BAFF was measured as described previously.25 Briefly, purified B cells were cultured in 100 μL of medium in 96-well U-bottomed culture plates with 100 ng/mL of recombinant human soluble BAFF (R&D Biosystems) or with anti-human IgM (10 μg/mL; BD Biosciences) or both for 72 hours. For blocking experiments, B cells pretreated with 2 μg/mL TACI-Ig or control-Ig were cultured with soluble BAFF and anti-human IgM. B-cell proliferation was quantitated by pulsing the cells during the last 18 to 24 hours of culture with 1 μCi (0.037 MBq) of [3H]-thymidine (Amersham Pharmacia, Piscataway, NJ) per well. Cells were harvested by an automated 96-well cell harvester (PerkinElmer, Shelton, CT), and incorporation of [3H]-thymidine was quantified by liquid scintillation counting (TopCount; PerkinElmer).
Induction of immunoglobulin production by B cells
Purified B cells (2.5 × 105) obtained from CB or AB were cultured in 24-well plates in the presence of human recombinant CD40L (1 μg/mL) and IL-10 (10 ng/mL). Some experiments used helper T cells to stimulate B cells in which freshly isolated T cells from AB and term CB were primed as described in “Purification and activation of T cells” and fixed with 1% paraformaldehyde. After washing with the medium, the fixed T cells from AB and term CB expressing CD40L were added to the B-cell culture from unrelated cord blood and adult blood. After 10 days of culture, supernatants were collected, and the concentration of Igs was measured by isotype-specific enzyme-linked immunosorbent assay (ELISA).
Gene expression by quantitative PCR
Adult, neonatal, and preterm neonatal T cells obtained as described in “Purification and activation of T cells” were activated for 0, 1, 3, 6, and 18 hours with PMA (10 ng/mL) and ionomycin (1 μg/mL). B cells were activated using anti-human IgM. Total RNA from activated and inactivated T and B cells was isolated using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. First-strand cDNA was synthesized from total RNA (1 μg) using oligo (dT) primers (50 μM) and Superscript III reverse transcriptase (200 U) as described by the manufacturer (Invitrogen). The primers for each gene were designed from the published human sequences using Primer3 software (http://primer3.sourceforge.net; open source) (Table 1). To establish a consistent standard curve for the quantitative PCR, 1 μg of Q-PCR reference RNA (Stratagene, La Jolla, CA) was used. Each cDNA sample was analyzed in triplicate by real-time quantitative reverse transcription (RT)–PCR using iCycler (Bio-Rad, Hercules, CA). Quantitative assessment of DNA amplification was detected through the dye SYBR Green according to the manuals provided by the manufacturer for SYBR Green QPCR Master Mix (Stratagene), using 75 to 250 nM individual primers (Proligo, Boulder, CO) for each gene. Data were normalized by amplifying the housekeeping gene β-actin during each PCR run and expressed as relative units as previously described.34,35
Sequences of oligonucleotides used as primers in quantitative PCR
| Oligonucleotide . | Sequence . |
|---|---|
| β-Actin sense | 5′-TCAGAAGGATTCCTATGTGGGCGA-3′ |
| β-Actin antisense | 5′-TTTCTCCATGTCGTCCCAGTTGGT-3′ |
| BCMA sense | 5′-CCAACCTGGTGAAACCCTGTC-3′ |
| BCMA antisense | 5′-AACGATTCTCCTGCCTCAGCC-3′ |
| BAFF-R sense | 5′-TGGAGTTTGGTGTGCTTGCC-3′ |
| BAFF-R antisense | 5′-TCATAGTGCCCCAGCTTTTG-3′ |
| TACI sense | 5′-TGACCATCTCCTGAGGGACTG-3′ |
| TACI antisense | 5′-TGGAAGGTTCACTGGGCTCC-3′ |
| B7–1 sense | 5′-ATGCTGCCTGACCTACTGCT-3′ |
| B7–1 antisense | 5′-ATCTTTTCAGCCCCTTGCTT-3′ |
| B7–2 sense | 5′-AAGTATATGGGCCGCACAAG-3′ |
| B7–2 antisense | 5′-CATTCCTGTGGGCTTTTTGT-3′ |
| CD40 sense | 5′-TTCTTTGTGCCAGCCAGGAC-3′ |
| CD40 antisense | 5′-CAGTGTGTCTCTCTATTCCAG-3′ |
| CD40L sense | 5′-CAGGGAAGTTTTGTGGAGGA-3′ |
| CD40L antisense | 5′-CACAATTCCACGCAATCAAG-3′ |
| CD69 sense | 5′-TCTCAATGCCATCAGACAGC-3′ |
| CD69 antisense | 5′-ACAAGCATTTTGGGCTGAAG-3′ |
| Oligonucleotide . | Sequence . |
|---|---|
| β-Actin sense | 5′-TCAGAAGGATTCCTATGTGGGCGA-3′ |
| β-Actin antisense | 5′-TTTCTCCATGTCGTCCCAGTTGGT-3′ |
| BCMA sense | 5′-CCAACCTGGTGAAACCCTGTC-3′ |
| BCMA antisense | 5′-AACGATTCTCCTGCCTCAGCC-3′ |
| BAFF-R sense | 5′-TGGAGTTTGGTGTGCTTGCC-3′ |
| BAFF-R antisense | 5′-TCATAGTGCCCCAGCTTTTG-3′ |
| TACI sense | 5′-TGACCATCTCCTGAGGGACTG-3′ |
| TACI antisense | 5′-TGGAAGGTTCACTGGGCTCC-3′ |
| B7–1 sense | 5′-ATGCTGCCTGACCTACTGCT-3′ |
| B7–1 antisense | 5′-ATCTTTTCAGCCCCTTGCTT-3′ |
| B7–2 sense | 5′-AAGTATATGGGCCGCACAAG-3′ |
| B7–2 antisense | 5′-CATTCCTGTGGGCTTTTTGT-3′ |
| CD40 sense | 5′-TTCTTTGTGCCAGCCAGGAC-3′ |
| CD40 antisense | 5′-CAGTGTGTCTCTCTATTCCAG-3′ |
| CD40L sense | 5′-CAGGGAAGTTTTGTGGAGGA-3′ |
| CD40L antisense | 5′-CACAATTCCACGCAATCAAG-3′ |
| CD69 sense | 5′-TCTCAATGCCATCAGACAGC-3′ |
| CD69 antisense | 5′-ACAAGCATTTTGGGCTGAAG-3′ |
Enzyme-linked immunosorbent assay
Ninety-six–well ELISA plates were coated with an anti-IgA, -IgG, and -IgM (Southern Biotechnology) at a concentration of 1 μg/mL in phosphate-buffered saline (PBS) and incubated overnight at 4°C. The plates were washed in wash buffer (0.05% Tween 20 in PBS) and blocked with the blocking buffer (1% BSA in PBS). The plates were washed again, and the culture supernatants were then added to the plates. Standards of purified human IgA, IgG, and IgM (Pierce Biotechnology, Rockford, IL) were diluted and added to the plates. Control wells with PBS alone were included in each plate. The plate with supernatant and standards were incubated overnight at 4°C and washed again, and then alkaline phosphatase–conjugated antibodies to IgA, IgG, and IgM (Southern Biotechnology) diluted to 1:2000 in blocking buffer were added and incubated on their respective plates for 2 hours at room temperature. The plates were washed again, and pNPP (p-nitrophenylphosphate; Sigma-Aldrich) substrate diluted in diethanolamine buffer (pH 9.8) was then added to the plates. The concentration of Ig in the supernatant was assessed by spectrophotometric analysis at 415 nm using a model 550 Microplate Reader (Bio-Rad Laboratories).36
Statistics
All data were expressed as mean plus or minus SEM, and analyses were performed by using the Statistical Analysis System, release 9.1 (SAS Institute, Cary, NC). When appropriate, logarithmic or square root transformations were used on the data to approximate normality. Group differences were tested by using analysis of variance (PROC GLM).37 To compare groups over time and to compare groups exposed to different experimental treatments, the PROC MIXED procedure was used.38 A conservative significance level was set at .001 to account for multiple comparisons.
Results
Naive B-cell population
The purity of enriched naive B cells was evaluated by flow cytometry by staining the cells with antibody against the B-cell lineage markers, CD19-FITC or CD20-PE. CD27 and IgD were used for the discrimination of naive B cells from other B cells (eg, CD27 for the identification of memory B cells and IgD for the identification of naive B cells) as previously reported.39,40 By surface staining 94% to 96% of the B-cell population was IgD positive, and very low numbers of cells were CD27+ (2%-3%). We consistently found that 95% or more CB total B cells were of naive phenotype; therefore, further sorting was not performed for these cells, but AB B cells used for each experiment were purified for their naive phenotype.
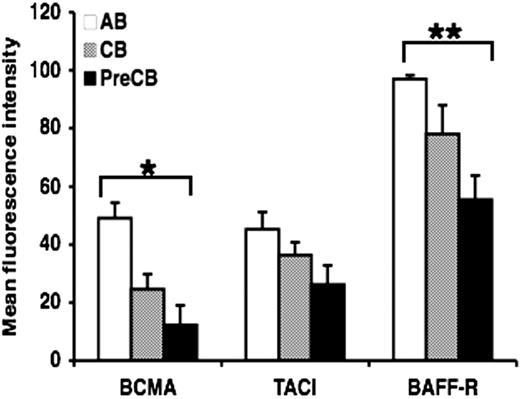
Surface expression of TACI, BAFF-R, and BCMA is lower in B cells from preterm cord blood compared with adult B cells
Because infants are particularly refractory to antigenic stimulation with TI antigens, and TACI and other TNFR are now thought to be crucial as costimulatory signals for B cells making antibody to TI antigens, we hypothesized that the ontogeny of these receptors might be delayed. Analysis by flow cytometry showed that the surface expression of BCMA and BAFF-R was decreased in B cells from neonatal cord blood, particularly preterm CB compared with B cells obtained from normal adult blood (Figure 1). Although surface expression of TACI was decreased in preterm CB B cells compared with AB B cells, statistical significance was not reached (Figure 1). Similar results were obtained using B cells activated with anti-IgM (data not shown). Isotype-matched controls were used throughout the study and always reacted with less than 3% of the cells.
Decreased expression of BAFF receptors on preterm cord blood–derived naive B cells compared with adult naive B cells. Freshly isolated naive B cells were assessed for the surface expression of BAFF-R, BCMA, and TACI by flow cytometry. Data are shown as mean (± SEM) of 10 independent experiments using 10 independent adult (AB), cord (CB), or preterm cord (PreCB) blood samples with each assay performed in triplicate. Statistically significant differences are shown. *P < .001; **P ≤ .005.
Decreased expression of BAFF receptors on preterm cord blood–derived naive B cells compared with adult naive B cells. Freshly isolated naive B cells were assessed for the surface expression of BAFF-R, BCMA, and TACI by flow cytometry. Data are shown as mean (± SEM) of 10 independent experiments using 10 independent adult (AB), cord (CB), or preterm cord (PreCB) blood samples with each assay performed in triplicate. Statistically significant differences are shown. *P < .001; **P ≤ .005.
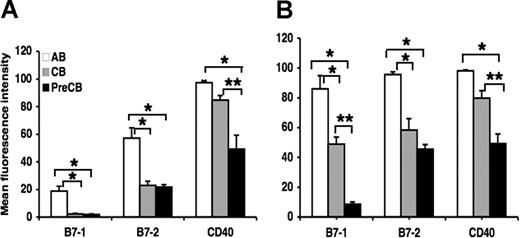
Surface expression of B7–1, B7–2, and CD40 is reduced in neonatal B cells and dendritic cells compared with adult cells
Surface expression of B7–1 and B7–2 was significantly reduced on B cells obtained from term and preterm CB compared with B cells obtained from AB, although the intensity of B7–1 staining was much lower than the intensity of B7–2 staining for term and preterm B cells (Figure 2A). CD40 expression in preterm neonatal B cells was less than that of B cells from adult and term neonatal B cells (Figure 2A). There were no significant differences between term and preterm B-cell expression of B7–1 and B7–2. Surface expression of B7–1, B7–2, and CD40 was significantly reduced on term and particularly preterm CB monocyte-derived dendritic cells compared with AB monocyte-derived dendritic cells. In addition, preterm CB dendritic cells expressed less B7–1 and CD40 than did term CB dendritic cells (Figure 2B).
Decreased surface expression of B7–1, B7–2, and CD40, cognate B-cell ligands for T-cell interaction, in term and preterm cord blood–derived B cells and dendritic cells compared with adult cells. Monocytes were purified from PBMCs and differentiated into dendritic cells as described in “Generation of monocyte-derived dendritric cells.” Data are shown as mean (± SEM) of 10 independent experiments using 10 independent adult (AB), cord (CB), or preterm cord (PreCB) blood samples with each assay performed in triplicate for cord blood–derived B cells (A) and dendritic cells (B). Statistically significant differences are shown. *P < .001; **P ≤ .005.
Decreased surface expression of B7–1, B7–2, and CD40, cognate B-cell ligands for T-cell interaction, in term and preterm cord blood–derived B cells and dendritic cells compared with adult cells. Monocytes were purified from PBMCs and differentiated into dendritic cells as described in “Generation of monocyte-derived dendritric cells.” Data are shown as mean (± SEM) of 10 independent experiments using 10 independent adult (AB), cord (CB), or preterm cord (PreCB) blood samples with each assay performed in triplicate for cord blood–derived B cells (A) and dendritic cells (B). Statistically significant differences are shown. *P < .001; **P ≤ .005.
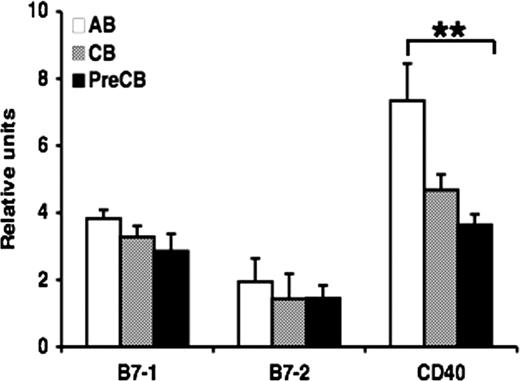
Gene expression of TNFR is reduced in neonatal B cells compared with adult B cells
Quantitative PCR was used to determine relative gene expression of TNFR family receptors in B cells obtained from CB or AB. There was significant reduction in gene expression of CD40 in cells from preterm CB compared with AB B cells, but no significant differences were observed in gene expression of B7–1 and B7–2 in neonatal B cells compared with AB B cells (Figure 3). Similar results were observed comparing anti-IgM–activated B cells from term CB or AB, suggesting that the differences in gene expression were not due to differential proportions of CB and AB B cells being in the resting state (data not shown).
Gene expression of CD40 is decreased in preterm neonatal cord blood–derived B cells compared with adult B cells. Real-time quantitative RT-PCR was performed on total RNA obtained from freshly isolated naive B cells. The amount of mRNA for the gene of interest was normalized relative to β-actin mRNA, and the graphs were generated with the relative values obtained after normalization. Data are shown as mean (± SEM) of 10 independent experiments using 10 independent adult (AB), cord (CB), or preterm cord (PreCB) blood samples with each assay performed in triplicate. Statistically significant differences are shown. *P < .001; **P ≤ .005.
Gene expression of CD40 is decreased in preterm neonatal cord blood–derived B cells compared with adult B cells. Real-time quantitative RT-PCR was performed on total RNA obtained from freshly isolated naive B cells. The amount of mRNA for the gene of interest was normalized relative to β-actin mRNA, and the graphs were generated with the relative values obtained after normalization. Data are shown as mean (± SEM) of 10 independent experiments using 10 independent adult (AB), cord (CB), or preterm cord (PreCB) blood samples with each assay performed in triplicate. Statistically significant differences are shown. *P < .001; **P ≤ .005.
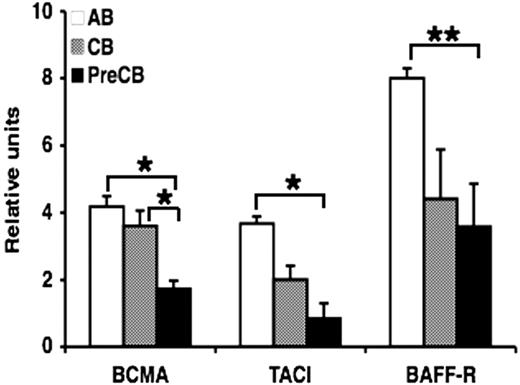
Gene expression of the BAFF and APRIL receptors BCMA, TACI, and particularly BAFF-R was also reduced in neonatal B cells, especially in preterm CB B cells compared with AB B cells. In addition, preterm CB B cells expressed significantly less BCMA compared with term CB B cells (Figure 4). Activation of B cells before measurement of gene expression yielded similar results (data not shown).
Gene expression of TNF family BAFF receptors, BCMA, TACI, and BAFF-R, is decreased in preterm neonatal cord blood–derived B cells compared with adult B cells. Real-time quantitative RT-PCR was performed on RNA obtained from freshly isolated naive B cells. The amount of mRNA for each gene of interest was normalized relative to β-actin mRNA, and the graphs were generated with the relative values obtained after normalization. Data are shown as mean (± SEM) of 10 independent experiments using 10 independent adult (AB), cord (CB), or preterm cord (PreCB) blood samples with each assay performed in triplicate. Statistically significant differences are shown. *P < .001; **P ≤ .005.
Gene expression of TNF family BAFF receptors, BCMA, TACI, and BAFF-R, is decreased in preterm neonatal cord blood–derived B cells compared with adult B cells. Real-time quantitative RT-PCR was performed on RNA obtained from freshly isolated naive B cells. The amount of mRNA for each gene of interest was normalized relative to β-actin mRNA, and the graphs were generated with the relative values obtained after normalization. Data are shown as mean (± SEM) of 10 independent experiments using 10 independent adult (AB), cord (CB), or preterm cord (PreCB) blood samples with each assay performed in triplicate. Statistically significant differences are shown. *P < .001; **P ≤ .005.
Gene expression of CD40L is significantly reduced in cord blood T cells compared with adult T cells
Because induction of CD40L expression on activated T cells is believed to play a key role in humoral and cell-mediated immune responses through engagement of CD40 on B cells, we determined the CD40L gene expression in purified neonatal and adult T cells activated by PMA and ionomycin at different time points by quantitative PCR. CD40L expression peaked at 3 hours of stimulation, and reduced gene expression was observed in term and preterm CB T cells at 3, 6, and 18 hours compared with AB T cells (Figure 5A). Expression of CD69, a control marker associated with the activation of T cells, was comparable in preterm and term CB T cells and in AB T cells at each time point (Figure 5B). In addition to CD40L, surface expression of additional functional molecules involved in cell activation (CD28, CD4, and CD8) was investigated, but no significant differences in the kinetics of expression were detected (data not shown).
Gene expression of CD40L but not CD69 is decreased in term and preterm neonatal cord blood–derived T cells compared with adult T cells during 18 hours after activation. Real-time quantitative RT-PCR was performed on RNA obtained from T cells activated at the indicated time. The amount of mRNA for CD40L (A) and CD69 (B) was normalized relative to β-actin mRNA, and the graphs were generated with the relative values obtained after normalization. Data are shown as mean (± SEM) of 10 independent experiments using 10 independent adult (AB), cord (CB), or preterm cord (PreCB) blood samples with each assay performed in triplicate. Statistically significant differences are shown. *P < .001; **P ≤ .005.
Gene expression of CD40L but not CD69 is decreased in term and preterm neonatal cord blood–derived T cells compared with adult T cells during 18 hours after activation. Real-time quantitative RT-PCR was performed on RNA obtained from T cells activated at the indicated time. The amount of mRNA for CD40L (A) and CD69 (B) was normalized relative to β-actin mRNA, and the graphs were generated with the relative values obtained after normalization. Data are shown as mean (± SEM) of 10 independent experiments using 10 independent adult (AB), cord (CB), or preterm cord (PreCB) blood samples with each assay performed in triplicate. Statistically significant differences are shown. *P < .001; **P ≤ .005.
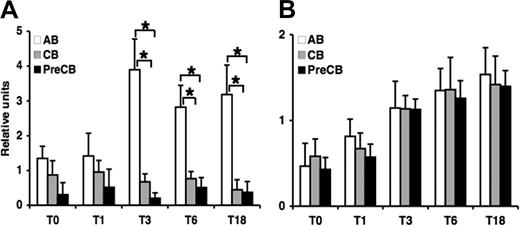
Preterm cord blood B-cell proliferation is reduced compared with adult cells in response to costimulation with anti-IgM and BAFF
To determine the functional effect of this decreased expression of TNF family receptors, we investigated whether exogenous BAFF could stimulate anti-IgM–activated CB B cells to proliferate in a normal fashion as previously described for AB B cells.25 BAFF plus anti-IgM strongly costimulated B-cell proliferation by 7- to 8-fold in AB B cells compared with term and preterm CB B cells (Figure 6). In the absence of costimulus, soluble BAFF induced a moderate increase in B-cell proliferation in AB B cells that was more than term or preterm CB B cells but did not reach statistical significance. The addition of TACI-Ig (10 μg/mL) but not irrelevant human Ig blocked the B-cell proliferation, showing that B-cell proliferation observed in this assay was mediated primarily by costimulation of BAFF.
BAFF-induced B-cell proliferation by neonatal cord blood–derived B cells is decreased compared with adult B cells. Naive B cells were stimulated with anti-IgM (10 μg/mL) in the presence or absence of BAFF (100 ng/mL) and tested for proliferation. For the blocking assay the TACI-Ig fusion protein was used at 2 μg/mL. Proliferation was measured by 3H-thymidine [1 μCi (0.037 MBq) per well] incorporation of B cells during the last 18 hours of a total of 96 hours. Data are shown as mean (± SEM) of 10 independent experiments using 10 independent adult (AB), cord (CB), or preterm cord (PreCB) blood samples with each assay performed in triplicate. Statistically significant differences are shown. *P < .001; **P ≤ .005.
BAFF-induced B-cell proliferation by neonatal cord blood–derived B cells is decreased compared with adult B cells. Naive B cells were stimulated with anti-IgM (10 μg/mL) in the presence or absence of BAFF (100 ng/mL) and tested for proliferation. For the blocking assay the TACI-Ig fusion protein was used at 2 μg/mL. Proliferation was measured by 3H-thymidine [1 μCi (0.037 MBq) per well] incorporation of B cells during the last 18 hours of a total of 96 hours. Data are shown as mean (± SEM) of 10 independent experiments using 10 independent adult (AB), cord (CB), or preterm cord (PreCB) blood samples with each assay performed in triplicate. Statistically significant differences are shown. *P < .001; **P ≤ .005.
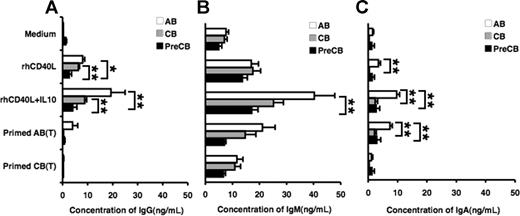
Production of immunoglobulin by stimulated B cells from preterm cord blood is reduced compared with adult B cells
Freshly isolated and mitogen-primed T cells were assayed for their ability to induce antibody production by B cells. T cells were activated with PMA and ionomycin, fixed with paraformaldehyde, and then added into the B-cell culture. CB B cells produced comparable amounts of IgM compared with adult B cells when stimulated with CD40L or CD40L and IL-10, whereas preterm CB B cells produced significantly less IgM than did AB B cells (Figure 7B). In addition, preterm CB B cells produced markedly less IgG after stimulation with CD40L or CD40L and IL-10 than either AB or term CB B cells (Figure 7A). Furthermore, IgA production after CD40L and IL-10 stimulation by term or preterm CB was significantly lower than that of AB B cells (Figure 7C). Although IgG production was decreased in term and preterm CB B cells in response to mitogen-primed AB T cells, it did not reach statistical significance (Figure 7A). On the one hand, IgA production was significantly lower in term or preterm CB B cells compared with AB B cells (Figure 7C). On the other hand, primed CB T cells were unable to stimulate significant amounts of IgG or IgA production in AB or CB B cells.
Induction of IgG, IgM, and IgA immunoglobulin production is decreased in preterm cord blood–derived B cells in response to CD40L plus IL-10 compared with adult blood B cells. Naive B cells were stimulated with the indicated ligands or medium alone for 10 days, and IgG (A), IgM (B), and IgA (C) production from the culture supernatants were measured by ELISA. Data are shown as mean (± SEM) of 10 independent experiments using 10 independent adult (AB), cord (CB), or preterm cord (PreCB) blood samples with each assay performed in triplicate. Statistically significant differences are shown. *P < .001; **P ≤ .005.
Induction of IgG, IgM, and IgA immunoglobulin production is decreased in preterm cord blood–derived B cells in response to CD40L plus IL-10 compared with adult blood B cells. Naive B cells were stimulated with the indicated ligands or medium alone for 10 days, and IgG (A), IgM (B), and IgA (C) production from the culture supernatants were measured by ELISA. Data are shown as mean (± SEM) of 10 independent experiments using 10 independent adult (AB), cord (CB), or preterm cord (PreCB) blood samples with each assay performed in triplicate. Statistically significant differences are shown. *P < .001; **P ≤ .005.
Discussion
Neonates remain particularly susceptible to infections with bacterial pathogens and often do not mount effective antibody responses. In particular, neonates are unable to mount effective antibody responses against bacterial PS and TI antigens. Previous hypotheses to explain impaired neonatal antibody responses suggested that immaturity of neonatal B and T cells, especially diminished expression of CD40L, might explain some but not all of the defects.15 Because decreased expression of CD40L results in defects in class switch recombination yielding human conditions such as hyper IgM syndrome in which B cells fail to switch from IgM to IgA and IgG, it seems logical that the immaturity in immunoglobulin production might in part be due to a decreased ability to provide cognate T-cell help to B cells. We found that CD40L gene expression by neonatal T cells was consistently lower compared with adult T cells as previously described.15 This finding does not represent a global activation defect in gene expression because of immaturity, because CD69 was expressed at similar levels by activated term, preterm, and adult T cells. Interestingly, we also found that neonatal, particularly preterm B cells and dendritic cells (DCs), underexpressed B7–1, B7–2, and CD40 compared with AB B cells and DCs. The B7/CD28 interaction along with the CD40-CD40L interaction, is crucial in stimulating proliferation, T-cell activation, and cytokine secretion, particularly, B7–2 that plays a dominant role in primary immune responses.41,42
The TNFR family member ligands BAFF and APRIL and their receptors, particularly TACI and BAFF-R, are also crucial for isotype switching of human B cells as well as for the antibody response to TI antigens such as bacterial PS.43,44 Mice with TACI deficiency cannot respond to TI antigens and do not isotype switch normally. Recently, it was reported that humans with mutations in TACI have decreased or defective isotype-switched memory B cells and manifest common variable immune deficiency with IgA deficiency and recurrent bacterial infections.27,44 Regulation of BCMA, TACI, and BAFF-R expression is poorly understood, and our data suggest that the expression of BCMA, TACI, and BAFF-R is decreased, particularly in preterm B cells compared with adult B cells. In addition, the decreased gene expression by both term and preterm B cells compared with adult B cells for these TNFRs was more pronounced than the decrease in surface expression. To distinguish between different mechanisms that can account for the apparent disparities in mRNA levels versus protein levels, future studies will be necessary to fully explore the mechanistic details accounting for decreased expression of receptors that participate in cognate T-B interactions in CB and AB B cells.
We were also able to show that decreased surface expression of the TNFR on preterm neonatal B cells translated into functional differences reflected by reduced proliferation after stimulation with BAFF and anti-IgM. Moreover, B-cell proliferation was inhibited by TACI-Ig, which binds to 2 known ligands, BAFF and APRIL. This finding is consistent with the previous reports that BAFF is the key factor for B-cell proliferation, and APRIL does not appear to play a major role in B-cell costimulation.27 Although we did not sort cells by isotype, both adult and term CB B cells but not preterm B cells yielded higher proliferation after stimulation with anti-IgM. We assume that many of the AB B cells and most of the neonatal and preterm B cells were IgM secreting. These data suggest preterm B cells are also deficient in activation after cross-linking surface immunoglobulin. Our current experiments only determined receptor expression and cellular function at the time of birth and did not investigate the time frame of their maturation. The ideal approach would be to use peripheral blood from neonates over time instead of only cord blood. Unfortunately, this approach is challenging because of the relatively large volumes of blood required for these experiments.
The term and preterm neonatal B cells were capable of making IgM after stimulation with CD40L and IL-10 or by exposure to primed T cells but had consistently lower levels of IgG and IgA, particularly in preterm CB, relative to those of adult B cells. These results are consistent with the observation that neonatal B cells are mostly uncommitted IgM+ and IgD+ cells45 and also that both the term and preterm newborns are capable of producing IgM in response to intrauterine challenge with pathogens. Primed adult T cells induced IgM production in both neonatal and adult B cells but little IgG or IgA in term or preterm B cells. Taken together, these results suggest neonatal immaturity not only because of T cells but also B cells, because term or preterm B cells were unable to support IgG or IgA production comparable to AB B cells despite being activated by primed T cells from adults. Because these T cells were fixed before stimulation, it is possible that the absence of cytokines secreted by T cells, which are required for isotype switching, caused these low levels of IgG and IgA production.16,46
We conclude that B cells derived from CB of term, and particularly preterm, neonates are deficient in expression of CD40, TACI, BCMA, and BAFF-R. This maturational delay in expression is associated with decreased B-cell proliferation in response to BAFF and anti-IgM and with decreased production of IgG and IgA on stimulation with CD40L plus IL-10. The ontogeny of the TNFR on B cells may in part explain the adaptive immune defects that have been observed in neonates to a variety of pathogens and TI antigens.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Heather Cohen for her technical assistance during the revision of this manuscript. We thank the nurse practitioners in the University of Minnesota Children's Hospital Neonatal Intensive Care Unit for their help in providing us with term and preterm cord blood. We thank Anne Jurek for her expert statistical analysis.
This work was supported by National Institutes of Health grants AI 46661 and AI32596 (J.R.S.).
National Institutes of Health
Authorship
Contribution: K.K. performed and designed the research and data analysis, and wrote portions of the manuscript; S.C. performed and analyzed research data and wrote portions of the manuscript; N.S.G. provided initial reagents, techniques, and scientific guidance during the first portion of research and was instrumental in the development of the final conclusions; J.R.S. was the principal investigator of this project and provided scientific guidance and critiques, reviewed data and data analysis, wrote portions of the manuscript, and edited all versions.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kulwant Kaur, Department of Pediatrics, University of Minnesota Medical School, 420 Delaware St SE, MMC 296, Minneapolis, MN 55455; e-mail: kambo002@umn.edu.

![Figure 6. BAFF-induced B-cell proliferation by neonatal cord blood–derived B cells is decreased compared with adult B cells. Naive B cells were stimulated with anti-IgM (10 μg/mL) in the presence or absence of BAFF (100 ng/mL) and tested for proliferation. For the blocking assay the TACI-Ig fusion protein was used at 2 μg/mL. Proliferation was measured by 3H-thymidine [1 μCi (0.037 MBq) per well] incorporation of B cells during the last 18 hours of a total of 96 hours. Data are shown as mean (± SEM) of 10 independent experiments using 10 independent adult (AB), cord (CB), or preterm cord (PreCB) blood samples with each assay performed in triplicate. Statistically significant differences are shown. *P < .001; **P ≤ .005.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/8/10.1182_blood-2007-01-069245/4/m_zh80210708800006.jpeg?Expires=1767710184&Signature=JXHrfo3vWgXUuy26zQdhYYioEOMixm5mOYXTm9kTaAGPcJHyIPP8Hq9qKfbtE11rdS09svk7BoGAXXg1x34ePAXBSAv9VLfD8NfJusq0zXWd1RsRhyY0Wp~gGJXJ5CP3izRQJExdnhNcSbGQ1SrXW8w~M53ZyVrKV70kWgCwjMNAzEJXLWtEI8qPcC9pxU~-AzmiUjUW69NGrR0C40fkcCUuSEU85SQWYllVbkVz8WWDLsQQadj6RiALgXSokRwCUISru6p9lb0OtkQK3IVYJiTgzV56k4Y69Dkte~xxQYGS~CwzOcGnZgD1IqwQaZBROFI4HlN5lpFVR3JcY1NU4Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal