In this issue, Jang and Sharkis show that hemopoietic stem cells (HSCs) can be fractionated into 2 major subpopulations based on the cellular content of reactive oxygen species (ROSs). Low ROSs predicted a high repopulating capacity along with multipotency, whereas high ROS content predicted relatively poorer HSC functions.
Attempts at precisely identifying rare HSCs within the hemopoietic heterogeneity have often been frustrating; many markers are shared by mature cells, but more importantly the most rigorous methods that separate HSCs from other cells yield a minuscule fraction that is nevertheless variable in terms of self-renewal.1 It is of paramount importance, therefore, to redefine the stem state and discover alternative HSC characterization tools (reviewed by Zipori2 ). In the Jang and Sharkis study, a novel cell feature, ROS content, is found to discriminate between mouse HSC subtypes. ROSlow HSCs have long-term repopulating capacity upon serial transplantations in irradiated recipients and are also multipotent. Conversely, the ROShigh subpopulation declines upon repeated transplantation and is partially lineage restricted. Importantly, the better functional performance of ROSlow cells corresponded to several properties ascribed to long-term repopulating HSCs, such as quiescence and capacity to interact with niche osteoblasts. By contrast, the stem-cell phenotype CD34−LSK was common to the ROShigh and ROSlow populations, highlighting the limitation of cell surface marker usage. The superior repopulating capacity of ROSlow cells suggests that they might be the HSCs found in the reduced oxygen sites within the bone marrow that constitute the stem-cell niches. Indeed, ROSlow cells exhibited a relatively higher adhesion capacity to osteoblastic cells. It is, however, yet to be demonstrated that ROSlow cells do indeed reside within the osteoblastic bone marrow niche in situ.
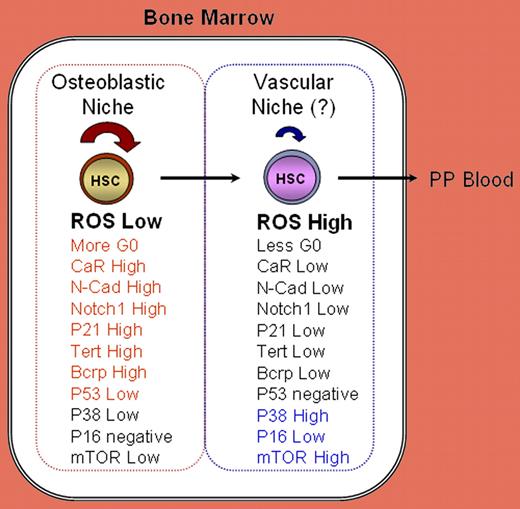
Although both ROSlow and ROShigh cells were found to share the HSC phenotype CD34−Lin−Sca+cKit+, they nevertheless markedly differ in essential stem-cell functions. As summarized here, they also differ in the cell-cycle status and in several key regulatory molecules. See the complete figure in the article beginning on page 3064.
Although both ROSlow and ROShigh cells were found to share the HSC phenotype CD34−Lin−Sca+cKit+, they nevertheless markedly differ in essential stem-cell functions. As summarized here, they also differ in the cell-cycle status and in several key regulatory molecules. See the complete figure in the article beginning on page 3064.
Stem-cell niches in general are constructed in such a way that the stem cell physically interacts with stromal cells that express antagonists of differentiation. The latter interfere with the activity of differentiation inducing cytokines to allow self-renewal. This was first demonstrated for mammalian HSCs3 and later substantiated by studies on Drosophila gonadal stem cells.4 Other components of the stem-cell niche include chemokine instructions and adhesion interactions. It has been further demonstrated that hypoxic areas within the bone marrow recruit hemopoietic progenitor cells.5 The present study raises the possibility that it is the abundance of ROSs within the stem cell, rather than in the microenvironment, that contributes to the maintenance of the stem state. These findings raise several questions that should be further experimentally examined: What is the molecular mechanism the controls ROSs in stem cells? Is the formation of ROSs in stem cells instructed by the stromal niche? Are ROShigh cells a differentiated product of ROSlow cells? Pharmacological inhibition of p38 or mTOR, which are found in elevated levels in ROShigh cells, restored their performance in LTC-IC culture assays. The latter are regarded as an in vitro corollary of in vivo repopulation tests. This suggests the need to develop strategies to convert ROShigh cells into ROSlow species, with the aim of improving HSC transplantation and subsequent engraftment in human patients.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal